* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Spring 2015 CH 421 Name ________________________________________ Section ___________ Post‐lab 3: The Grignard Reaction: Preparation of an Alcohol
Marcus theory wikipedia , lookup
Homoaromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Metal carbonyl wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Stille reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Hydroformylation wikipedia , lookup
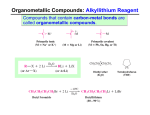
Spring 2015 CH 421 Name ________________________________________ Section ___________ Post‐lab 3: The Grignard Reaction: Preparation of an Alcohol (Due at the start of lab following this experiment) MgBr 1) Consider the Grignard reagent tert‐butylmagnesium bromide: a. What is the formal oxidation state of magnesium? b. What is the formal oxidation state of bromine? c. What is the formal oxidation state of carbon bonded to Mg? 2) Outline how to prepare the Grignard reagent phenethylmagnesium bromide in two steps from phenethyl alcohol. Be sure to include all reagents and draw the starting material, intermediate, and product structures. 3) What type of solvent is required for the synthesis and reaction of a Grignard reagent? You can provide a general solvent class, or name a specific solvent in that class. 4) Aldehydes undergo reaction with a Grignard reagent to provide an alcohol product. Many aldehydes are prone to air oxidation. For instance, a bottle of benzaldehyde will turn from a clear liquid to a white solid if left open over time. What is the oxidation product generated in this case? 5) Grignard reagents can also react with other electrophiles, such as epoxides. Draw the ring‐opened alcohol product that would be isolated after aqueous workup. PhMgBr + O OH 6) Outline three different methods of preparing the following tertiary alcohol from a Grignard reaction with a ketone. Ph a. b. c. 7) Esters undergo reaction with a Grignard reagent to form an intermediate ketone, which then undergoes further attack by a second equivalent of the Grignard reagent. Indicate the ester starting material and the Grignard reagent that would provide the following alcohol after aqueous workup. 8) Grignard reactions with carbonyl compounds are performed under anhydrous conditions, as they MgBr D2O would otherwise react with water. Draw the product formed from treatment of a Grignard Et2O reagent with deuterium oxide (“heavy water”). 9) Most often, Grignards are reacted with carbonyl compounds, such as aldehydes, ketones and esters. NOTE: each of these electrophiles differs in its reactivity based on both steric effects and electronic effects that influence the electrophilicity (i.e. + character) of the carbonyl carbon. a. Which is the most electrophilic of these three carbonyl compounds? b. Which is the least electrophilic of these three carbonyl compounds? 10) Aldehydes and ketones can be prepared from alcohols via oxidation. Indicate an appropriate reagent for the following transformation. (HINT: see Chapter 17 in McMurry.) OH O