* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Halogenoalkanes
Aromaticity wikipedia , lookup
Homoaromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Marcus theory wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
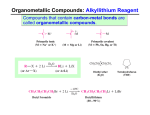
www.thisisrevision.com for tests, flashcards and revision courses Reactions of grignard reagents Nucleophilic substitution reactions of halogenoalkanes - S ubstitution with OH ions (hydrolysis). Reagents and conditions are… - CH3CH2Br + OH → heat and reflux with dilute aqueous sodium hydroxide solution. CH3CH2OH + Br - CH3CH2Br + CN - → CH3CH2Br + NH3 CH3CH2Br + 2NH3 → → With carbonyl compounds (aldehydes and ketones). CH3CH2-MgBr + CH3CHO heat and reflux with potassium cyanide in ethano l. CH3CH2CN + Br - + + CH3CH2-CH(CH3)-O MgBr + H Cl - - S ubstitution with NH 3 (aminolysis). Reagents and conditions are… Because grignard reagents are prepared in-situ, other substances are usually added to them in dry ether. Gases, such as methanal and carbon dioxide, can be bubbled through. All the reactions occur in two stages, with dilute aqueous acid such as HCl being added in the second stage. S ubstitution with CN- ions (cyanolysis). Reagents and conditions are… www.thisisrevision.com for tests, flashcards and revision courses heat in a sealed tube with excess concentrated ammonia in ethanol. CH3CH2NH2 + HBr or… CH3CH2NH2 + NH 4Br - → CH3CH2 -CH-O- MgBr+ CH3 → CH3CH2 -CH-OH + MgBrCl CH3 With CO2 CH3CH2-MgBr + CO2 - + - + CH3CH2-CO2 MgBr + H Cl - + → CH3CH2 -CO2 MgBr → CH3CH2 -CO2H Note that this reaction is very exothermic so the solution must be cold, or dry ice (solid CO2 at –78o C used instead). Why is water not used as a solvent for the reactions with NH3 and CN ? - Both species are basic and react with water to give OH ions, so some of the halogenoalkane is made into an alcohol instead: NH3 + H 2 O → NH 4+ + OH- Formation of grignard reagents Reactions of grignard reagents cont… Grignard reagents can be formed from both alkyl and aryl halogeno compounds. Reagents and conditions are… gently warm the halo-compound with magnesium turnings in dry ether, using a crystal of iodine if necessary. C 6H5 -Br + Mg → C 6H5 -MgBr CH3CH2-Br + Mg → CH3CH2-MgBr How and why are the conditions kept anhydrous? Grignard reagents react with water and it inhibits their formation. Calcium chloride guard tubes are attached to the apparatus (reflux condenser) and the halo-compound and they are dried beforehand. What is the purpose of the iodine crystal? To initiate the reaction. How does the reaction depend on the choice of halogen atom? Bromo and iodo compounds are better choices as the C-Halogen bonds are longer and weaker, making the compounds more reactive. www.thisisrevision.com for tests, flashcards and revision courses With epoxy ethane. O CH3CH2-MgBr + CH2-CH2 - + + CH3CH2-CH2-CH2 -O MgBr + H Cl - - + → CH3CH2 -CH2-CH2-O MgBr → CH3CH2 -CH2-CH2-OH + MgBrCl Notes These reactions are classified as nucleophilic addition. When methanal (CH2 O) is added, a primary alcohol with one extra carbon atom is formed. When other aldehydes are used (e.g. ethanal), secondary alcohols are formed. When ketones are used (e.g. pro panone), tertiary alcohols are formed. When CO2 is used, a carboxylic acid with one extra carbon is formed. When epoxy ethane is used, a primary alcohol is formed with two extra carbons. Because grignard reagents are prepared in-situ, yields of subsequent reactions are high as there are no mechanical losses as a result of isolating the grignard reagent. Grignard reagents can be used instead of toxic reagents such as KCN in order to introduce an extra carbon atom, usually in fewer steps, which also improves yield. www.thisisrevision.com for tests, flashcards and revision courses