* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download thebacterialflagellum
Magnesium transporter wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Protein moonlighting wikipedia , lookup
Endomembrane system wikipedia , lookup
Signal transduction wikipedia , lookup
Protein phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein domain wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Cytokinesis wikipedia , lookup
List of types of proteins wikipedia , lookup
Type three secretion system wikipedia , lookup
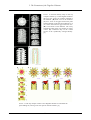
Helsinki University of Technology Laboratory of Computational Engineering S-114.500 Basics for Biosystems of the Cell Fall 2004 6.1.2005 ASSIGNMENT REPORT THE BACTERIAL FLAGELLUM Sebastian Köhler 55017P Index INDEX 1 Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 The Flagellum. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.1 The Engine and Hook. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2.2 The Propeller. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 The Swimming of a Peritrichous Bacterium. . . . . . . . . . . . . . . . . . . . . . 4 The Structure of Flagellin. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 The Formation of the Flagellar Filament . . . . . . . . . . . . . . . . . . . . . . . . 5.1 Self-Assembly and the Export System. . . . . . . . . . . . . . . . . . . . . . 5.2 The Cap Complex. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 References. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 2 3 3 4 6 7 9 9 9 12 1. Introduction 1 INTRODUCTION The dutch scientist Antonie van Leeuwenhoek was the first to describe bacteria in the late 17th century [1]. He manufactured microscopes and discovered small animals in his well water. He described them as “little eels, or worms, lying all huddled up together and wriggling” [2]. The bacteria he was observing was spirillum, probably Spirillum Volutans. However, Leeuwenhoek did not see its flagellum; the propulsion system of bacteria. The bacterial flagellum was first seen on Chromatium okenii by the german naturalist and zoologist Christian Ehrenberg about 150 years later in 1836. In 1872 Ferdinand Cohn also saw it on S. Volutans [2]. The bacterial flagellum is a large organelle consisting of over 20 different proteins, some of them present in only a couple of ten molecules, while others form huge complexes consisting of tens of thousands of molecules. The flagellum looks like a long thin string protruding from the bacterium. Different species have different numbers and arrangements of flagella, largely affecting the way in which the bacterium swims in a viscous fluid. Bacteria are classified into four groups based on the flagellum: Monotrichous, Amphitrichous, Lophotrichous and Peritrichous. Amphitrichous Picture 1. A collage of pictures of the four groups of bacteria classified according to the number and arrangement of flagella [3]. These groups are illustrated in picture 1. Monotrichous bacteria have a single flagellum. If the flagellum is located at either end of the bacterium it has a monotrichous polar distribution. An amphitrichous bacterium has one flagellum at both ends of the body. Lophotrichous bacteria have several flagella in a close formation at one end of the oblong body and if the flagella are evenly distributed over the whole body, then the bacterium is called peritrichous. In this assignment report the structure and composition of the bacterial flagellum will be discussed, as well as the means by which a peritrichous bacterium can swim and change direction of movement. Furthermore, the self-assembly of the 2 2. The Flagellum long, stringlike propeller will be discussed. This report is based on the material stated in the References - section. 2 THE FLAGELLUM The propulsion system of bacteria, or the flagellum as it is called, consists of three parts as illustrated in picture 2. The basal body embedded in the cell wall constitutes the engine. A short “hook” or “universal joint” is attached to the engine and a long helical filament: the propeller. Picture 2. The structure of the bacterial flagellum. The stators (green) and the rotor (red) embedded in the cell membrane constitute the engine. The protruding hook (dark orange) is followed by the propeller (lighter orange). 2.1 THE ENGINE AND HOOK The rotary engine consists of a rotor and stators. It is approximately 30 nm in diameter. Picture 3 gives a more detailed view of the engine, and as can be seen, the stators are made of two proteins, MotA and MotB, anchored to the inner membrane. Two other proteins, S ring and M ring, form the rotor inside the cylindrical stator complex. There are also many other proteins attached to the rotor, essential to the operation of the engine. Torque is generated between the rotor and the stator by the flow of protons from the outside to the inside of the cell. This is a result of a concentration gradient between the bacterium and its environment, probably evoked by the cell’s metabolism. As the protons flow across the cell’s membrane through the stators, 3 2. The Flagellum which are proton channels, it is thought [2] that a part of the protein MotA moves or undergoes a conformational change, which exerts a force on the protein FliG. FliG is attached to the rotor and therefore this would cause the rotor to rotate. The engine is fully reversible and can reach speeds of ~20000 rpm [4] by itself, but with an attached filament (propeller) it only reaches 200 – 1000 rpm [5]. Picture 3. A detailed picture of the structure of the engine and hook. [4] The hook is a joint between the engine and propeller. It is attached to the rotor through a series of different proteins and allows the helical filament to point away from the cell. The hook is approximately 55 nm long and consists of a single protein, FlgE. The hook, like the propeller, which will be discussed next, is hollow. 2.2 THE PROPELLER The propeller, a helical filament, is a hollow tube 20 nm thick and 10 – 15 µm long [4]. It is composed of a single protein: flagellin, or FliC, as it is also called. There are about 30000 flagellin subunits forming the filament. At the end of the filament there is a cap protein complex. Its importance, as well as the tube’s hollowness, will be explained later. The helical filament consists of 11 protofilaments of flagellin subunits twisted together to form the compact structure. There are about 11 subunits per 2 turns. The protofilaments, however, come in two different types, resulting in two different conformations of a straight filament (all 11 protofilaments are of the same type): Rtype (right-handed twist) and L-type (left-handed twist). 4 2. The Flagellum A study [4] of these two straight filaments revealed that the repeat distance along the protofilament in the L- and R-type straight filament differ with 0,8 Å (see picture 5). Picture 4. A detailed picture of the structure of the helical filament, which is the propeller. [4] Picture 5. Comparison between the repeat distance along the protofilament in the L- and R-type straight filaments. [4] Picture 6. 3D electron density maps of the Land R-type straight filaments. Resolution ~10 Å. Scale bar, 100 Å. [4] The repeat distance in the L-type is 52,7 Å and 51,9 Å in the R-type. This means that the flagellin subunits are more thightly packed in the R-type protofilaments. 5 3. The Swimming of a Peritrichous Bacterium There are no wild-type bacteria with straight filaments, since these would not be very effective propellers. A more effective propeller is supercoiled, i.e. it twists around itself like a corkscrew. The study of the two straight filaments is the key to understanding what causes the supercoiling of the filaments in wild-type bacteria. 3 THE SWIMMING OF A PERITRICHOUS BACTERIUM As previously mentioned, a peritrichous bacterium has flagella all over its body, protruding in different directions. A question now arises: How can it move in a specific direction and how does it change direction? The helical filament of each flagellum consists of different numbers of L- and R-type protofilaments, and since the flagellin subunits are more dense in the R-type protofilaments, these are also slightly shorter. This causes the supercoiling of the flagellar filament. Depending on the combination of different protofilaments there are two helical forms of supercoiled filament: left- and right-handed. A normal filament of wild-type flagellin, has two R-type protofilaments and nine L-type protofilaments if it is left-handed. The right-handed form is obtained by switching all R-type protofilaments to L-type, and vice versa [6]. Picture 7. The swimming motion of the peritrichous bacterium. The movement alternates between two modes: “run” and “tumble”. [7] When the bacterium moves forward it is said to “run”. In this mode several left-handed flagellar filaments are bundled together generating the force which allows the bacterium to move in a specific direction. During this mode of movement the engine of each flagellum in the bundle rotates counter clockwise, as seen from outside the cell. To change direction the bacterium quickly reverses the rotors of the flagella. This sudden twist causes the left-handed supercoiled filaments to be transformed into right-handed filaments. This, in turn, causes the bundle to rapidly fall apart. Now the bacterium is said to be in “tumble” mode. The individual flagella cannot move the bacterium in any given direction, but the resulting erratic movement alters the orientation of the bacterium. When the engines are once again 6 4. The Structure of Flagellin reversed it is headed in a new direction (see picture 8). The average run lasts for about 1 s and the tumble for only about 0,1 s [2]. Picture 8. The swimming pattern of a peritrichous bacterium. [4] In the next section we’ll take a look at how the filament can switch between its left- and right-handed forms as quickly as it does. 4 THE STRUCTURE OF FLAGELLIN In order to find out how the switching between different supercoiled filaments is accomplished, the atomic structure of flagellin (the monomer of the filament) had to be defined. This was done by Namba et al. [6]. They crystallized a fragment of Salmonella flagellin by clipping of peptides from the carboxy- and aminoterminal ends. Then, with an improved cryocrystallographic technique, they obtained an atomic model at 2.0 Å resolution. Picture 9. The structure of the fragment of Salmonella flagellin. The Cα backbone is to the left and all hydrophobic side-chains are displayed with the backbone to the right. [6] This fragment consists of three distinct domains, labeled D1, D2 and D3. The fragment looks like a boomerang, or a pair of airplane wings, each about 70 Å long 7 4. The Structure of Flagellin and about 20 Å wide at the center. An interesting part of the flagellin fragment is the β-hairpin (140-160) in domain D1. Two parallel distorted β-turns are linked at the tip by a crossed loop (146-153). The atomic model of the flagellin fragment was docked into an electron density map of the R-type straight filament (see picture 10) and the fit was almost perfect. The docking revealed that the flagellin subunits in a single protofilament are connected through interactions between domain D1 (near the β-hairpin) of the upper subunit and domain D1 (upper part) and a small part of domain D2a of the lower subunit. Picture 10. Docking of the atomic model of flagellin into an electron density map of the filament. [6] Because the L-type protofilament repeat is only 0,8 Å longer than the R-type, the conformational change between these forms are relatively small. To study the nature of the conformational change Namba et al. used a model of three consecutive protofilament subunits. The top subunit was fixed and then the lower subunit was pulled down to see what would happen in the middle subunit. The lower subunit’s Cα backbone was translated 0,1 Å at each step and energy minimization was carried out. Up to a dislocation of 4,5 Å the middle subunit stretched elastically, but after this point over the next 0,2 Å a sudden conformational change was observed in the β-hairpin in domain D1. This has lead to the belief that it is, in fact, the βhairpin which is responsible for the to distinct conformations of the protofilament and that it is also the switch which alters the protofilament at subångström precision. A more extensive model simulation is still needed to prove that this is actually true. 8 5. The Formation of the Flagellar Filament 5 THE FORMATION OF THE FLAGELLAR FILAMENT 5.1 SELF-ASSEMBLY AND THE EXPORT SYSTEM The bacterial flagellum is formed from the inside of the cell outward, by a process called self-assembly. In self-assembly the component proteins interact spontaneously without the aid of enzymes or other factors. In this section the selfassembly of the flagellar filament will be described. Not until the basal body and the FliF ring has been formed at the inner membrane (see picture 11) does the self-assembly of the hook and the filament begin. This is carefully regulated by genetic control; for example, no FliC proteins are made until the basal body is complete. It is believed that a protein export system is attached to the FliF ring and that it selectively exports flagellar proteins into the hollow tube by using the energy of ATP hydrolysis [8]. The hollow tube of the hook and the filament is approximately 30 Å wide [8]. Picture 11. The bacterial flagellum with the protein export system attached to the FliF ring. The export system exports flagellin subunits through the central channel to the distal end of the flagellar filament. [8] 5.2 THE CAP COMPLEX In order for the growing process of the filament at its distal end to proceed, a protein cap complex has to stay attached to the growing end. The cap, made of the protein HAP2 (Hook Associated Protein 2), is an assembly promoter, and the flagellin subunits which travel through the central channel polymerize underneath it. The cap complex is vital to the self-assembly, without it the flagellin subunits 9 5. The Formation of the Flagellar Filament would simply leak out of the hollow filament. A dimer of the cap complex is shown in picture 12. Picture 12. A dimer of the cap complex. (A) Electronmicroscope image. Scale bars: left long 290 Å; left short 100 Å; bottom 120 Å. (B) Solid surface representation of the 3D density map. [8] Picture 13. (B) Electron cryomicrographs of the cap-filament complex. (C) Averaged image of the cap-filament complex. The inner tube and the plate of the cap complex can clearly be seen. Scale bar, filament diameter: 230 Å. [8] As picture 12B shows, the cap complex is a pentameric plate with 5 leg-like anchor domains. The plate is 120 Å wide and 25 Å thick. Picture 13C shows that there is a cavity right beneath the pentameric plate of the cap complex. This cavity inside the filament is roughly 40 Å wide and 70 Å deep [8]. All domains of the folded flagellin protein, except domain D2, are small enough to pass through the hollow tube through the hook and the filament. This suggests that the flagellin subunit is not completely folded when the export system inserts it into the central channel. This cavity seems to have the right size to allow a flagellin subunit to fold without interference into its final conformation before it is polymerized into the filament. The five legs of the cap complex are also anchored inside the cavity. This leaves five gaps between the plate of the cap and the filament. These gaps are of varying shapes and sizes (see picture 14C), but only one is large enough to allow a flagellin subunit to fit into it. This is the binding site for the next flagellin protein. As the flagellin binds to its proper place in the growing filament it forces the cap complex to rotate into the next energetically stable and equivalent position to the current one. This is accomplished by the “walking” of the anchor legs inside the cavity. Thus only one flagellin binding site is left open for the next protein subunit. 10 5. The Formation of the Flagellar Filament Picture 14. Electron density maps of the cap complex and the tip of the flagellar filament. (A) Top view of the cap complex attached to the filament. (B) Side view of the filament. (C) Five views of the gaps between the plate and the filament in the directions labeled 1-5 in (A). Notice the inverted L gap in the first view. (D) Cross-section of the filament. The cavity beneath the plate of the cap complex is clearly visible. (E) Contoured map of the central section of the cylindrically averaged density. [8] Picture 15. The cap complex rotates as new flagellin subunits are inserted at the open binding site, leaving a new site open for the next subunit. [8] 11 6. References 6 REFERENCES [1] [2] Bra Böckers Lexikon, Vol 14, Bokförlaget Bra Böcker, 1987 Physics today on the Web: Howard C. Berg, Motile Behavior of Bacteria, http://www.aip.org/pt/jan00/berg.htm Mary Johnson, Mechanisms of Bacterial Motility, http://www.indstate.edu/thcme/micro/flagella.html Keiichi Namba, Dynamic Aspects of the Bacterial Flagellum, Macromolecular Architecture, 2004; 333-344 Wikipedia - The Free Encyclopedia, http://www.wikipedia.com Samatey F, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K, Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling, Nature, 2001; 410, 331-337 http://microbiology.okstate.edu/faculty/demed2/lecture_notes/ cell%20biologyppt.html Yonekura K, Maki S, Morgan DG, DeRosier DJ, Vonderviszt F, Imada K and Namba K, The Bacterial Cap as the Rotary Promoter of Flagellin Self-Assembly, Science, 2000; 290, 2148-2152 [3] [4] [5] [6] [7] [8] 12