* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Stereoselective Reduction of Ketones with Sodium Borohydride
Marcus theory wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Elias James Corey wikipedia , lookup
George S. Hammond wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Petasis reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
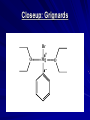
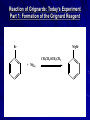
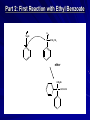
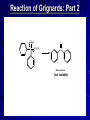
The Grignard Synthesis Miniscale Synthesis of Triphenylmethanol from Ethyl Benzoate MgBr Organic Chemistry Lab II, Spring 2010 Dr. Milkevitch March 29 & 31, 2010 Today’s Experiment Take a look at some very important chemistry Prepare a Grignard Reagent – Group of very important synthetic reagents React it with an ester – Make an alcohol Purpose: – Learn about organometallic reagents Prepare one: phenylmagnesium bromide – Accomplish a reaction in low water conditions – Use the Grignard reagent to make an alcohol Background Organometallic Reagents – Extremely useful group of organic compounds – Allow for some interesting chemistry to take place – Their use: Make carbon-carbon bonds Grignard Reagents – Developed by Victor Grignard in the turn of the 20th century – Discovered: Organic halides and magnesium form organomagnesium reagents Called organometallic compounds Organometallic Compounds Contain metal-carbon bonds Many examples in chemistry In Organic Chemistry: – 2 main “classes” of organometallic compounds Organomagnesium reagents (“Grignards”) Organolithium reagents Usefulness of these compounds – The carbon atoms is nuclophilic – Widely used to make new carbon-carbon bonds – Used to attach carbonyl groups of aldehydes, ketones, and esters Formation of the Reagent Formed by reaction of an alkyl halide with magnesium metal – Reaction commonly done in ether solvents – Under very low water conditions Br MgBr CH 3CH 2OCH 2CH 3 + Mg(s) Result: Grignard Reagent Carbon-magnesium bonds are polar – Carbon atom has a partial negative charge – Makes the carbon nucleophilic Its going to “look” for a positive charge – Capable of attacking electrophilic carbons Such as carbons in carbonyl groups Br Mg Function of the Ether Solvent Grignard reagents – Commonly formed in ether solvents They stabilize the Grignard reagent – Protect it from oxidation Must use anhydrous solvents – Grignards are very sensitive reagents – Grignards are strong bases – Will react with any reagent with an acidic proton Water Alcohols Carboxylic acids – Destroys the reagent Closeup: Grignards Br O Mg O Reaction of Grignards: Today’s Experiment Part 1: Formation of the Grignard Reagent Br MgBr CH 3CH 2OCH 2CH 3 + Mg(s) Part 2: First Reaction with Ethyl Benzoate MgBr O C OCH 2CH 3 ether OMgBr C OCH 2CH 3 Reaction of Grignards: Part 2 OMgBr O C C OCH2CH3 Benzophenone (not isolable) Reaction with 2nd Equivalent of Grignard Reagent O MgBr C Benzophenone ether OMgBr OH + H2O triphenylmethoxymagnesium bromide triphenylmethanol Procedure I All glassware must be dry – Dried in the 120 deg C oven overnight – Already done! RB flask, stir bar, condenser, drying tube 125 ml erlenmeyer flask, Claisen adaptor, grad cylinder, several small erlenmeyer flasks Remove from oven, allow to cool, assemble according to diagram on next slide – CAUTION: It will be hot!!!!!!!! Reaction Setup Procedure II Allow glassware to cool before assembling Weigh out 960 mg of Mg turnings – Place in RB flask with stir bar Assemble apparatus In the addition funnel – Place 4.2 ml of dry bromobenzene – 20 ml of anhydrous ethyl ether – Swirl to mix Have a ice bath standing by to cool reaction if necessary Add about 1 ml of bromobenzene/ ether to the RB flask – Open stopcock, let ~ 1 ml of bromobenzene /ether to go into the flask Turn on cooling water in the condenser Look for cloudiness/ bubbles on the metal surface – Indicates the reaction has begun! If after about 5 min you don’t see anything – Add a small crystal of iodine Wait another 5 min, if again no reaction occurs….. Procedure III Ask for assistance – I will come over and work some type of magic in the attempt to get your reaction started – I won’t tell you what I’m doing, because I’m not sure what I will do to get your reaction going – I’ll giggle it around and look at it a lot, and say “hmmmm” a great deal – And your reaction will probably, eventually start – We hope! In all likelihood, it will eventually begin Once the reaction has started, turn on the stirrer Add the remainder of the bromobenzene/ ether solution dropwise to maintain a steady reflux Addition should take ~ 45 min When addition is done, add 3 ml of ether to the addition funnel – To rinse down any residual bromobenzene – Add this rinse to the RB flask Fit the flask with a heating mantle – Reflux the mixture gently for 15 min When the 15 min is over, most of the Mg should be gone – But its not likely – Proceed with the experiment Procedure IV Add 1.41 ml of ethyl benzoate to 10 ml of anhydrous ether Place this in the addition funnel Add the ethyl benzoate solution to the Grignard reagent at rate that will maintain a steady reflux After all the ethyl benzoate has been added – Reflux the solution (with stirring) for 30 min When complete, remove from heat and allow to cool Parafilm flask and set it aside until the next lab period DO NOT USE A GLASS STOPPER Place flask inside a beaker – Place in the hood until next week