* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 3.4 Diffusion and Osmosis
Cell nucleus wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Programmed cell death wikipedia , lookup
Signal transduction wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
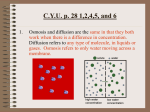
DIFFUSION AND OSMOSIS 3.4 KEY CONCEPT • Materials move across membranes because of concentration differences. Diffusion and Osmosis • Molecules can move across the cell membrane through passive transport. • Passive transport does not require energy input from a cell • There are two types of passive transport 1. Diffusion 2. Osmosis Diffusion • Diffusion is the movement of molecules in a fluid or gas from high concentration to low concentration • Molecules diffuse down a concentration gradient. • “Move downhill” or from high to low Diffusion • Diffusion stops when dynamic equilibrium (spread evenly, but molecules still moving) is reached Diffusion • Concentration • Number of molecules in a substance in a given volume • Concentration gradient • Difference in concentration from one area to another Diffusion • Diffusion plays a key role in cells ability to move substances into and out of the cell • Small lipids and nonpolar molecules like O2 and CO2 diffuse easily • Cells continually consume O2 • Therefore the concentration of O2 is almost always higher outside the cell • Result O2 diffuses into cell without the need of energy Osmosis • Osmosis is the diffusion of water molecules across a semipermeable membrane from high water molecule concentration to low H20 concentration. • Continues until dynamic equilibrium is reached Osmosis • Homeostasis • Biological Balance • Key concept of selectively permeable membrane and osmosis is to maintain homeostasis! Osmosis • Tonicity • A measure of water pressure against a semipermeable membrane • Three types of Tonicity 1. Isotonic • Concentration of solute is equal inside and outside of cell Cell in Isotonic Solution 10% NaCl 90% H2O ENVIRONMENT CELL 10% NaCl 90% H2O What is the direction of water movement? The cell is at _______________. equilibrium NO NET MOVEMENT Osmosis 2. Hypertonic • The concentration of solute is greater outside the cell • Plasmolysis – The process by which the cell shrinks from losing water Cell in Hypertonic Solution 15% NaCl 85% H2O ENVIRONMENT CELL 5% NaCL 95% H2O What is the direction of water movement? Osmosis 3. Hypotonic • The concentration of solute is greater inside the cell • Cytolysis – The process by which a cell bursts from water entering • Turgor pressure – Pressure exerted on the cell wall of plants due to water pushing out Cell in Hypotonic Solution 10% NaCl 90% H2O CELL 20% NaCl 80% H2O What is the direction of water movement? Osmosis • Some single celled organisms and animals are adapted to survive hypotonic solutions • They have structures (cell wall and vacuole) to store or remove (contractile vacuole) excess water Practice • Draw arrows to indicate the direction of water movement into cell, out of cell, or both! • The 10% solution represents a cell Video • Tonicity and osmosis Some Molecules diffuse through transport proteins • Facilitated Diffusion • Some molecules cannot easily diffuse across the cell membrane. • A transport or carrier protein provides a door for a substance to enter the cell. Square peg in a round hole idea! • Still No energy is used