* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download rev1
Survey
Document related concepts
Transcript
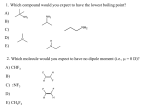
Introduction to Organic and Biochemistry Fall 2016 Review for Exam One About the exam: this exam will consist of multiple choice, nomenclature, structure drawing, finishing reactions and short answer questions. It will cover chapters 11 and 12 Chapter 11: Saturated Hydrocarbons 1. Know that Wohler is considered to be the father of organic chemistry. Know why that is. (Know how he disproved Vitalism theory.) 2. Know what organic chemistry is and is not: CO2, CO, CN are not considered organic. 3. Know what a hydrocarbon is. Where do hydrocarbons come from? How are they purified from one another? 4. Know how to draw the structural formula, the condensed structural formula, the skeletal formula and the line structural formula for any hydrocarbon. 5. Be able to use table 11.2 to identify major families (functional groups) for organic compounds. 6. Know how sp3 hybridization is achieved for carbons to make alkanes. Know that there is free rotation about the sigma bonds and know the bond angles and geometry for an alkane. 7. Be able to name alkanes by IUPAC rules. Know methane through nonane for parent name, know halogens, know the substituents we used in class. 8. Know what constitutional and geometrical isomers are. 9. Be able to name cycloalkanes includes the use of cis and trans. 10. Know and be able to explain the physical properties of alkanes and cycloalkanes 11. Know the chemical properties of alkanes and cycloalkanes. Be able to finish and balance reactions concerning: a. combustion b. halogenation Chapter 12: Unsaturated Hydrocarbons 1. Know that these include alkenes, alkynes and aromatics. Know what those are 2. Know how sp2 hybridization is achieved for alkenes. Know that double bonds are made from one sigma and one pi bond and the pi bond restricts rotation about the double bond. Know the bond angles and geometry for an alkene. 3. Know how to name alkenes and cycloalkenes by IUPAC rules, including use of cis and trans for geometric isomers. 4. Know how cis and trans are important to the vision cycle. 5. Know about the chemical properties of alkenes and cycloalkenes. If more that one organic product is made, show the major product, as applies, for the following: a. addition of hydrogen b. addition of a halogen c. addition of hydrogen halides using Markovnikov’s rule d. addition of water using Markovnikov’s rule e. polymerization f. combustion 6. Know how sp hybridization is achieved for alkynes. Know that triple bonds are made from one sigma and two pi bonds. Know the geometry and bond angles for alkynes. 7. Know how to name alkynes by IUPAC rules. 8. Know that the chemical properties are the same as for alkenes, but 2 moles of reactant per mole of hydrocarbon are required since we have two pi bonds to break. 9. Know what an aromatic compound is, the geometry and the bond angles.