* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Photo Album
Environmental enrichment wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Subventricular zone wikipedia , lookup
Electrophysiology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Synaptogenesis wikipedia , lookup
Central pattern generator wikipedia , lookup
Apical dendrite wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Biological neuron model wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Optogenetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural coding wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Channelrhodopsin wikipedia , lookup
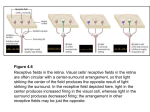
Chapter 19. Information Processing in Neural Networks Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.1 (A) The lateral geniculate nucleus (LGN) of the thalamus receives input from the eyes. (B) Two possible representations of visual information that may be encoded in a visual region: local luminance across the image (top) or local contrast in luminance (bottom). A key step in understanding the processing of a neural circuit is to determine explicitly what information is represented in the circuit. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.2 In the stretch reflex, the sensory neuron uses a rate code to represent the change in length of the muscle. The motor neuron represents the amount of force needed to contract the muscle to counteract the stretch. The motor neuron also uses a rate code, and must compute its appropriate firing rate based on the firing rate of the sensory neuron. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.3 The motor cortex represents body movements using a topographic map of the body surface along the cortical surface. This figure shows a cross-section through primary motor cortex. Electrical stimulation of the medial wall of the cortex produces movements of the foot or leg, whereas stimulation of the lateral cortex produces movement of the face or hand. Notice that more cortex is devoted to body parts that make finescale movements, such as the hand, compared to body parts that make only coarse movements, such as the leg. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.4 The primary visual cortex represents the visual field (top) as a distorted topographic map (bottom). The right visual field (shaded gray) is represented in the left hemisphere. The upper visual field (light gray) is represented ventral to the lower visual field (dark gray). Like motor cortex (Fig. 19.3), more cortical space is devoted to regions that require fine-scale processing. Thus, the region of high-acuity vision (the fovea, denoted by a star, and the surrounding 10°) takes up ~50% of the primary visual cortex, whereas the far periphery takes up only a small fraction of cortical space. From Van Essen et al., 1984. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.5 Neural tuning curves based on rate coding. (A) Orientation tuning curve from visual cortex. The firing rate of the neuron changes as a function of the orientation of the bar of light presented in the cell’s receptive field. The top shows peristimulus time histograms of the firing rate of the neuron as a function of time; the shaded regions represent the stimulus presentation time. The bottom shows a tuning curve based on the spike firing histograms. Note that the shape of the tuning curve can vary based on cognitive factors such as attention, as black indicates conditions when the monkey paid attention to the stimulus and white indicates conditions when the stimulus was unattended. Modified with permission fromMcAdams and Maunsell, 1999. (B) A tuning curve for direction of motion from the primary motor cortex. Reproduced with permission fromGeorgopoulos et al., 1982. (C) A tuning curve for sound frequency from auditory cortex. Note that the tuning curves vary as a function of sound intensity (in decibels) as well as frequency. Modified with permission fromPhillips and Orman, 1984. (D) A tuning curve for head direction from the anterior dorsal thalamus of an awake, behaving rat. From Yoganarasimha and J. J. Knierim, unpublished data. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.6 Varieties of tuning curve shapes. (A) Gaussian. (B) Linear. (C) Inhibitory. (D) Sigmoidal. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.7 Temporal coding of checkerboard-like stimuli called Walsh patterns by inferotemporal cortex neurons. (A) The top graph shows a spike density function and the bottom graph shows raster plots of individual spikes on each presentation. The horizontal line under each graph represents the stimulus duration. The cell fired an initial transient burst, was quieter for ~100 ms, then fired again. (B) The same cell fired at a similar overall rate to a different pattern, but the temporal pattern of activity was different from that of A. For pattern B, the cell did not produce the initial transient burst but instead fired only during the second interval after a delay of ~100 ms relative to pattern A. Reproduced with permission from Richmond et al., 1987. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.8 Temporal coding in the locust olfactory system. (Top) Four oscillations are apparent in the local field potential (LFP). (Bottom) Peristimulus histograms of firing probability as a function of time for two neurons (PN1 and PN2) presented with nine different odor combinations. Each odor stimulus produces a different combination of firing rates (probabilities) and temporal patterns relative to the oscillations of the LFP in the two projection neurons. Reprinted from Wehr and Laurent, 1996, by permission from Macmillan Publishers Ltd. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.9 Temporal coding in the rat hippocampus place cell system. (A) A rat runs back and forth on a linear track for food reward at each end. (B) A firing rate map for one hippocampal cell shows a firing field of elevated activity at a single location on the track. The color code denotes mean firing frequency at each location. (C) The local field potential (black trace) shows the characteristic theta rhythm. Each burst of spikes from the place cell (red ticks) occurs at successively earlier phases of the theta rhythm (compare relative locations of red and blue ticks). Parts A–C reprinted fromHuxter et al., 2003, by permission from Macmillan Publishers Ltd. (D) Because of the theta “phase-precession” effect shown in C, place fields that occur in a particular order on the track (ABCD) preserve their firing order in a temporally compressed manner within each theta cycle, which may be important for the storage of spatiotemporal sequences of activity in the hippocampus. Part D reproduced with permission from Skaggs et al., 1996. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.10 Population coding. Three hypothetical motor cortex cells are demonstrated with coarse tuning curves. The activity of each cell is ambiguous as to the precise direction of motion of a limb. For example, if cell 1 is active at half of its maximal firing rate, it is ambiguous as to whether the direction of movement is to the right or left of its preferred firing direction. However, knowing the activity of a population of neurons allows a more precise decoding of movement direction. If both cells 1 and 2 are firing at their half-maximal rate, and cell 3 is inactive, then the possible direction of movement that can cause this activity pattern is limited to a smaller range (shaded region). The population activity of many such cells can encode the movement direction with high precision. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.11 (A) Population vectors from motor cortex. Each blue line indicates the preferred firing direction of a motor cortex neuron. The length of the line indicates the neuron’s mean firing rate for a particular movement. Calculating the average of all blue vectors produces the red population vector, which is very close to the actual movement of the subject (yellow line). Reproduced with permission fromGeorgopoulos et al., 1988. (B) Activity from a population of 80 simultaneously recorded hippocampal place cells. Many cells fire in restricted locations in a square box (red and yellow regions; blue indicates regions where a cell was silent). A population vector analysis shows that the firing of these place cells can reproduce a rat’s trajectory with high precision (blue line indicates rat’s actual trajectory, red line indicates the reconstructed trajectory from the population code). Modified with permission from Wilson and McNaughton, 1993. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.12 Iconic neural circuits. Green neurons are excitatory and red neurons are inhibitory. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.13 Circuitry of the Aplysia siphon-gill withdrawal circuit. A stimulus to the siphon causes the gill and siphon to withdraw in a reflex action. After repeated stimulation, the behavioral response decreases because less transmitter is released from the sensory neurons. This simple form of learning is called habituation. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.14 Pyloric rhythm circuit of the lobster stomatogastric ganglion. (A) Schematic diagram of the three cell types that produce the rhythm. Note that the synapses are inhibitory. (B) The cells fire in a stereotyped sequence of activity, with the electrotonically coupled AB/PD cells firing first, followed by the LP cells, and finally the PY cells. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.15 Circuit of the locust antennal lobe–mushroom body system. Antennal lobe projection neurons make sparse connections with Kenyon cells of the mushroom body and strong connections with feedforward inhibitory neurons of the lateral horn. The combination of temporally specific firing, sparse connectivity, and strong feedforward inhibition turns the coarse-coded antennal lobe representation into a sparse-coded mushroom body representation. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.16 Delay lines and coincidence detection in the barn owl sound localization system. Axons from the ipsilateral nucleus magnocellularis innervate the nucleus laminaris from the dorsal side. Axons from the contralateral nucleus magocellularis innervate the nucleus laminaris from the ventral side and send interdigitating projections parallel to the contralateral projections. Individual neurons in the nucleus laminaris receive input from both projections. Because the contralateral and ipsilateral projections convey sound information from different ears, the cells in nucleus laminaris are excited when they receive coincident input from both ears. Cells at the dorsal part of the nucleus will receive coincident input when the contralateral ear receives sound before the ipsilateral ear, as it takes longer for the signal to be transmitted over the longer axons. Cells in the ventral part of nucleus laminaris will receive coincident input when the contralateral and ipsilateral ears receive sound simultaneously. Thus, the nucleus laminaris contains an auditory map of contralateral and frontal space, computed from interaural time differences. The owl uses this representation to detect the location of the sound source in the azimuthal (horizontal) plane. Adapted with permission from Carr and Konishi, 1988. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.17 Model of orientation tuning proposed by Hubel and Wiesel (1962). The center-surround receptive fields of LGN neurons are shown on the left. If a primary visual cortex neuron received input only from LGN cells that were aligned in a particular orientation, then the cortical neuron would be maximally excited when a bar of light of the same orientation is presented. Bars of light at other orientations would excite fewer of the LGN afferents, causing the cortical cell to fire at a lower rate. Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.18 Schematic diagram of some main features of cerebellum circuitry. The cerebellum receives input from mossy fibers and climbing fibers. The mossy fibers synapse onto cerebellar deep nuclei cells and onto granule cells of the cerebellar cortex, which send parallel fibers that synapse onto the inhibitory output neurons of the cortex, the Purkinje cells. Climbing fibers synapse onto deep nuclei cells and also make powerful synapses directly onto Purkinje cells and act as an error signal to drive cerebellar learning. (See Chapter 20 for a more detailed treatment of the circuitry of the cerebellum and its role in learning.) Copyright © 2014 Elsevier Inc. All rights reserved Figure 19.19 Schematic diagram of the recurrent collateral system of the CA3 field of the hippocampus. CA3 neurons receive direct input from the medial entorhinal cortex (MEC) and the lateral entorhinal cortex (LEC), as well as a strong projection from the dentate gyrus (DG). CA3 sends output to CA1 (the Schaffer collaterals), but also sends recurrent collaterals that innervate other CA3 cells. This recurrent collateral system has been hypothesized to endow the CA3 network with the properties of an autoassociative memory system, including the ability to reconstruct complete output patterns when presented with only partial input. Copyright © 2014 Elsevier Inc. All rights reserved