* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Grignard Reactions - faculty at Chemeketa
Survey
Document related concepts
Marcus theory wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Wolff rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Stille reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Transcript
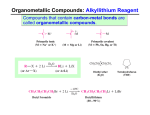
Grignard Reactions A Grignard reagent is an organic magnesium halide. It can be either an alkyl or an aryl compound (RMgX or ArMgX). Grignard (pronounced green yard) reagents were first prepared in France around 1900 by Victor Grignard (1871-1935). Grignard reagents are usually made by reacting an organic halide and magnesium metal in an ether solvent: RX + ArX + Mg Mg ether ether RMgX X = Cl, Br, or I ArMgX X = Br In the Grignard reagent, the bonding electrons between carbon and magnesium are shifted away from the electropositive Mg to form a strongly polar covalent bond. As a result the charge distribution in the Grignard reagent is such that the organic group (R) is partially negative and the –MgX group is partially positive. This charge distribution directs the manner in which Grignard reacts with other compounds. – + RMgX The Grignard reagent is one of the most versatile and widely used reagents in organic chemistry. We will consider only its reactions with aldehydes and ketones at this time. Grignards react with aldehydes and ketones to give intermediate products that form alcohols when hydrolyzed. With formaldehyde, primary alcohols are formed; with other aldehydes, secondary alcohols are formed; with ketones, tertiary alcohols are formed. Grignard reagent + formaldehyde → 1º ROH Grignard reagent + other aldehydes → 2º ROH Grignard reagent + ketones → 3º ROH Specific examples of each type of reaction follow: CH3 H2C O formaldehyde + CH3MgBr ether H2C OMgBr H2O CH3CH2OH + Mg(OH)Br 2 H H C O + CH3MgBr ether C OMgBr CH3 benzaldehyde H2O CHOH + Mg(OH)Br CH3 CH2CH3 acetone CH3CCH3 + ether CH3CH2MgBr CH3CCH3 O CH2CH3 H2O CH3CCH3 + Mg(OH)Br OH OMgBr The Grignard reaction with acetone may be explained in this way. In the first step of the addition of ethyl magnesium bromide, the partially positive –MgBr of the Grignard bonds to the oxygen atom, and the partially negative CH3CH2– bonds to the carbon atom of the carbonyl group of acetone. CH2CH3 CH3CCH3 + CH3CH2MgBr CH3CCH3 _ O O +MgBr In the hydrolysis step, a proton [H+] from water bonds to the oxygen atom, leaving the hydroxyl group [–OH] to combine with the +MgBr. So, the alcohol is formed. CH2CH3 CH2CH3 CH3CCH3 _ O + +MgBr H OH CH3CCH3 OH + Mg(OH)Br 3