* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Grignard Reaction - This is Synthesis
Fischer–Tropsch process wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Marcus theory wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Discodermolide wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
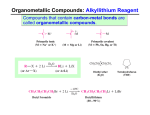
::: Application Report Efficient Grignard Reaction under Sealed Vessel Conditions The Grignard reaction is a powerful, frequently used organometallic transformation to add important structural motifs to carbonyl compounds resulting in secondary or tertiary alcohols. Since usually the low boiling THF is used as a solvent, closed vessel conditions and elevated temperatures can significantly enhance the reaction. This is best done in a conventional synthesis reactor. © Shutterstock.com 1 Introduction 3 Reaction Conditions The formation of organometallic Grignard reagents from halides and elemental magnesium is a rather tricky and often slow starting process. Prevention of moisture and air is crucial. Therefore standard reaction conditions in open vessels require special precautions to activate the magnesium and to keep the moisture away from the reagents. 110 mg (4.5 mmol) Mg turnings in 2 mL dry THF were admixed with 0.42 mL (4 mmol) bromobenzene in an argon-flushed reaction vial. After the exothermic reaction has stopped, the vial is sealed with the silicone cap, placed in the reactor and heated for 20 min at 90 °C to form the Grignard reagent (see Figure 2, step 1). After cooling 300 mg (2 mmol) ethylbenzoate dissolved in 1 mL dry THF are added and the mixture is heated for another 10 min at 90 °C. After cooling the mixture is treated with 5 mL HCl (2 M) and then evaporated. The residue is extracted with diethyl ether and dried over MgSO4. Evaporation of the solvent furnishes triphenylmethanol in 56% yield (292 mg). Sealed vessel conditions allow for simple creation of inert atmosphere and elevated reaction temperatures, thus successful initiation and short reaction times are granted. 2 Equipment The preparation of the Grignard reagent and the subsequent Grignard reaction was performed as a one-pot two-step sequence in a Monowave 50 synthesis reactor utilizing 10 mL glass vials with silicone cap and PTFE septum. Fig. 2 One-pot, two-step reaction to form triphenylmethanol 4 Results Heating beyond the boiling point of the employed solvent allows reduction of the reaction time. Typically the formation of the Grignard reagent as well as the Grignard reaction itself require 30-60 minutes at reflux. Under sealed vessel conditions both steps, especially the Grignard reaction, can be significantly shortened. Due to the short reaction process the isolated product can be obtained within only one hour. Fig. 1 Conventionally heated closed vessel synthesis reactor Monowave 50 D91IA003EN-A 1 www.anton-paar.com ::: Application Report This is because of the high field density in modern microwave reactors, which leads to rapid and tremendous heating of the magnesium and causes degradation and carbonization of the solvent on the surface of the magnesium turnings.2 Comparison experiments with a closed vessel immersed in a preheated oilbath showed that identical reaction times at identical temperatures lead to identical yields (see Table 1). Table 1: Comparison of conditions and outcome under oilbath conditions, microwave heating and conventional autoclave heating (Monowave 50) Oilbath Microwave Monowave 50 Temp. 90 °C 90 °C 90 °C Reaction Time 1 20 min 20 min 20 min Reaction Time 2 10 min -- 10 min Yield 55 % n.a. 56 % 5 Conclusion Rapid heating under sealed vessel conditions allows a quick, reproducible and failsafe procedure to generate Grignard reagents. The subsequent Grignard reaction furnishes the desired product in much shorter time compared to classical reflux conditions. 6 References The significant advantage of Monowave 50 compared to oilbath heating lies in the easy and quick handling, the safe and trouble-free operation at elevated temperatures, and the permanent and comprehensive recording of data. Experimental work from the Karl-Franzens University Graz, Institute of Chemistry [1] D. Obermayer, J. M. Kremsner, A. Stadler, Minutes, not Hours! A Practical Guide to High-speed Organic Synthesis in Modern Microwave Reactors, Anton Paar selfpublishing, 2016, p 58-59, ISBN 978-3-200-04434-0 Using microwaves as heating source for the formation of the Grignard reagent (with the potential to activate the Mg turnings) has proven unsuccessful. While microwaves can be nicely used for the Grignard reaction, using the already prepared magnesium bromide reagent,1 the direct preparation of the Grignard reagent can be troublesome. D91IA003EN-A [2] B. Gutmann et al., Angew. Chem. Int. Ed. 2011, 123, 7778 2 www.anton-paar.com