* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organometallic Reagents: Sources of Nucleophilic Carbon for
Homoaromaticity wikipedia , lookup
Metal carbonyl wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Elias James Corey wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Stille reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
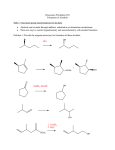
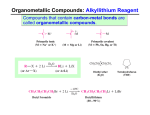
8-7 Organometallic Reagents: Sources of Nucleophilic Carbon for Alcohol Synthesis If the carbonyl carbon of an aldehyde or ketone could be attacked by a nucleophilic carbon atom, R:-, instead of a hydride ion, both an alcohol and a new carbon-carbon bond would be formed. The class of compounds called organometallic reagents are strong bases and good nucleophiles and are useful in this kind of synthesis. Alkyllithium and alkylmagnesium reagents are prepared from haloalkanes. Alkyllithium and alkylmagnesium compounds can be prepared by reaction of alkyl halides with lithium or magnesium in ethoxyethane (diethylether) or oxacyclopentane (THF). The order of reactivity of the haloalkane is: Cl < Br < I (CH3CH2 )2 O, 0o 10o C CH3Br + 2 Li CH3Li + LiBr Methyl-lithium Grignard reagents, RMgX, can be formed from primary, secondary, and tertiary haloalkane, as well as from haloalkenes and halobenzenes. Grignard reagents are very sensitive to moisture and air and are formed in solution and used immediately. The metal atoms in a Grignard reagent are electron-deficient and become coordinated to two solvent molecules: The alkylmetal bond is strongly polar. The carbon-lithium bond in CH3Li has about 40% ionic character, and the carbonmagnesium bond in CH3MgCl has about 35% ionic character. The metal atom is strongly electropositive and is at the positive end of the dipole. The formation of a Grignard reagent is an example of reverse polarization. In the haloalkane, the carbon atom attached to the halogen was electrophilic. In the Grignard reagent, the carbon atom has become nucleophilic. The alkyl group in alkylmetals is strongly basic. Carbocations are the conjugate bases of alkanes (estimated pKa’s of about 50), and as a result are extremely basic, much more so than amines or alkoxides. Because of their basicity, carbocations are extremely sensitive to moisture or other acidic functional groups. This reaction is one method which can be used to convert alkylhalides into alkanes. A more direct way of producing an alkane from a haloalkane is by an SN2 displacement of the halide by a hydride ion from LiAlH4. NaBH4 is not reactive enough to carry out this displacement. A deuterium atom can be introduced into an alkane by the reaction of D2O with an organometallic reagent: 8-8 Organometallic Reagents in the Synthesis of Alcohols One of the most useful reactions of organometallic reagents is the reaction with aldehydes and ketones to produce an alcohol containing a new C-C bond. Reaction with formaldehyde produces a primary alcohol. Aldehydes other than formaldehyde form secondary alcohols. Ketones react to form tertiary alcohols. Alkyllithium and Grignard reagents cannot be used to displace halide ions from haloalkanes as the reaction is too slow.