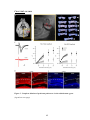
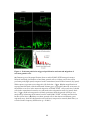
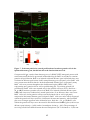
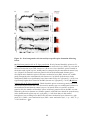
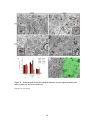
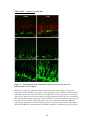
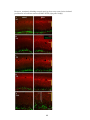
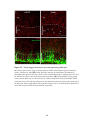
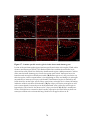
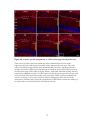
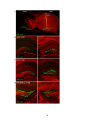
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lesion of the perforant path triggers a biphasic neurogenic response
Electrophysiology wikipedia , lookup
Haemodynamic response wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Apical dendrite wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Axon guidance wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Chemical synapse wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neuroanatomy wikipedia , lookup
Subventricular zone wikipedia , lookup
Oregon Health & Science University OHSU Digital Commons Scholar Archive December 2012 Lesion of the perforant path triggers a biphasic neurogenic response in the adult dentate gyrus Julia V. Perederiy Follow this and additional works at: http://digitalcommons.ohsu.edu/etd Recommended Citation Perederiy, Julia V., "Lesion of the perforant path triggers a biphasic neurogenic response in the adult dentate gyrus" (2012). Scholar Archive. Paper 918. This Dissertation is brought to you for free and open access by OHSU Digital Commons. It has been accepted for inclusion in Scholar Archive by an authorized administrator of OHSU Digital Commons. For more information, please contact [email protected]. O REGON H EALTH & S CIEN CE U N IVERSITY S CHOOL OF M ED ICIN E – G RAD UATE S TUD IES Ph.D . D issertation LESION OF THE PERFORAN T PATH TRIGGERS A BIPHASIC N EUROGEN IC RESPON SE IN THE AD ULT D EN TATE GYRUS by Julia V. Pered eriy Presented to the N euroscience Grad uate Program and the Oregon H ealth & Science University School of Med icine in partial fulfillm ent of the requirem ents for the d egree of Doctor of Philosophy Decem ber, 2012 OREGON HEALTH & SCIEN CE UN IVERSITY SCHOOL OF MED ICIN E – GRAD UATE STUD IES Approval for Completion of Ph.D . D egree School of Med icine Oregon H ealth & Science University CERTIFICATE OF A PPROVAL ___________________________________ This is to certify that the PhD d issertation of Ju lia V. Pered eriy has been ap p roved ______________________________________ Gary L. Westbrook – Mentor/ Ad visor ______________________________________ Craig Jahr – Mem ber ______________________________________ Phillip Cop enhaver – Mem ber ______________________________________ Tianyi Mao – Mem ber ______________________________________ Law rence Sherm an – Mem ber ______________________________________ Mary Logan – Mem ber TABLE OF CON TEN TS A CKN OWLED GEMEN TS ………………………………………………………………….. iii A BSTRACT ………………………………………………....……………………………... iv IN TROD UCTION STRUCTURAL PLASTICITY IN THE D EN TATE GYRUS - REVISIN G A CLASSIC IN JURY MOD EL Plasticity in the adult brain, implications for injury-induced circuit reorganization …………………………………………….…………………… 1 Synaptic and d end ritic plasticity in the ad ult brain Sprouting and the axonal response to injury Glial and extracellular response to injury Perforant path lesion as a model of post-injury plasticity in the adult brain ………………………………………...……………………. 6 Ad vantages of m od el Post-lesion circuit reorganization – axons Post-lesion circuit reorganization – d end rites/ spines Post-lesion glial and extracellular m atrix response Figures ……………………………………………………….……………………. 11 CHAPTER 1 N EURAL IN JURY TRIGGERS A BIPHASIC RESPON SE IN AD ULT-GEN ERATED N EURON S IN THE D EN TATE GYRUS Introduction ………………………………………………………………………. 15 Methods …………………………………………………………………………... 17 Results ……………………………………………………………………………. 24 Proliferation and Migration of new born neurons follow ing perforant path lesion Dend ritic outgrow th and synapse form ation post-lesion i de novo dend ritic spines in the d enervated zone ii D iscussion …………………………………………………………………...…... 31 An initial neurogenic response to perforant path lesion Circuit reorganization follow ing d enervation Is a spine a m arker of a functional excitatory synapse post-lesion? Lim its to grow th Figures ………………………………………………………………………….... 36 CHAPTER 1 – AD D EN D UM M ECHAN ISMS OF LESION -IN D UCED PROLIFERATION AN D D ISPERSION OF N EWBORN GRAN ULE CELLS IN THE D EN TATE GYRUS Possible mechanisms of injury-induced proliferation and dispersion of new born granule cells in the dentate gyrus ………………………………….... 46 Circuit activity Grow th factors Reelin Rad ial glia Figures ………………………………………………………………………….. 52 CHAPTER 2 ROLE OF EXTRACELLULAR EN VIRON MEN T IN POST-LESION CIRCUIT REORGAN IZATION Laminar borders and axonal sprouting follow ing lesion ……………………. 56 Lam inar specificity of excitatory afferents Pre- and postsynaptic com ponents of lesion -ind uced circuit reorganization Extracellular matrix in development and follow ing lesion ………………….. 58 Role of the extracellular m atrix in generation and m aintenance of lam inar bord ers Manipulation of the post-lesion extracellular environm ent Dend ritic outgrow th and spine d ensity follow ing d igestion of CSPG Figures …………………………………………………………………………. 66 G EN ERAL D ISCUSSION ……………………………………………………………… 71 LIMITS OF PLASTICITY iii S UMMARY AN D CON CLUSION S …………………………………………………….. 73 REFEREN CES …………………………………………………………………………. 75 iv A CKN OWLED GMEN TS This w ork w ould not have been possible w ithout the m ultitud e of people around m e that, despite their ow n d ynam ic lives, had m om ents for d eeply m eaningful philosophical conversations and shared laughs. These people are family, friend s, m entors, and even strangers, that all inspired m e in m any w ays. The exam ples they set forth w ill be forever integrated into my path forw ard and I w ill continue to be thankful for their guid ance. This w ork w as also supported by N IH grant MH 46613 and by Oregon Partnership for Alzheim er’s Research Tax Check-Off grant. We thank Osw ald Stew ard for training in the perforant path lesion proced ure. We thank Stephanie Kaech -Petrie w ith the Jungers Center Microscopy Core for assistance w ith im aging param eters and Sue Aicher, Melissa William s, Lisa Dirling, and Robert Kayton w ith the Electron Microscopy Core for tissue preparation and assistance w ith im aging equipment (supported by N IH , P30 N S06180). v A BSTRACT The ad ult brain is in a continuous state of rem od eling. This is now here m ore true than in the d entate gyrus, w here com peting forces such as neurod egeneration and neurogenesis d ynam ically m od ify neuronal connectivity, and can occur sim ultaneously. This plasticity of the ad ult nervous system is particularly im portant in the context of traum atic brain injury or d eafferentation because it illustrates the potential for regeneration in the central nervous system . H ere, w e sum m arize a classic injury m od el, lesioning of the perforant path, w hich rem oves the m ain extrahippocam pal input to the d entate gyrus. Early stud ies revealed that in response to d eafferentation, axons of rem aining fiber system s and d end rites of m ature granule cells undergo lam ina -specific changes, providing one of the first exam ples of structural plasticity in the ad ult brain. Given the increasing role of ad ult neurogenesis in the function of the d entate gyrus, w e also com pare the response of new born and m ature granule cells follow ing lesioning of the perforant path. We find that the lesion triggered a m arked proliferation and increased outw ard m igration of new born neurons in the d eafferented d entate gyrus. The d end rites of these cells show red uced com plexity w ithin the d enervated zone, but d end ritic spines w ith intact post-synaptic d ensities still form , d espite the absence of glutamatergic nerve term inals. Follow ing entorhinal lesion, n ew born neurons, but not m ature granule cells, have a higher d ensity of d end ritic spines in the non -d enervated inner m olecular layer, accom panied by an increase in m iniature EPSC am plitud es and rise tim es. Lam inar bord ers are m aintained post-lesion and show lam ina-specific reactive gliosis and changes in the extracellular m atrix. Our results d em onstrate that injury causes an increase in new born neurons and lam ina-specific synaptic reorganization, indicative of enhanced plasticity. The presence of de novo d end ritic spines in the d enervated zone suggests that the post-lesion environment provid es the necessary signals for spine form ation even if excitatory innervation is absent. These stud ies provid e insights not only to plasticity in the d entate gyrus, but also to the response of neural circuits to brain injury. vi IN TROD UCTION STRUCTURAL PLASTICITY IN THE DEN TATE GYRUS REVISIN G A CLASSIC IN JURY MODEL (The follow ing w ill be p u blished as a review in Frontiers in N eu ral Circu it s in 2013) Plasticity in the adult brain The ability of the m am malian brain to change w ith experience is perhaps its m ost im portant feature. At the organism al level, the positive (ad aptive) benefits of experience d epend ent changes und erlie our abilities to learn, speak m ultiple languages, rid e a bicycle and so on. H ow ever, equally im portant are end uring negative (malad aptive) effects that are associated w ith experience-d ependent changes, includ ing benign habits as w ell as m ore d isruptive cond itions such as anxiety, post-traum atic stress, and d rug ad d iction. In both cases, these changes are m anifested at the level of circuits and ind ividual neurons as a reord ering of gene expression profiles, synaptic strength, and circuit connectivity. Reorganization reflects the ad aptation of the netw ork to a changing environm ent, either encod ing new inform ation or com pensating for injury -ind uced d egeneration. Reorganization follow ing a brain injury inevitably perturbs the d ynam ic equilibrium , w hich can affect many aspects of neuronal structure and function , includ ing intrinsic neuronal properties, synaptic interactions, and connectivity w ithin and betw een netw orks. The cellular and m olecular land scape can im pose lim its on plasticity and regenerative capacity of the ad ult brain. A variety of injury m od els have been used to exam ine the response of the brain , such as crush injuries to peripheral nerves, cortical stab w ound s, and spinal cord injury (SCI) m od els. For exam ple, SCI m od els have been extensively exam ined for factor s that 1 lim it the grow th of axons follow ing d am age or transection (Tuszynski and Stew ard , 2012; Akbik et al., 2012). H ere w e focus on the perforant path lesion, a brain injury m od el that interrupts the m ain excitatory input to the d entate gyrus of the hippo campus. This m od el has the experim ental advantages of a highly lam inated structure and allow s analysis of not only the axonal response to injury, but also changes in d end rite m orphology and synaptic reorganization. This classic lesion provided som e of the first evidence for structural plasticity follow ing injury in the CN S, and also provid es an opportunity to exam ine the injury response of som e of the m ost highly plastic neurons in the brain, ad ult generated new born granule cells. We first highlight features of neuronal and non -neuronal plasticity that d rive ad aptive and m aladaptive changes in brain circuits. Subsequently, w e d iscuss the perforant path lesion m od el as an exam ple of injury -ind uced plasticity in the ad ult brain. Synaptic and dendritic plasticity in the injured brain It is w ell know n that synaptic and d end ritic plasticity occur in sensory system s follow ing d eprivation, and in m otor system s follow ing d isuse (H ickm ott and Steen, 2005; H ofer et al., 2006). H ow ever, spines and dend rites also und erg o d ynam ic functional and structural changes follow ing acute injury or neurod egeneration. These changes fall into several categories, includ ing retraction of d end ritic arbors follow ing loss of inputs; com pensatory increases in d end ritic arbors in d om ains of afferent inputs unaffected by the injury; transient changes in spine d ensities; and alterations in the types or shapes of d end ritic spines. For exam ple, d end ritic reorganization occurs after ischem ia (H osp and Luft, 2011), but the d egree of rem od eling d ep end s on the proxim ity of dend rites to the site of infarction. Brow n et al. (2010) rep orted d end ritic retraction follow ing ischem ic injury in 2 cortex ad jacent to the infarct, but com pensatory dend ritic outgrow th aw ay from the site of injury. On the other hand , Mostany and Portera-Cailliau (2011) saw only d end ritic pruning at cells in peri-infarct cortex. Dend ritic spine d ensity is also sensitive to ischem ia (Brow n et al., 2008) and spinal cord injury (Kim et al., 2006), both of w hich lead to a red uction in sp ine d ensity and elongation of the rem aining spines, albeit at d ifferent tim e scales. Because spine elongation is associated w ith synaptogenesis, the und erlying m echanisms for these changes are in many cases thought to be sensitive to injury -ind uced alterations in netw ork activity. For exam ple, the intense neuronal activity associated w ith kainate-ind uced seizures triggers bead ing of d endrites and subsequent loss of spines (Drakew et al., 1996; Zeng et al., 2007). H ow ever, brief seizure activity can also trigger m ore ‘physiological’ responses, such as the ind uction LTP in CA3 pyram id al neurons (Ben-Ari and Gho, 1988). This d ichotom y suggests that netw ork responses to injury are likely to be context-specific, and m ay reflect exaggerations of the norm al ad aptiv e responses to stim uli (Figure 1). Sprouting and the axonal response to injury Axons can also recover and / or reorganize follow ing injury, although the extent of regeneration varies. In the peripheral nervous system , regenerating axons can grow long d istances and re-innervate their targets, thus leading to functional recovery. H ow ever, regenerating axons in the central nervous system are often unable to penetrate the lesion, thus limiting long-range axonal outgrow th. Perhaps m ost extensively stud ied exam ples are experim ental m od els of spinal cord injury, in w hich cut or d am aged axons of the corticospinal tract form retraction bulbs and eventually m ove aw ay from the lesion site unable to penetrate the gliotic scar (H ill et al., 2001; Fitsch and Silver, 2008). H ow ever, if 3 the transection is incom plete, sprouting of uninjured axons, as w ell as cortical reorganization can lead to partial functional recovery follow ing injury (Raineteau and Schw ab 2001; Maier and Schw ab 2006). The d ifference in the capacity for axonal regeneration in the peripheral and central nervous system s reflects differences in intrinsic neuronal properties (Liu et al., 2011) and in post-injury changes in the extracellular environm ent (Giger et al., 2010). Whereas d egenerating material in th e peripheral nervous system is effectively cleared follow ing injury (Chen et al., 2007; Bosse, 2012), these processes are m uch slow er in the central nervous system (Vargas and Barres 2007; Giger et al., 2010), and m ay thus interfere w ith reinnervation of d eafferented target areas. Axonal structural plasticity m ay also be m alad aptive follow ing injury, as can occur in the brain of patients w ith tem poral lobe epilepsy. Follow ing seizures, m ossy fiber axons sprout recurrent collaterals that synapse onto granule cell d end rites in the inner m olecular layer, thereby increasing excitatory connectivity w ithin the d entate gyrus (Sutula and Dud ek, 2007). Such structural reorganization can lead to an imbalance betw een excitation and inhibition in the circuit, w hich m ay und erlie recurrent seizures. Glial and extracellular response to brain injury Glial cells are intim ately involved in function and plasticity of the healthy ad ult brain; how ever, their contribution to recovery follow ing injury is even m ore striking. Brain and spinal cord traum a, neurod egeneration, ischem ia, and infection all stim ulate m orphological and m olecular changes in surround ing astrocytes, often referred to as reactive gliosis. Depending on the triggering m echanism and its d uration, the glial response can prom ote or inhibit recovery (Figure 2; Sofroniew , 2009). For exam ple, d uring m ild insults to the CN S, such as the im m une reaction that follow s a viral infection or as 4 occurs in areas d istant to a lesion site, astrocytes hypertrophy but rem ain tiled (Figure 2B; Wilhelm sson et al., 2006). In such cases, tissue reorganization is m inim al, and reactive astrogliosis resolves w ithin a few w eeks. H ow ever, follow ing m ore severe CN S insults such as m ajor traum a, stroke, or neurod egeneration, astrocytes acquire expansive reactive m orphology, and their processes extend beyond their original bord ers (Sofroniew and Vinters, 2010). The resulting d ense netw ork of new ly proliferated astrocytes can recruit other cell types, includ ing fibrom eningeal cells and m icroglia, resulting in the form ation of a perm anent and im penetrable glial scar (Figure 2C). Reactive astrogliosis has trad itionally been view ed as m alad aptive because gliosis can contribute to glutam ate toxicity (Takano et al., 2005), generation of seizures (Tian et al., 2005; Jansen et al., 2005), inflam m ation (Bram billa et al., 2005), and chronic pain (Milligan and Watkins, 2009). Furtherm ore, the glial scar can inhibit axonal regrow th (Silver and Miller, 2004). Interestingly, astrocytes have also been im plicated in lym phatic d rainage in the central nervous system , a process that is likely d isrupted during reactive astrogliosis (H am by and Sofroniew , 2010; Iliff et al., 2012). Although experim ental interference w ith glial scar form ation can increase axonal regeneration, it can also increase lesion size and d im inish functional recovery (Sofroniew , 2009). The latter suggests that the presence of reactive astrocytes, d epend ing on the context, can have positive effects on neuronal reorganization by stabilizing the extracellular ion balance, red ucing seizure likelihood , and d am pening excitotoxicity (Rothstein et al., 1996; Sw anson et al., 2004; Koistinaho et al., 2004). An im portant prod uct of glial cells, the extracellular m atrix (ECM), surround s the synapse (Dityatev et al., 2006; Dityatev et al., 2010b) and is instrum ental in synaptic plasticity both in the healthy and injured brain (Dityatev, et al., 2010a; Dityatev and Fellin, 5 2008; Frischknecht and Gund elfinger, 2012). For exam ple, astrocyte-d erived ECM com ponents, such as throm bospond ins, initiate synaptic d evelopm ent (Christopherson et al. 2005; Xu et al., 2010) as w ell as regulate synaptic plasticity (Eroglu, 2009). In ad d ition, inactive perisynaptic m atrix m etalloproteases are transiently activated follow ing ind uction of LTP in the hippocam pus (Bozd agi et al., 2007; N agy et al., 2006). Because extracellular m atrix com ponents at least partially originate from glia, activation of astrocytes follow ing injury can affect expression of ECM m olecules and thus post-injury neuroplasticity. Like the astroglial response, these m olecules can have a d ual role in recovery. For exam ple, expression of chond roitin sulfate proteoglycan s is beneficial in containing the size of a lesion, but a few d ays later can inhibit axonal grow th (Zuo et al., 1998; Galtrey and Faw cett, 2007; Rolls et al., 2008). Likew ise, m atrix m etalloproteinases have a positive effect on reactive synaptogenesis w hen transiently upregulated (Falo et al., 2006), but persistent and w id espread MMP expression lead s to regression of d end ritic spines, degeneration of synapses, and neuronal apoptosis (Falo et al., 2006; H untley, 2012). The com plexity of the glial and ECM response und erscores both the potential for, and the lim itations of, repair and regeneration follow in g brain injury. Perforant path lesion as a model of post-injury plasticity in the adult brain A dvantages of model Lesioning of the perforant path w as one of the first m od els to d ocum ent injury ind uced plasticity in the ad ult brain . This lesion of the m ajor excitatory input into the d entate gyrus affects the trisynaptic hippocam pal circuit, d isrupting the d istinctly unid irectional progression of excitatory activity arriving from other brain regions (Know les, 1992). Because the entorhinal lesion site is d istant from the dentate gyrus, local 6 d egenerative/ inflam m atory effects at the lesion site can be easily separated from the regenerative effects of post-lesion circuit reorganization. The sim ple cyto- and fiber architecture and lam ination pattern of the d enta te gyrus also provid es an experim ental ad vantage because the lesion affects only one of m any afferent fiber system s. Each afferent input term inates in a specific lamina of the m olecular layer (H jorth -Sim onsen and Jeune, 1972) and each is functionally and m olecularly d istinct (Leranth and H ajszan, 2007). This d iversity allow s a com parison of heterotypic and hom otypic sprouting post -lesion (Ram irez, 2001), as the balance of these inputs may have a role in functional recovery. Post-lesion circuit reorganization – axons Afferents to the d entate gyrus have d iverse origins and neurotransm itter phenotypes that converge on the hippocam pus (Figure 3, left panel). Glutam atergic inputs to the outer 2/ 3rd s of the d entate m olecular layer includ e the entorhinod entate per forant path (H jorth-Sim onsen and Jeune, 1972; van Groen et al., 2003) and a w eak species-specific com m issural projection from the contralateral entorhinal cortex (van Groen and Kadish, 2002). Glutam atergic input to the inner m olecular layer consists of the m ossy cell axons from the com m issural/ associational (C/ A) collaterals (Gottlieb and Cow an, 1973; Soriano and Frotscher, 1994). These excitatory synaptic inputs are com plem ented by cholinergic, GABAergic, norad renergic, d opam inergic, and serotonergic projections that term inate throughout the m olecular layer (Leranth and H ajszan, 2007). Because the entorhinod entate projection is the largest glutam atergic afferent fiber system , a perforant path lesion severs the m ajority of excitatory innervation in the d entate gyrus, thus effectively d enervating the rd s outer 2/ 3 of the m olecular layer and vacating 80-90% of all synapses in that region (Matthew s et al., 1976a; Stew ard and Vinsant, 1983). Subsequent d egeneration of 7 excitatory synapses triggers com pensatory axon al sprouting that can be either hom o- or heterotypic, d epending on the neurotransm itter involved . H om otypic sprouting, i.e. glutam atergic axons, includ es the w eak entorhinod entate projection from the contralateral, non-lesioned entorhinal cortex that norm ally term inates in the d eafferented region (Stew ard et al., 1973; Stew ard , 1976a; Cotm an et al., 1977; Deller et al., 1996a), and the glutam atergic com ponent of the com m issural/ associational fiber system that norm ally term inates in the inner molecular layer (Gall and Lynch, 1981; Deller et al., 1996b). Although hom otypic reactive sprouting can partially replace lost synapses in the d enervated zone (Marrone et al., 2004b), the d egree of excitatory reinnervation is species specific (d el Turco et al., 2003; Deller et al., 2007). H om otypic sprouting can also partially restore postsynaptic function , as w ell as am eliorate som e behavioral d eficits (Ram irez, 2001). Lesion of the perforant path also triggers reactive heterotypic sprouting of non glutam atergic afferen ts, such as the cholinergic septod entate projection. Sprouting of this fiber system w as initially d etected as an increase in acetylcholinesterase (AChE) staining in the d enervated zone (Figure 4, left panel; Lynch et al., 1972; N ad ler et al., 1977a,b). The w id th of the AChE band w as subsequently correlated w ith the extent of the lesion and the tim e course of reorganization (Zim m er et al., 1986; Stew ard , 1992), and therefore has been used as a m arker for the extent and com pleteness of a perforant path lesion . Although the increase in AChE staining d ensity in the d enervated region has been corroborated (Vuksic et al., 2011), it rem ains uncertain w hether this increase indicates actual cholinergic sprouting or is a consequence of post-lesion tissue shrinkage (Ph inney et al., 2004). Perforant path lesions also cause sprouting of GABAergic C/ A axons (Deller et al., 1995), 8 as w ell as trigger receptor reorganization and new inhibitory synapse form ation on m ature granule cells (Sim bürger et al., 2000; 2001). In com bin ation w ith a d ecrease in glutam atergic innervation, these results suggest that lesions of the perforant path can alter the excitation/ inhibition balance in the d entate gyrus (Clusm ann et al., 1994), w hich can potentially com plicate functional recovery. H ow ever, heterotypic sprouting m ay also serve an adaptive purpose in post-lesion circuit reorganization by reinnervating vacated synapses and thus preventing or d elaying transsynaptic cell d eath. Post-lesion circuit reorganization – dendrites/spines Interrup tion of the perforant path d enervates one of the m ain inputs to the principal neurons in the ad ult d entate gyrus – the m ature granule cells. These cells are part of the trisynaptic hippocam pal circuit, w ith their d end rites receiving afferent input from the entorhinal cortex and other brain regions, and their axons form ing the m ossy fibers that synapse w ith pyramid al cells in CA3. The tw o subd ivisions of the perforant path, m ed ial and lateral, synapse w ith m ature granule cell d end rites in the midd le and oute r m olecular layers, respectively (H jorth -Sim onsen and Jeune, 1972; van Groen et al., 2003). Follow ing a perforant path lesion, these axons d egenerate (Matthew s et al., 1976a), thus elim inating the m ajority of excitatory input onto d end ritic segm ents in the outer tw o third s of the m olecular layer (Figure 3). The loss of excitatory input initiates a series of m orphological and functional changes in the post-synaptic m ature granule cells. Dend rites retract, resulting in less com plex d end ritic arbors in the d en ervated region (Caceres and Stew ard , 1983; Diekm ann et al., 1996; Schauw ecker and McN eill, 1996; Vuksic et al., 2011). Distal d end ritic segm ents are progressively lost for period s up to 90 d ays post -lesion, w ith som e recovery by 180 d ays post-lesion (Figure 4, right panel). H ow ever, this recovery 9 m ost likely reflects the extension of existing d endrites, rather than form ation of new branches (Vuksic et al, 2011). Similarly, the density of d end ritic spines – the postsynaptic target of the entorhinod entate projection – is significantly red uced follow ing lesion, but only in the d eafferented zone (Parnavelas et al., 1974; Vuksic et al., 2011). Surprisingly there is relatively little data assessing the functional state of the d entate gyrus circuit follow ing such lesions. H ow ever, spontaneous neural activity in m ature granule cells post lesion appears to transiently d ecrease im m ediately follow ing lesion, then grad ually returns to pre-lesion levels by 8 d ays (Reeves and Stew ard , 1988). The source of this activity presum ably reflects reorganization of synaptic inputs that follow s excitatory reinnervation by sprouting afferents. Post-lesion glial and extracellular matrix (ECM ) response Post-lesion structural reorganization in the ad ult d entate gyrus is influenced by th e post-injury d ynam ics of the extracellular environm ent. Reactive gliosis follow ing perforant path lesion is both rapid and sustained , and is consid ered ad aptive in this context. Gliosis serves to clear d egenerating d ebris, m aintain laminar bord ers, and to aid reactive synaptogenesis in the d eafferented region. For exam ple, microglia proliferate and acquire reactive m orphology w ithin three d ays post-lesion and return to baseline by d ay ten (H ailer et al., 1999). H ow ever, activation of astrocytes in the d enervated zone is d elayed relative to m icroglia and persists for at least 30 d ays post -lesion (H ailer et al., 1999). Together, m icroglia and astrocytes participate in phagocytosis of d egenerating axons (Bechm ann and N itsch, 2000) and may regulate axon sprouting and reactive synaptogenesis (Gage et al., 1988; Ullian et al., 2004). The efficiency of phagocytosis follow ing injury, especially of d egenerating m yelinated axons, generally correlates w ith 10 the d egree of regeneration in the CN S (N eum ann et al., 2009). Because the glial response is lim ited to the d enervated region, w ith relatively little reactive gliosis in the inner m olecular layer, this lamina-specific reaction m ay und erlie the lack of sprouting across lam inar bord ers into the d enervated zone. Reactive gliosis also triggers lamina-specific changes in the extracellular m atrix, w hich m ay affect the m aintenance of lam inar bord ers follow ing lesion. For exam ple, tenascin -C (Deller et al., 1997) and chond roitin sulfate proteoglycans (H aas et al., 1999) are secreted by reactive astrocytes follow ing perforant path lesion. Both these factors affect axon al outgrow th d uring d evelopm ent and follow ing injury (Bovolenta and Fernaud -Espinosa, 2000; Bartus et al., 2012). Similarly, reactive astrocytes can secrete throm bospond ins or m atrix m etalloproteases (Christopherson et al., 2005; Warren et al., 2012), w hich can provid e a scaffold for lesion -ind uced synaptogenesis (Deller et al., 2001; Mayer et al., 2005). In sum m ary, lesion of the perforant path elim inates the m ain excitatory input in the outer tw o-third s of the d entate m olecular layer, thus partially d enervating d end rites of m ature granule cells. This lesion m od el illustrates both the potential for regeneration in the CN S, but also som e of the limits. Within tw o w eeks post-lesion, rem aining afferent hom o- and heterotypic system s can sprout, but reorganization of axons and synaptic term inals is lim ited by lam inar bord ers, w hich are likely m aintained by lesion -ind uced gliosis and changes in the extracellular m atrix. Experim ents d escribed in this d issertation revisit the classic perforant path lesion m od el, but also includ e an ad ditional com ponent of the hippocam pal circuit in the ad ult brain – ongoing neurogenesis in the dentate gyrus. Specifically, w e d elineate the m aturation and functional integration of new born granule cells follow ing rem oval of their m ain excitatory input. The d end ritic spine reorganization 11 and de novo synaptogenesis w e observed follow ing lesion bring into light several im portant aspects of the role of excitatory activity in d end ritic outgrow th and the form ation of d end ritic spines. Sim ilarly, these results provid e a found ation for further exploration of signals that m ay serve as replacements for excitatory activity d uring synaptogenesis. IN TROD UCTION : FIGURES Figure 1 – Plasticity in the central nervous system. (A) Axons from tw o d ifferent pathw ays synapse onto spines on the sam e d end rites. Each synapse is surround ed by astrocytes (red), m icroglia (green), and extracellular m atrix. (B) Increases in activity, such as occur d uring learning, can strengthen connections by axonal sprouting (blue) as w ell as form ation of new filopod ia and d end ritic spines (*). Adjacent afferents, surround ing glia, and extracellular m atrix are relatively unaffected . (C) Disruption of afferents, such as follow ing injury, lead s to d egeneration of d am aged axons (d otted lines), activation of astrocytes, m icroglia, and extracellular m atrix, as w ell as retraction of d end ritic spines (*). Com pensatory sprouting of undam aged afferents from 12 another brain region can form new synapses, includ ing contacts w ith d enervated spines (#). 13 Figure 2 – Adaptive and maladaptive glial changes follow ing injury. The d egree of astrogliosis d epend s on the severity of injury. (A) Glia and extracellular m atrix at baseline. Astrocytes are tiled , i.e. their processes d o not overlap w ith neighboring astrocytes. Microglia are interspersed throughout the region. (B) Mild injury triggers activation of m icroglia and astrocytes. Astrocytes and m icrog lia increase in size and acquire m ore com plex process m orphology, but astrocytes m aintain their tiled form ation. This response is consid ered ad aptive because it lim its the spread of d egeneration aw ay from the site of injury, dam pens excitotoxicity, and prom otes tissue regeneration. Such glial activation typically resolves w ithin a few w eeks after a mild , transient injury. (C) In contrast severe injury causes reactive astrocytes to invad e neighboring d om ains, recruit reactive microglia, and increases secretion of extracellular m olecules. This results in form ation of a persistent glial scar that can be im penetrable to sprouting axons. 14 Figure 3 – Lamina-specific axon sprouting and reactive gliosis follow ing perforant path lesion. The m olecular layer of the ad ult d entate gyrus is a highly lam inated structure w ith afferent inputs segregated based on their origin and neurotransm itter phenotype. All afferent axons form either sym m etrical or asym metrical synapses w ith m ature granule cells (black traces) in a lam ina-specific m anner. Left panel: The inner m olecular layer (IML) is occupied by the glutam atergic com missural/ associational fibers (C/ A) that arise from m ossy cells in the ipsi- or contralateral hilus. The m id d le and outer m olecular layer (MML, OML) are occupied pred om inantly by the glutamatergic perforant path (MPP, LPP), w hich originates in the ipsilateral entorhinal cortex. In rats (but not in m ice), there is also a crossed glutam atergic projection from the contralateral entorhinal cortex (cEC) that term inates in the outerm ost m olecular layer (OML). Cholinergic axons (ACh) from the septal nuclei/ d iagonal band of Broca are interspersed throughout the m olecular layer, as are astrocytes (red) and quiescent microglia (green). Right panel: Lesion of the en torhinal cortex (red X, left panel) transects both m ed ial and lateral perforant path, thus eliminating the m ajority of excitatory input into the d entate gyrus. Degeneration of these axons ind uces lam ina-specific sprouting of the rem aining septohippocam pal (ACh), com m issural/ associational (C/ A), and crossed entorhino-d entate (cEC) afferents. In the rat, the contralateral entorhino-d entate projection (cEC) partially restores excitatory innervation of the mature granule cells (black trace), how ever, their d end ritic length and com plexity are still red uced . The microglia (green) and astrocytes (red) becom e ‘activated ’ follow ing lesion, but this response is lim ited to the d eafferented zone. N ote the expansion of the inner m olecular layer and shrinkage of the outer layers. 15 Figure 4 – Structural plasticity follow ing perforant path lesions. Left panels (m od ified from Stew ard , O. and J. A. Messenheimer, 1978): Mature cat hippocam pus histochemically stained for acetyl cholinesterase (AChE) activity at 60 d ays post-lesion. The d ensity of AChE is d ramatically increased in the d enervated outer m olecular layer (top right, d ark band), consistent w ith sprouting of the cholinergic septohippocam pal axons follow ing lesion. Also note that the thickness of the inner m olecular layer is increased d ue to sprouting of the glutam atergic com m issural/ associational fibers (bottom right, double arrow s). Right panels (m odified from Matthew s, D. A., Cotm an, C., and Lynch, G., 1976b): Ultrastructural evid ence for synaptic regeneration in the d enervated zone at 60 d ays post-lesion in the m ature rat. Serial sections through a com plex spine (green) show synaptic contacts w ith a d egenerating bouton (D) as w ell as w ith a regenerating axon (*). a = spine apparatus. 16 CHAPTER 1 N EURAL IN JURY TRIGGERS A BIPHASIC RESPON SE IN AD ULT-GENERATED N EURON S IN THE D ENTATE GYRUS (These d ata w ill be p u blished in the Jou rnal of N eu roscience in 2013) Introduction Traum atic brain injury, neurod egeneration, and ischem ia all involve d enervation, the extent of w hich contributes to the severity of clinical sym ptom s. H ow ever, brain injury can also stim ulate plasticity. An exam ple of such plasticity in the ad ult brain is the synaptic reorganization follow ing lesions in the entorhinal cortex, w hich irreversibly interrupt the m ain excitatory input into the d entate gyrus – the perforant path. The d entate gyrus provid es a particularly ad vantageous m od el system for exam ining brain injury because its highly lam inated m olecular layer segregates inputs from d ifferent sources. Perforant path axons from the m ed ial and lateral entorhinal cortex term inate in the m id dle and outer third of the m olecular layer, respectively (H jorth-Sim onsen and Jeune, 1972; H jorth-Sim onsen, 1972, van Groen et al., 2003), w here they synapse w ith apical d end rites of m ature granule cells. When these inputs are severed , rem aining intact fiber system s that also synapse onto m ature granule cells reorganize by sprouting and reactive synaptogenesis (Deller and Frotscher, 1997). These changes are m axim al w it hin tw o w eeks post-lesion but grad ually stabilize by several m onths post -lesion (Vuksic et al., 2011). In ad d ition to m ature cells, there is ongoing neurogenesis in the dentate gyrus, generating 5-10 thousand new granule cells per d ay (Cam eron and McKay, 2001). A subset of these new born neurons survive and integrate into the hippocam pal circuit, show 17 enhanced short-term synaptic plasticity, and m ay contribute to norm al function of the hippocam pus (Dayer et al., 2003; Ge et al., 2008). Because new born cells are exquisitely sensitive to intrinsic and extrinsic stim uli (van Praag et al., 1999; Overstreet et al., 2004; Tashiro et al., 2007; Balu and Lucki, 2009; Ming and Song, 2011), they are a potentially im portant source of neurons for replacem ent and repair follow ing neural injury. N ew born granule cells go through a series of highly stereotyped stages, d uring w hich they extend their d end rites through the m olecular layer, d evelop spines, and acquire excitatory synaptic inputs over a period of several w eeks (van Praag et al., 2002; Overstreet-Wadiche and Westbrook, 2006). This pattern of functional m aturation provid es an opportunity to exam ine w hether new born cells integrate into a recently d eafferented circuit. H ere, w e focused on a population of new born granu le cells that w ere d ivid ing at the time of a unilateral perforant path lesion. We hypothesized that new neurons w ould show enhanced structural plasticity com pared to m ature neurons. To id entify new born granule cells w e used a POMC-EGFP transgenic m ouse, in w hich EGFP expression is d riven by transient activity of the pro-opiom elanocortin (POMC) prom oter that labels these cells at approxim ately 14 d ays post-m itosis (Overstreet et al., 2004b). We also used intrahippocam pal injections of a retrovirus expressing GFP to label cohorts of new born neurons at later time points. The com pleteness of the lesion w as confirm ed m orphologically and by the absence of extracellular field responses in the outer m olecular layer in acute brain slices. In the ipsilateral d entate g yrus, lesions caused proliferation of new born neurons by 2 w eeks, follow ed by a lamina -specific rearrangem ent of d end ritic spines and excitatory synaptic activity by 3 w eeks post-lesion. Surprisingly, confocal im ages and im m unoelectron m icrographs of the d enervated zone revealed that new born 18 neurons form ed new d end ritic spines post-lesion w ithout norm al apposing presynaptic term inals. 19 Methods M ice: All proced ures w ere perform ed in accord ance w ith OH SU IACUC and Biosafety com m ittee protocols and in accord w ith the N IH guid elines for handling of anim als. C57Bl6 m ice w ere six to eight w eeks of age at the tim e of the proced ure. Both m ale and fem ale mice w ere included , and w ere d erived from m ultiple breedings. POMC-EGFP transgenic mice in C57Bl6 background w ere used for stud ies of new born granule cells at 14 d ays post-mitosis, the peak of EGFP expression (Overstreet et al., 2004b). We also used retroviral labeling of w ild -type C57Bl6 m ice for visualization of new born granule cells at 21 d ays post-mitosis, and lentiviral labeling for visualization of m ature granule cells at 21 d ays post-lesion. For GFP labeling of new born granule cells, w e used a replicationd eficient Moloney Murine Leukem ia Virus-based retroviral vector (Lew is and Emerm an, 1994) d esignated pRubi (Retrovirus w ith internal ubiquitin prom oter, Luikart et al., 2011b). Isoflurane-anesthetized w ild -type m ice received bilateral stereotaxic injections d irectly into the d orsal hippocam pus (2 l per hem isphere, see below ). For GFP labeling of m ature granule cells, w e used a lentiviral vector that contains the H IV-1 Flap elem ent, the hum an Ubiquitin-c promoter, GFP, and the Woodchuck hepatitis virus posttranscriptional regulatory elem ent (FUGW, Lois et al., 2002; Luikart et al., 2011a). Volum e for lentiviral injections w as 1 l per hem isphere. Stereotaxic coord inates w ere: AP -1.9, LM 1.1, DV -2.5 and -2.3, w ith an injection rate of 0.25 l per m inute. The need le w as left in place for one m inute before w ithd raw ing from the injection site. Viral particles w ere prepared using 6 established protocols w ith titers of 1-10 X 10 (Luikart et al., 2011a). Unilateral Lesions of the Perforant Path: Mice w ere anesthetized in an ind uction chamber filled w ith 4% isoflurane/ oxygen and placed in a Kopf stereotaxic apparatus w hen 20 rend ered unresponsive. During surgery, 2% isoflurane/ oxygen mixture w as continuously ad m inistered through a nose cone. Prior to incision, the scalp w as treated w ith 2% lid ocaine, iod ine m ixture, and a d ouble antibiotic. Skull w as exposed and a rectang ular access w ind ow w as d rilled using a 0.275m m diam eter carbid e d rill (Kemm er Praezision, Placentia, CA), starting 2m m lateral to lam bd a, 1m m rostral to the transverse suture, and spanning the w id th of the hem isphere. Lesion w as perform ed m anually, using a 15 alum inum m icroscalpel (N o. 715, Oasis Med ical, San Dim as, CA), w ith m ed ial to lateral path trajectory, at a d epth of ~ 4.5m m . Care w as taken to avoid scraping the scalpel along the bottom of the skull. Lesions alternated betw een left and right hemisph ere w ith the contralateral hem isphere serving as the control. In prelim inary experim ents, a few anim als d id not survive the proced ure, how ever refinem ent of the lesion site resulted in no m ortality. For m orphological analysis at 14 and 21 d ays post-lesion, animals w ere d eeply anesthetized w ith 2% Avertin (2,2,2-Tribrom oethanol, Sigma-Ald rich, St. Louis, MO), transcard ially perfused w ith PBS + 4% sucrose follow ed by 4% paraform ald ehyd e (PFA) + 4% sucrose. Brains w ere extracted and post-fixed overnight in 4% PFA+sucrose at 4 C. After fixation, each brain w as split in the coronal plane just anterior to the site of the lesion and each half w as sectioned separately, using a vibratom e (VT-1000S, Leica Microsystems Inc., Buffalo Grove, IL). The rostral half, containing the d orsal hippocam pus, w as sectioned coronally (100 m sections) and these sections w ere used for all m orphological analysis. The caud al half, containing the lesion site, w as cut horizontally into serial 300 m sections to assess the extent of lesion. Anim als w ere exclud ed from analysis if the lesion injured the ventral hippocam pus, w hich led to hippocam pal atrophy, or if the lesion w as incom plete (see Figure 1). A lesion w as consid ered com plete if it spanned the entire height of the entorhinal cortex and w as visible even in the m ost ventral of the horizontal sections. 21 Immunohistochemistry: 100 m coronal sections w ere perm eabilized for 30 m inutes w ith 0.4%-TritonX in PBS (PBS-T), blocked for 30 m inutes w ith 10% H orse Serum in PBS-T, and stained overnigh t w ith prim ary antibod y in 1.5% H orse Serum / PBS-T. Stains requiring second ary antibod ies w ere w ashed in PBS and incubated w ith second ary antibod y in 1.5% H orse Serum / PBS-T for 4 hours at room tem perature. Prim ary antibodies: rabbit anti GFP (Alexa 488-conjugated ; 1:500; A21311; Invitrogen, Carlsbad , CA); rabbit anti glial fibrillary acid ic protein (GFAP; 1:500, Z-0334; DAKO, Carpinteria, CA); rabbit anti vGlut1 (1:500; 135 303; Synaptic Systems, Goettingen, Germ any); rabbit anti vGlut2 (1:400; 135 404; Synaptic System s, Goettingen, Germ any). Secondary antibod ies (1:200 dilution): goat anti rabbit (Alexa 568; A11011; Invitrogen, Carlsbad , CA). Sections w ere m ounted on glass slid es and coverslipped using VectaShield w ith DAPI (H -1200; Vector Laboratories Inc., Burlingam e, CA). Imaging and M orphological A nalysis: Im aging w as done on an LSM 7 MP laser scanning m icroscope (Carl Zeiss MicroIm aging; Thornw ood , N Y). All cell counting, d end ritic arbor/ spines tracing, and Sholl analysis w ere d one m anually w ith Im ageJ (N ational Institutes of H ealth; Bethesd a, MD). At 14 d ay post-lesion, sections from POMC-EGFP m ice w ere im aged w ith : 40x objective, 0.7x optical zoom , z-stacks of 50 m , 1 m betw een planes. At 21 d ays post-lesion, sections from retro- and lentiviral-injected m ice w ere im aged for d end ritic arborization w ith a 40x objective and 0.7x optical zoom . Thickness of z-stack w as d esigned to accom m od ate the d end ritic span of labeled granule cells, w ith 1 m d istance betw een im age planes. For analysis of spine d ensity of new born and m ature granule cells at 21 d ays post-lesion, w e used a 63x objective w ith 3x optical zoom . Span of 22 z-stack w as tailored to the thickness of a single segm ent of d end rite w ith 0.5 m d istance betw een planes. Dend ritic spines w ere im aged and analyzed in inner and outer m olecular layers of the d entate gyrus. Laser intensity and gain w ere adjusted to accom m od ate staining efficiency, but the sam e microscope settings w ere used for both control (non lesioned ) and the lesioned hem isphere. BrdU: For proliferation m easurem ents, tw o injections of Brd U (300 m g/ kg) w ere ad m inistered to POMC-EGFP m ice at three separate intervals post-lesion: (i) 0 and 4 hours post-lesion (hpl), (ii) 16 and 20 hpl, and (iii) 40 and 44 hpl. Anim als w ere perfused w ith sucrose-containing PBS follow ed by PFA (see above) at 8, 24, and 48 hours post -lesion (four hours after the last Brd U injection). For survival m easurem ents, BrdU injections w ere at 40 and 44 hpl, and the tissue w as exam ined at 28 d ays post-lesion. For Brd U im m unostaining, sections w ere w ashed tw ice in KPBS for 10 m inutes each. Antigen retrieval w as perform ed using an acid / base sequence: 30 m inutes in 2N H Cl (at 36 C) follow ed by 10 m inutes in pH 8.5 KPBS (at room tem perature). The sections w ere then perm eabilized for 30 minutes in 0.4% KPBS-T and blocked for 30 m inutes w ith 5% H orse Serum (H S) in KPBS-T. Prim ary antibod y (rat anti Brd U; ab6326; AbCam , Cam brid ge, MA) w as applied at 1:2000 in 5% H S/ KPBS-T overnight at 4 C. The sections w ere w ashed in KPBS and incubated for 4 hours w ith Rhod am ine Red second ary antibod y (goat anti rat; 112-295-003; Jackson Imm unoResearch, West Grove, PA), at 1:200 in 5% H S/ KPBS-T at room tem perature. The sections w ere subsequently stained w ith Alexa 488-conjugated rabbit anti GFP (as d escribed above). Sections w ere im aged on a Zeiss LSM 780 confocal m icroscope, using a 20x objective. Z-stacks w ere 60 m , w ith im age planes 2 m apart. All Brd U-positive cells w ithin the granule cell layer of the d orsal hippocam pus w ere counted 23 for every third 100 m section and averaged for each animal. Because all sections contained both hem ispheres, lesion and control hippocam pi w ere processed in parallel. Electrophysiology: Extracellular field potentials and w hole cell record ings w ere perform ed at 14 d ays post-lesion, from acute slices of d orsal hippocam pus, at the same antero posterior level used for m orphom etric analysis. The anim als w ere d eeply anesthetized w ith an intraperitoneal injection of Avertin (2,2,2-Tribrom oethanol, Sigm a-Ald rich, St. Louis, MO) and transcard ially perfused w ith ice-cold cutting solution, containing 110m M choline Cl, 7m M MgCl2, 2.5m M KCl, 1.25mM N aH 2PO 4*2H 2O, 0.5mM CaCl2, 1.3m M N aascorbate, and 25mM N aH CO 3, bubbled w ith 95% O 2-5% CO 2. For field record ings, 400 μm coronal slices w ere m ad e using a Leica vibratom e in artificial cerebrospinal fluid (ACSF) containing 119 m M N aCl, 2.5 m M KCl, 2.5 m M CaCl 2, 1.3 m M MgSO 4, 1 mM N aH 2PO 4, 26.2 m M N aH CO 3, and 11 m M glucose, bubbled w ith 95%O 2-5%CO 2. After 1 hour recovery, field potentials w ere record ed w ith ACSF-filled 2-3MΩ glass pipettes placed in the outer m olecular layer of the d entate gyrus. In pilot experim ents in the d eafferented d entate gyrus, w e looked for field excitatory postsynaptic potentials (fEPSPs) in m ultiple locations and at varying d epths in the supra and infrapyram id al blad e, and w ere unable to elicit responses at the usual range of stim ulus intensities. To quantify fEPSPs w e constructed input/ output curves after stim ulation of the infrapyram id al blad e, thus red ucing contamination from the adjacent CA1 region. Stim uli w ere d elivered at 0.1H z (d uration 100 µs, AMPI Iso-Flex constant current stim ulator) via a bipolar tungsten electrod e (FH C, Bow d oin, ME) placed in the outer m olecular layer, approxim ately 150 μm d eep into slice and approxim ately 500 μm from the record ing electrod e. At each stim ulus intensity, ten responses w ere averaged using IgorPro. The am plitud es of the fiber volley 24 and initial slope of the fEPSPs w ere graphed relative to the stim ulation intensity. For each slice, responses w ere record ed in the d entate gyrus from the lesioned and non -lesioned hem ispheres. For w hole-cell record ings, the hem ipheres w ere separated and sectioned at 350 m in the transverse hippocam pal plane. Slices w ere stored and recordings perform ed in a solution containing 125m M N aCl, 2.5m M KCl, 2.0m M CaCl2, 1.0m M MgCl2, 1.25m M N aH 2PO 4, 25m M N aH CO 3, and 25 m M glucose, bubbled w ith 95%O 2-5%CO 2. For m EPSC record ings, w e ad d ed 10µM SR95531 to block GABA A channels and 1μM tetrod otoxin to block action potentials. The Cs-gluconate w hole-cell pipette solution contained 100m M gluconic acid, 0.2m M EGTA, 5m M H EPES, 2m M Mg-ATP, 0.3m M Li-GTP, pH =7.2, 295m Osm (pH to 7.2 using 50%CsOH for a final concentration of 100-120 Cs-gluconate). Series resistance (5–20 MΩ) w as m onitored , and experim ents w ere d iscarded if the resistance increased by >10 MΩ. Currents w ere filtered at 4 kH z and sam pled at 40 kH z (MultiClam p 700A Molecular Devices, Union City, CA). m EPSCs w ere d etected using a tem plate w ith rise tim e=1m s, d ecay tim e=6ms, baseline=10ms, length=30m s and a threshold of three (Axograph X, Syd ney, AU). Captured events w ere m anually review ed . Rise tim es w ere d efined as the tim e betw een 10% and 90% of the m axim al am plitud e. Electron M icroscopy: For ultrastructural analysis of m ature granule cells at 21 d ays post lesion, anim als w ere transcard ially perfused w ith PBS follow ed by a 0.1M Sod ium cacod ylate buffer containing 1.5% glutarald ehyd e, 1.5% PFA, 0.05M sucrose and 0.25% CaCl2 (pH 7.4). Extracted brains w ere post-fixed overnight in the sam e solution. The hem ispheres w ere separated along the m id line and each w as cut sagittally into 1m m sections. The hippocam pi from each 1m m section w ere m icrod issected out and further cut into 1x1x2m m blocks that includ ed the d entate gyrus and CA1. Blocks w ere em bed d ed in 25 coffin m old using Spurr:Epon, polym erized overnight at 60 C, trim m ed, and cut along the long axis into coronal 700nm sections using a Leica EM UC6 vibratom e (Leica Microsystem s, Inc., Buffalo Grove, IL). Som e sections w ere placed on glass slid es and stained w ith 0.5% Toluidine blue in 0.5% Sod ium Tetra Borate for region selection. Sections from lesioned and non -lesioned hem ispheres containing the suprapyram id al blad e of the d entate gyrus w ere em bed d ed in resin, and further cut into 70nm sections on an ultram icrotom e (Leica Microsystem s, Inc., Buffalo Grove, IL). The sections w ere placed on 200-square m esh Copper/ Rhod ium grid s and counterstained w ith 5% uranyl acetate and Reynold ’s lead citrate. The inner and outer molecular layers of the suprapyram id al blad e of the d entate gyrus from both lesioned and unlesioned hem ispheres w ere im aged at 13,000x on an FEI Technai G 2 12 Biotw in at 80kV. Survey im ages that show reactive astrocytes w ere acquired at 6,800x. For ultrastructural analysis of 21-d ay-old granule cells, w e im m unolabeled sections from retrovirus-injected animals at 21 days post-lesion. Anim als w ere d eeply anesthetized w ith N a-Pentabarbitol (150mg/ kg) and , w hen rend ered unresponsive, w ere transcard ially perfused w ith heparinized saline, follow ed by a 3.8% Acrolein in PFA and 2% PFA in PB (H egarty et al., 2010). Tissue blocks containing both d orsal hippocam pi w ere postfixed in 2% PFA (no acrolein) for 30 m inutes; em bed d ed in agar ; and cut coronally into 40 m sections on a vibrating microtom e (Leica Microsystem s, Inc., Buffalo Grove, IL). H em ispheres w ere not separated for this experiment. Sections w ere stored in sucrose solution at -20 C until need ed . Sections w ere rinsed 2x in 0.1M PB to clear the sucrose solution; placed in 1% sod ium borohyd rid e for 30 m inutes; rinsed w ith PB until bubbles w ere gone; treated w ith a cryoprotectant solution; and freeze-thaw ed to perm eabilize. 26 Once at room tem perature, sections w ere rinsed 2X in 0.1M Tris-saline and incubated in 0.5% BSA for 30 m inutes. Rabbit anti-GFP prim ary antibod y (Millipore, AB3080, Tem ecula, CA) w as applied at 1:1000 for 36 hours at 4 C. Follow ing incubation, sections w ere rinsed 3X in 0.1M Tris-saline, 5-10 m in each, follow ed by biotinylated goat anti-rabbit second ary antibod y (Vector Labs, BA-1000, Burlingam e, CA; 1:400 in 0.1% BSA, 30 m inutes, room tem p). Sections w ere incubated in ABC solution (Elite Vectastain, Vector Labs, Burlingam e, CA; 30 m in), rinsed w ith 0.1M Tris-saline, and then treated w ith d iam inobenzid ine-H 2O 2 solution (5 m in). Follow ing w ashes in 0.1M Tris-saline and then in 0.1M PB, sections w ere incubated in 2% osm ium tetroxid e (60 m in), d ehyd rated , flat em bed d ed in EMBed -812 (RT 14120; Electron Microscopy Sciences, H atfield , PA) betw een 2 sheets of ACLAR flurohalocarbon film (#50425; Electron Microscopy Sciences, H atfield , PA), and cured at 60 C for 48 hours (Figure 8A). Regions of interest from lesioned and unlesioned hem ispheres containing labeled new born neurons in the suprapyram id al blad e of the dentate gyrus w ere cut out and superglued onto cured resin blanks. Tissue w as thin-sectioned on an ultram icrotom e (Leica Microsystem s, Inc., Buffalo Grove, IL) into 75nm sections and placed onto N ickel/ Copper mesh grid s. Grid s w ere counterstained and im aged as d escribed above. Statistical A nalysis: All values are expressed as m ean±SEM. A Generalized Linear Mod el (GLM) w as used for analysis of POMC-EGFP cell counts, total d end ritic length and arborization, and d end ritic spine d ensity. Kolm ogorov-Sm irnov (KS) test w as used to analyze d istributions of granule cell d ispersion and of m EPSC am plitud es and rise tim es. Repeated Measures/ ANOVA w as used for field potentials and Sholl analysis. Paired t-test w as used for analysis of single m EPSCs, BrdU cell counts, and EM synaptic com position. 27 Chi-squared test w as used to com pare proportions of surviving BrdU+ cells. Significance cutoff w as p < 0.05. p values are reported in the text. Results The perforant path from the entorhinal cortex is the m ajor afferent input to hippocam pus, form ing excitatory synapses in the outer 2/ 3rd s of the m olecular layer of the d entate gyrus. To exam ine the im pact of d eafferentation on ad ult -generated new born neurons in the d entate gyrus, w e m ad e unilateral surgical lesions of the perforant path in young ad ult m ice (Figure 5A). Because surgical lesions can be variable, w e verified the com pleteness of each lesion by serially sectioning the caud al half of the brain from d orsal to ventral in the horizontal plane (Figure 5B). In this exam ple of a com plete lesion at 14 d ays post-lesion (d pl), the location of the surgical cut w as visible even in the ventral-m ost horizontal sections of the caud al half of the m ouse brain (arrow head s). To account for system ic factors such as anesthesia or surgical stress, the contralateral dentate gyrus w as used as an in-anim al control. Anim als w ith incomplete lesions or w ith d am aged hippocam pi w ere exclud ed from the analysis. We confirm ed d egeneration of perforant path fibers by extracellular field recordings in acutely prepared coronal brain slices at 14 d pl. We m easured the fiber volley that represents action potential propagation in perforant path axons, and field excitatory postsynaptic potentials (fEPSPs) that refle ct granule cell d epolarization in response to excitatory synaptic transmission. As expected , stim ulation contralateral to the lesion evoked a short latency fiber volley follow ed by a fEPSP as record ed w ith an extracellular electrod e in the outer m olecular layer. H ow ever, both the fiber volley and fEPSP am plitud es w ere greatly red uced or com pletely absent on the lesioned sid e (Figure 5C) over a w id e range of stim ulation intensities. For exam ple, at 28 1.0 μA stim ulation intensity, the fiber volley am plitud e w as d ecreased 76.1% (control: -0.30 m V; lesion: -0.07 m V, p < 0.05, Repeated Measures/ AN OVA; n=12 slices from 5 anim als). Sim ilarly, the fEPSP am plitud e at this stim ulation intensity w as decreased 88.2% (control: 0.34 m V/ m sec; lesion: -0.04 m V/ m sec, p < 0.0001, Repeated Measures/ ANOVA; n=12 slices from 5 anim als). To assess the integrity of perforant path nerve term inals post-lesion, w e im m unolabeled the vesicular glutam ate transporters, vGlut1 and vGlut2, w hich often show com plem entary d istributions at excitatory synapses. vGlut1 is expressed in perforant path fibers as w ell as the m ossy fibers of d entate granule cells, w hereas vGlut2 is expressed in hilar m ossy cells and extrahippocampal inputs from the supram am m illary nuclei (Leranth and H ajszan, 2007). At 21d pl, vGlut1 im m unolabeling w as relatively hom ogeneous throughout the m olecular layer and hilus, but w as not present w ithin the granule cell layer (Figure 5D, left panel). In the d eafferented d entate gyrus, there w as no d etectable labeling in the outer 2/ 3rd s of the m olecular layer (OML staining ratio lesion/ control = 0.17, n=5 animals), although labeling w as preserved in the inner m olecular layer (IML). vGlut2 im m unolabeling show ed the expected exp ression in a narrow band of the supragranular layer (Boulland et al., 2009), but no d etectable expression in the d enervated zone (Figure 5E, right). The lack of vGlut1 (or vGlut2) staining post-lesion is consistent w ith com plete perforant path transection. Proliferation and migration of newborn neurons following perforant path lesion To exam ine the response of new born neurons follow ing perforant path lesion, w e used POMC-EGFP transgenic m ice that transiently label new borns neurons at 29 approxim ately 10-14 d ays post-m itosis (Overstreet et al., 2004b, Figure 6A, left panel). There w as an increase in the num ber of EGFP + cells in the d entate gyrus post-lesion (Figure 6A, right panel) com pared to the contralateral control (Figure 6A, left panel) in the sam e anim al (control: 74±11 X 103 cells/ m m 3; lesion: 107±22 X 103 cells/ mm 3, p < 0.05, GLM; n=8 anim als). In ad d ition to the increased num ber, EGFP + cells w ere no longer lim ited to the subgranular zone, but had migrated w ithin the granule cell layer as show n in the close-up of the suprapyram id al blad e of the d entate gyrus (Figure 6B). To quantify the m igration, w e m easured the perpendicular d istance betw een the center of each EGFP + cell bod y and the bord er betw een the subgranular zone and hilus (Figure 6C). N ew born granule cells in the d eafferented d entate gyrus w ere nearly three times farther from the hilar bord er than those in the contralateral control, thus shifting the d istribution of new born neurons w ithin the granule cell layer to the right (Figure 6D; control: 9.16±0.81 m ; lesion: 25±2.47 m , p < 0.0001, KS-test; n=8 anim als). The increase in new born neurons on the sid e of the lesion could result either from an increase in proliferation or an increase in survival. Thus w e injected Brd U into lesioned m ice to label divid ing cells in the d entate gyrus. Tw o injections of BrdU, separated by 4 hours, w ere used in ord er to saturate labeling d uring one cell cycle (see m ethod s). We counted the number of Brd U-labeled cells w ithin the granule cell layer four hours after the last Brd U injection (at 8, 24, and 48 hours post-lesion) as an estimate of proliferation and at 1 m onth post-lesion as an estimate of survival. At 8 and 24 hours post-lesion there w as only a slight increase in Brd U-labeled cells (not show n; 8 hours – control: 6.3±1.1 X 103 cells/ m m 3; lesion: 7.2±1.4 X 103 cells/ m m 3, N S, paired t-test, n=4 anim als; 24 hours – control: 6.2± 0.8 X 103 cells/ m m 3; lesion: 7.3±1.4 X 103 cells/ m m 3, N S, paired t-test, n=5 30 anim als). H ow ever, by 48 hours post-lesion, there w as a d ou bling of BrdU-labeled cells (Figure 7A, control: 10.6±1.3 X 103 cells/ m m 3; lesion: 21.6±2.6 X 103 cells/ m m 3, p < 0.001, paired t-test; n=7 anim als), suggesting that the increase in EGFP + cells w as the result of a lesion-ind uced proliferative response. As expected , the m ajority of Brd U + cells w ere in the subgranular zone at the hilar bord er. As show n in Figure 7A (right panel), Brd U -labeled cells w ere also observed in the d enervated region, consistent w ith a glial response follow ing lesion (H ailer et al., 1999, see also below ). At 1 m onth, post-lesion, there w as a correspond ing increase in surviving BrdU + cells (Figure 7B, control: 4.2±0.8 X 103 cells/ m m 3; lesion: 7.5±1.2 X 103 cells/ m m 3, p < 0.01, paired t-test; n=7 anim als). Despite the increase in proliferation, the percentage of surviving new born granule cells w as sim ilar betw een hem ispheres (Figure 7C, control: 39.6%; lesion: 34.7%, N S, Chi-squared test), suggesting that the survival rate w as not altered by the lesion. Dendritic outgrowth and synapse formation post-lesion Ad ult-generated new born granule cells extend d end rites into the inner m olecular layer by 2 w eeks post-m itosis, but have yet to receive excitatory inputs. By the 3rd w eek, the d end rites reach the outer 2/ 3rd s of the m olecular layer and a re contacted by the perforant path (Overstreet, 2006). Unlike m ature granule cells that are d enervated by the perforant path lesion, w e analyzed new born cells that w ere d ivid ing at the tim e of the injury. This approach allow ed us to exam ine the effect of the perforant path lesion on d end ritic outgrow th and synapse form ation after perforant path fibers have alread y d egenerated . Dend ritic arbors at 14 d ays post-lesion w ere m easured using POMC-EGFP m ice. Dend rites and synaptic activity at 21 d ays post-lesion w ere m easured in neurons that had been labeled w ith GFP using retroviral vectors at the tim e of lesion (pRubi, see 31 m ethod s). As expected , the total length and branch num ber of d end rites increased betw een d ay 14 and 21 in the control (Figure 8A, left panels). H ow ever, the grow th of d end rites and branches w as significantly less post-lesion (Figure 8A, right panels and Figure 8B; 14 days – control: 239.5±26.7 m ; lesion: 202.0±26.4 m , p < 0.05, GLM, n=5 anim als, 7-15 neurons per d entate gyrus; 21 days – control: 1189.7±119.3 m, lesion: 807.2±109.8 m , p < 0.0001, GLM, n=5 anim als, 5-10 neurons per d entate gyrus). Sholl analysis of all traces revealed that d end ritic com plexity w as not affected in 14-d ay-old neurons, w hen the d end rites are confined to the inner m olecular layer (Figure 8C, N S, Repeated Measures/ ANOVA), but there w as a significant red uction in the d istal d end ritic com plexity in 21 d ay-old neurons (Figure 8D, p < 0.001, Repeated Measures/ AN OVA), correspond ing to the d enervated zone. In ad d ition to the perforant path, d entate granule cells receive com m issural/ associational excitatory inputs located in the inner m olecular layer, w hich are not interrupted by the lesion and thus could provid e synaptic input to ad ult-generated new born neurons. To exam ine th is possibility, w e record ed m iniature excitatory synaptic currents (m EPSCs) from 21-d ay-old granule cells that had been GFP-labeled w ith the pRubi retrovirus on the d ay of the lesion. m EPSCs w ere present at 21 days post-lesion, but the frequency w as d ecreased in the denervated dentate gyrus com pared to the contralateral control (Figure 9A, control: 0.13±0.01 H z; lesion: 0.09±0.02 H z, p < 0.05, paired t-test; n=11 and 12 neurons, respectively). The m EPSC am plitud e w as increased on the lesioned sid e (Figure 9B, right panel, control: 13.5±0.7 pA; lesion: 16.5±0.9 pA, p < 0.05, paired t-test; n=11 and 12 neurons, respectively). The d istribution of a rand om sam ple of m EPSC am plitud es show ed a rightw ard shift w ith few er sm all events (5-15pA) and m ore 32 large events (15-50pA; Figure 9B, center panel, p < 0.0001, KS-test). The rise-tim e of m EPSCs w as also faster (Figure 9C, right panel, control: 1.46±0.08 m s; lesion: 1.21±0.06 ms; p < 0.01, paired t-test; n=11 and 12 neurons, respectively). The d istribution of rise-tim es show ed a leftw ard shift w ith increase in fast-rising (0-1.5m s) and a decrease in slow -rising (1.5-3m s) events (Figure 9C, center panel, p < 0.0001, KS-test). The larger, m ore rapid ly rising m EPSCs in the d enervated d entate gyrus m ay indicate preferentia l input from synapses in the inner m olecular layer that are closer to the som atic record ing site. These results are consistent w ith the lack of vGlut1/ 2 staining in the d enervated zone and thus the absence of sprouting across lam inar bound aries (see Figure 1). de novo dendritic spines in the denervated zone The perforant path forms excitatory axons onto dend ritic spines of m ature granule cells. Follow ing lesioning, d end ritic spines are transiently red uced (Matthew s et al., 1976a; Stew ard et al., 1983; Vuksic et al., 2011). We com pared the im pact of lesioning on preexisting d end ritic spines on m ature granule cells w ith its im pact on de novo form ation of d end ritic spines on new born neurons. Mature and new born granule cells w ere GFP labeled w ith retroviral (p Rubi) and lentiviral (FUGW) vectors, respectively. At 21 days post-lesion, new born neurons in the contralateral control had a large number of d end ritic spines in the outer m olecular layer (Figure 10A, top left). H ow ever there w ere few er new ly form ed spines on the lesioned sid e (Figure 10A, top right, control: 1.53±0.17 spines/ µm ; lesion: 1.22±0.12 spines/ µm , p < 0.001, GLM; n=7 anim als). There w as a sim ilar red uction in the num ber of existing spines on m ature granule cells follow ing d eafferentation (Figure 10B, top row , control: 2.36±0.18 spines/ µm ; lesion: 1.61±0.13 spines/ µm , p < 0.001, GLM; n=5 anim als). In contrast, there w as a 45% increase in d end ritic spines on new born 33 neurons in the inner m olecular layer (Figure 10A, low er row , control: 1.11±0.17 spines/ µm ; lesion: 1.61±0.12 spines/ µm , p < 0.001, GLM; n=7 anim als). Mature granule cells, how ever, d id not show a change in spine density in the inner m olecular layer (Figure 10B, low er panel; control: 2±0.16 spines/ µm ; lesion: 2.02±0.23 spines/ µm , N S, GLM; n=5 anim als). These results ind icate that, follow ing lesion, spine form ation on new born granule cells can occur throughout the m olecular layer, but w as m ost robust in areas that had intact glutam atergic axons. We w ere surprised that new born granule cells d eveloped d end ritic spines in the outer m olecular layer in the absence of functional perforant path activity as m easured by the field EPSPs. Likew ise, m ature granule cells show an initial d ecrease in spines follow ing perforant path lesion, but then nearly com pletely recover (Stew ard et al., 1983; Vuksic et al., 2011). To evaluate w hether these spines are associated w ith presynaptic term inals w e exam ined the synaptic ultrastructure in the suprapyram id al blad e of the d entate gyrus at 21 d ays post-lesion. In the d entate gyrus contralateral to the lesion, intact asym m etric (excitatory) synapses w ith presynaptic term inals and postsynaptic d ensities w ere easily apparent throughout the m olecular layer (Figure 11A, left colum n) as w ell as the inner m olecular layer in the lesioned hemisphere (Figure 11A, top right). H ow ever, synaptic structures w ere d isrupted in the d enervated zone. Degenerating nerve term inals w ere electron-d ense and w ithout clearly d efined presynaptic vesicles, w hereas post synaptic d ensities w ere p reserved (Figure 11A, bottom right). To quantify synaptic structures in each layer, 10 regions of interest from 3 non -adjacent sections in each d entate gyrus w ere im aged . A synapse w as consid ered intact if there w as a presynaptic term inal containing synaptic vesicles, a subm embrane post-synaptic d ensity (PSD), and close 34 apposition of pre- and post-synaptic mem branes. As show n in Figure 11B, the num ber of postsynaptic d ensities w as unaffected by the lesion (PSDs – control: 0.6/ m 2; lesion: 0.51/ m 2, N S, paired t-test; n=4 anim als), but presynaptic term inals and intact/ norm al synapses w ere m arked ly d ecreased in the outer molecular layer of the d enervated d entate gyrus (axon term. w/vesicles – control: 0.85/ m 2; lesion: 0.24/ m 2, p < 0.001, paired t-test, n=4 anim als; synapses: control: 0.28/ m 2; lesion: 0.06/ m 2, p < 0.001, paired t-test, n=4 anim als). Reactive gliosis w as also apparent in the d enervated zone as previously d escribed (Figure 11C, shad ed region; also H ailer et al., 1999). Because synapses on new born neuron constitute only a sm all fraction of all synapses in the m olecular layer, the analysis in Figure 11 largely, if not com pletely, represents synapses on m ature granule cells. The relative retention of postsynaptic structures could sim ply represent slow turnover of pre-existing PSDs that are no longer innervated . H ow ever, the protocol w e used for retroviral labeling of new born neurons sam ples only new d end ritic spines and synapses that form ed after the lesion. To exam ine w hether the d end ritic spines on new born neurons have intact synaptic ultrastructure, w e perform ed im m unoelectron m icroscopy on GFP-labeled new born neurons using anti-GFP antibod ies. Because of the relatively sparse labeling of new born neurons w ith the retrovirus, sections of the m olecular layer w ere chosen that contained several labeled d end ritic trees (Figure 12A, black boxes). Occasional d end rites w ith punctate or electron labeling w ere seen in the inner and outer m olecular layer in the control and d eafferented d entate gyrus (Figure 12B). The low frequency of im m unolabeled d end rites preclud ed a quantitative analysis of spines d irectly attached to labeled dend rites, thus w e counted the num ber of postsynaptic d ensities (PSDs) contiguous w ith im m unolabeled areas. Although 35 d ecreased in num ber, PSDs w ere found on new ly form ed d end ritic spines in the d enervated zone (Figure 12B, right panel, control: 0.71 0.1 PSDs/ m 2; lesion: 0.38 0.06 PSDs/ m 2, n=4 anim als, 10 regions of interest from 4 non-adjacent sections per d entate gyrus), how ever they w ere not associated w ith norm al presynaptic structures (control: 0.04 0.01 presynaptic term inals/ m 2; lesion: 0 term inals/ m 2; n=4 anim als, 15 regions of interest from 5 non-ad jacent sections per d entate gyrus) or w ith intact synapses (control: 0.03±0.01 synapses/ m 2; lesion: 0 synapses/ m 2; n=4 anim als, 15 regions of interest from 5 non-ad jacent sections per d entate gyrus). Given the sem i-quantitative nature of this experim ent, p -values could not be d eterm ined . D iscussion We used a classic injury m od el – unilateral lesion of the perforant path – to d em onstrate that selective d eafferentation of the ad ult d entate gyrus alters the m aturation and functional integration of new born granule cells. We focused on new born neurons d ivid ing at the tim e of lesion and thus evaluated the initial neurogenic response as w ell as de novo dend ritic outgrow th and synaptogenesis in the absence of excitatory input from the entorhinal cortex. This approach allow ed us to com pare m ature granule cells that w ere d enervated by the lesion, w ith new born granule cells that w ere never innervated by the perforant path. A n initial neurogenic response to perforant path lesion Ad ult-generated new born neurons are highly sensitive to environm ental and m olecular stim uli (Gould and Tanapat, 1999; Vivar et al., 2012) as w ell as brain injury (Liu et al., 1998; Dash et al., 2001; Parent, 2003; Parent and Murphy, 2008). In our experim ents, a 36 chronic d enervating injury triggered proliferation of new born granule cells, consistent w ith previous stud ies (Cam eron et al., 1995; Gould and Tanapat, 1997). Although a substantial portion of new born neurons norm ally und ergo cell death (Kuhn et al., 2005; Sierra et al., 2010), in our experim ents the sam e fraction of new born cells survived at 30 d ays in the lesioned and non-lesioned hem isphere, indicating that the ‘extra’ new neurons had an equal chance of integrating into the circuit. The factors contributing to proliferation of new neurons are likely m ultifactorial and context -d epend ent. Seizures, traum a and ischem ia acutely increase glutam ate release and neural activity, w hereas d enervating injury m ight be expected to d o the opposite. H ow ever, d entate granule cells show a transient increase in activity for 2-4 hours post-lesion as m easured by c-fos im m unoreactivity (H aas et al., 1999; and n=6 anim als, not show n). Although the NMDA receptor antagonist MK-801 can block the post-lesion increase in activity (N itsch and Frotscher, 1992), in our experim ents it did not block the proliferation of new born granule cells observed at 14 d ays post-lesion (1 m g/ kg IP, n=4 anim als, Figure 13). Proliferation d id correspond w ith time of m icroglial activation in the d enervated zone (d ay 2-3, Figure 17; Gehrm an et al., 1991; H ailer et al., 1999; Dissing-Olesen et al., 2007), and the subsequent appearance of reactive gliosis (day 7, H ailer et al., 1999). Reactive astrocytes prod uce grow th inhibitors, such as cytokines, as w ell as trophic factors and grow th substrates cond ucive to regeneration (Sofroniew , 2009; Sofroniew and Vinters, 2010). For exam ple, astrocytes show increased expression of bFGF follow ing injury (Gom ez-Pinilla et al., 1992), thus potentially d riving stem cell proliferation, differentiation, and survival (Gage et al., 1995). Circuit reorganization following denervation 37 Although brain injury can stim ulate neurogenesis, relatively little is know n about the integration of new born neurons into functional circuits post -injury. Perforant path lesions have trad itionally been used to characterize m ature granule cells and the reorganization of their inputs in the context of structural plasticity in the ad ult brain. For exam ple, d end ritic com plexity and spine d ensity of m ature granule cells decrease in the outer m olecular layer follow ing rem oval of the perforant path (Parnavelas et al., 1974; Matthew s et al., 1976a; Caceres and Stew ard , 1983; Diekm ann et al., 1996; Schauw ecker and McN eill, 1996; Vuksic et al., 2011), d espite injury-ind uced sprouting of afferent axons from uninjured brain regions (Deller and Frotscher, 1997), such as the com m issural/ associational axons (Gall and Lynch, 1981; Schauw ecker and McN eill, 1995), septohippocam pal cholinergic fibers (Stanfield and Cow an, 1982), and central norad renergic fibers (Peterson, 1994). Thus, although d entate circuitry can reorgan ize post-lesion (Deller and Frotscher, 1997; Deller et al., 2007), sprouting of rem aining axons is insufficient for com plete m orphological recovery (Diekm ann et al., 1996). Labeling new born neurons that w ere d ividing at the tim e of the lesion allow ed us to exam ine their d end ritic outgrow th and synapse form ation in the absence of a m ajor excitatory input. The d end rites of new born neurons penetrated into the d enervated zone but had sim pler m orphology, ind icating that local presynaptic activity is im portant in the elaboration of d end rites on new born neurons (e.g. Tashiro et al., 2006). The sim pler m orphology of new born d end rites w as com parable to the retraction of distal d end rites in m ature granule cells, suggesting that presynaptic activity also plays a role in m aintainance of preexisting d end rites (Diekm ann et al., 1996). The increase in de novo d end ritic spines in the inner m olecular layer and the increase in the am plitud e and rise tim e of EPSCs in 38 new born neurons ind icated that excitatory axons could form new synapses w ith new born granule cells, how ever synaptogenesis appeared to be localized to the uninjured region. Whether sprouting axons cross lam inar bord ers and form synapses on d enervated m ature granule cells has been controversial (Deller et al., 2001; d el Turco et al., 2003; Phinney et al., 2004). H ow ever, in our experim ents intact excitatory nerve term inals w ere absent from the outer m olecular layer post-lesion, indicating that glutamatergic axons d id not cross lam inar barriers. Whether the laminar barriers reflect resid ual preexisting lam inar cues or are prod uced post-lesion by m icroglia and reactive astrocytes is not yet clear (Bechm ann and N itsch, 2000; Collazos-Castro and N ieto-Samped ro, 2001; Deller et al., 2001; Deller et al., 2006). Is a spine a marker of a functional excitatory synapse post-lesion? The presence of d end ritic spines is usually taken as a proxy for the presence of a functional glutam atergic excitatory synapse. Although d ecreased in num ber, d end ritic spines on new born neurons w ere present in the outer m olecular layer in the absence of excitatory input. Im portantly, unlike m ature neurons, spines on new born neurons form ed >2 w eeks after the lesion. Thus, one m ust conclude that in the setting of d enervation or neural injury, d end ritic spines can form on new born neurons w ithout intact perforant path axon term inals. In ad dition, a substantial num ber of d end ritic spines on mature cells in the outer m olecular layer w ere present three w eeks after d enervation in the absence of any functional or m orphological excitatory synaptic input, w hich m ay represent either m aintenance of existing spines or form ation of new spines. Interestingly, postsynaptic specializations (junctional fold s) at the frog neurom uscular junction are m aintained follow ing d enervation (Birks et al., 1960), possibly because perisynaptic Schw ann cells 39 com e in d irect contact w ith d enervated junctional fold s, release acetylcholine , and trigger sm all postsynaptic potentials (Katz and Miledi, 1959; Miled i and Slater, 1968). Spine d ensities on m ature granule cells in vivo have been reported to recover to near-norm al level by 30-180 d ays post-lesion (Parnavelas et al., 1974; Caceres & Stew ard, 1983; Vuksic et al., 2011), although it is not clear w hether these spines are associated w ith presynaptic nerve term inals or w hether regeneration requires presynaptic input. Sim ilarly, d end ritic spines form on d eafferented cerebellar Purkinje cells of weaver transgenic m ice, d espite the lack of presynaptic parallel fibers (H irano and Dem bitzer, 1973; Sotelo, 1990). In organotypic entorhino-hippocam pal cultures, in vitro d enervation of m ature granule cells d id not affect new spine form ation, but the authors observed changes in spine stability and synaptic activity at 3-4 d ays post-lesion (Vlachos et al., 2012a; 2012b). In our in vivo experim ents, there w as no evid ence of intact presynaptic term inals in the d enervated zone at 21 d ays post-lesion. Thus, m ature granule cells can retain and regenerate spines w ithout perforant path input post-lesion, w hereas new born neurons d o not require perforant path input for spine form ation in the outer m olecular layer. It is interesting to consider w hat signals might substitute for presynaptic term inals in the ind uction of de novo d end ritic spines, or the m ainten ance of m ature spines, follow ing d enervation. Because of the tissue reaction to d enervation or neural injury, the environm ent for d end ritic spine and synapse form ation may d iffer m arked ly from norm al d evelopm ent (Mori et al., 2005). For exam ple, in ad d ition to glutam atergic inputs, other transm itters - includ ing GABAergic or cholinergic inputs - could serve as trophic signals follow ing injury (Laud er, 1993; Ben-Ari, 2002; Overstreet-Wad iche et al., 2005). It is also possible that signals from reactive astrocytes or m icroglia that invad e the d enervated zone 40 could ind uce or m aintain d end ritic spines. For exam ple, glia-d erived signals m ight be neurotransm itters or trophic factors (Sofroniew and Vinters, 2010) as w ell as extracellular m atrix proteins such as throm bospondins (Möller et al., 1996; Christopherson et al., 2005) or m atrix m etalloproteinases (Warren et al., 2012). 41 CHAPTER 1: FIGURES Figure 5 – Complete ablation of perforant path axons in the adult dentate gyrus. (legend on next page) 42 Figure 5 – Complete ablation of perforant path axons in the adult dentate gyrus. (A) A unilateral lesion of the entorhinal cortex (EC) transects the perforant path (red lines). Left panel: H orizontal section of a m ouse brain from Allen Brain Atlas illustrating r ostrocaud al position of the lesion (red line). Orange box outlines the position of the middle panel, w hich show s close-up of the lesioned perforant path (red lines, MPP and LPP) input to the d entate gyrus (DG). (B) Lesioned brains w ere cut in half along the top bord er of the orange box in (A), the cerebellum w as rem oved , and the caud al half w as horizontally sectioned d orsal to ventral (num bered in ord er of sectioning). Arrow head s indicate the location of the lesion, w hich is visible even in the ventral-m ost section (11). (C) Left panel: Exam ple traces of field record ings from an acute brain slice at 14 d ays post-lesion. Whereas in the control d entate gyrus traces show ed a robust fiber volley and an increase in the field EPSP w ith increasing stim ulus intensity (black trace), the d eafferented d entate gyrus show ed no response regard less of stim ulation intensity (red trace). Scale bar = 5m s/ 50 V. M iddle panel: Measurem ents of fiber volley am plitud e across a range of typical stim ulation intensities. Fiber volley am plitud es in the d eafferented d entate gyrus (w hite circles) w ere significantly red uced follow ing lesion, as com pared to the contralateral control (black circles, p < 0.05). The sam e w as observed for field EPSPs (right panel), w hich w ere alm ost entirely absent from the lesioned tissue (w hite circles) com pared to the contralateral control (black circles, p < 0.001). (D ) . Left panel: vGlut1 im m unostaining of glutam atergic nerve term inals typically labels the entire d entate m olecular layer (IML, MML, OML), an d CA1. Right panel: vGlut1 expression w as d rastically d ecreased at 21 d ays post -lesion in the d enervated zone (MML, OML, CA1), but w as unaffected in the intact inner m olecular layer (IML, scale bar = 50 m ). (E) Left panel: vGlut2 expression in the control d entate gyrus w as localized to the supragranular zone of the granule cell layer (GCL), the m id d le/ outer m olecular layers (MML, OML), and S.l-m of CA1. Right panel: At 21 d ays post-lesion, vGlut2 expression w as decreased in the d enervated region (MML, OML, and S.l-m of CA1), but w as unchanged in the supragranular zone (bright band ad jacent to the granule cell layer). Dashed lines separate d entate gyrus from CA1. Scale bar = 50 m . CA1 = cornu am m onis1, CA3 = cornu am m onis3, DG = d entate gyrus, EC = entorhinal cortex, GCL = granule cell layer, IML = inner m olecular layer, LPP = lateral perforant path, MPP = m ed ial perforant path, MML = mid d le m olecular layer, OML = outer m olecular layer, S.lm = stratum lacunosum -m oleculare, S.rad = stratum rad iatum . 43 Figure 6 – Perforant path lesion triggered proliferation and outw ard migration of new born granule cells. (A) Dentate gyrus (10x m agnification) from an ad ult POMC-EGFP transgenic m ouse show ed increased proliferation of new born granule cells at 14 d ays post -lesion of the perforant path (Right panel) com pared to the contralateral, non -lesioned control (Left panel). White squares represent area enlarged in (B). Scale bar = 50 m . (B) 40x m agnification of suprapyram id al blad e of the d eafferented d entate gyrus (right panel) show ed an increase in + the num ber as w ell as in the outw ard migration of POMC-EGFP cells post-lesion. Labeled cells in the contralateral control w ere restricted to the subgranular zone (left panel). Scale bar = 20 m . (C) N ew born granule cell bod y position w ithin the granule cell layer w as analyzed by m easuring the d istance betw een each POMC-EGFP + cell body and the hilar bord er (w hite lines). (D ) Many new born granule cells in the d enervated d entate gyrus m igrated aw ay from the subgranular zone (red bars) com pared to control (black bars), as revealed in the frequency d istribution (p < 0.0001). 44 Figure 7 – Perforant path lesion caused proliferation of new born granule cells in the ipsilateral dentate gyrus, but did not affect the fraction that survived. Com pressed 60 m z-stacks from d entate gyrus of a POMC-EGFP transgenic m ouse w ith new born neurons labeled in green and im m unolabeling for BrdU in red . (A) To exam ine granule cell proliferation, Brd U w as injected at 40 and 44 hours post -lesion and tissue w as evaluated at 48 hours post-lesion. In the control dentate gyrus (left panel), a few BrdU + cells w ere present near the hilar bord er. In the d eafferented d entate gyrus (right panel), m any Brd U + cells w ere present throughout the d eafferented region (MML, O ML, CA1), representing new granule cells as w ell as proliferating glia. To evaluate granule cell + proliferation, Brd U cells w ere counted only in the granule cell layer (GCL). Scale bar = 50 m . (B) To m easure granule cell survival, BrdU w as injected at 40 and 44 hours postlesion, and tissue w as evaluated at 1 m onth post-lesion. As at 48 hours, there w ere m ore Brd U + cells (red) in the granule cell layer on the lesioned sid e as w ell (right panel) com pared to the control d entate gyrus (left panel). Brd U + cells w ere m ore w id ely dispersed w ithin the granule cell layer (arrow s), consistent w ith neuronal m aturity, w hereas Brd U + + glia w ere no longer apparent in the m olecular layer. Scale bar = 50 m . (C) Brd U cells w ithin the granule cell layer w ere increased in th e d eafferented d entate gyrus (red bars) at 48 hours post-lesion (p < 0.001) and at 1 m onth post-lesion (p < 0.01). The percentage of surviving cells d id not d iffer betw een the tw o hem ispheres (34.7% lesioned vs. 39,6% non - 45 lesioned ). ** = p < 0.01, *** = p < 0.001. Yellow cells are the result of z-stack com pression and d o not ind icate co-localization. 46 Figure 8 – Perforant path lesion reduced outgrow th and complexity of new born granule cell dendrites. (A) Upper panel: At 14 d ays post-lesion, dend ritic arbors of 14-d ay-old POMC-EGFP + neurons w ere greatly reduced in total length and com plexity on the lesioned sid e (right) com pared w ith the contralateral control (left). Lower panel: Retrovirally infected 21-day-old granule cells at 21 d ays post-lesion also show ed red uced d end ritic outgrow th in the d eafferented d entate gyrus (right). Scale bar = 50 m (B) Top panel: At both 14 and 21 d ays post-lesion, total d end ritic length w as significantly d ecreased (p < 0.05, p < 0.001). Bottom panel: The num ber of branches in 14- and 21-d ay-old granule cells (POMC-EGFP and Retrovirus, respectively) w as d ecreased on the lesioned sid e (p < 0.001). (C,D ) Sholl analysis of 14-d ay-old POMC-EGFP + granule cell d end rites at 14 d ays post-lesion (n=5 anim als, 7-15 neurons per hem isphere), show ed an apparent shift in d end ritic com plexity tow ard the granule cell/ inner m olecular layer, w here incoming fibers are intact (but these changes d id not reach significance). H ow ever, Sholl analysis of retrovirally infected 21d ay-old granule cell d end rites at 21 d ays post-lesion, show ed a d ecrease in com plexity of d istal d end ritic segm ents in the d eafferented zone († = p < 0.001), relative to granule cells in the contralateral hem isphere, n=5 animals, 5-10 neurons per hem isphere). * = p < 0.05; *** = p < 0.001. Scale bars = 50 m . 47 Figure 9 – Perforant path lesion decreased mEPSC frequency, but the amplitudes w ere increased and rise-times w ere faster in new born granule cells. (A) Left panel: Representative traces of spontaneous activity in 21-d ay-old granule cells at 21 d ays post-lesion (scale bar = 2s/ 20pA). Granule cells in the d eafferented d entate gyrus (bottom trace) had few er m iniature EPSCs than the contralateral control (top trace). Right panel: m EPSC frequency on the lesioned hem isphere w a s significantly low er (p < 0.05). (B) Left panel: Exem plar traces of m EPSCs from lesioned and control hem ispheres (scale bar = 5m s/ 2pA). Miniature events w ere larger in granule cells from the lesioned hem isphere (red trace) than from the control (black trace). M iddle panel: The am plitud e distribution of m EPSCs w as shifted to the right in the lesioned hem isphere (red bars, p < 0.0001). Right panel: Mean m EPSC am plitud e w as significantly increased follow ing lesion (p < 0.05). (C) Left panel: m EPSCs in 21-d ay-old granule cells from the d eafferented (red) show ed faster rise-tim es than control (black, scale bar = 2m s). M iddle panel: Distribution of m EPSCs risetim es. Lesion shifted the d istribution to the left (red bars) relative to the contralateral control (black bars, p < 0.0001). Right panel: m EPSC rise tim e w ere significantly faster follow ing lesion (p < 0.01). * = p < 0.05, ** = p < 0.01. 48 Figure 10 – N ew born granule cells show ed layer-specific spine formation follow ing lesion. (A) N ew born granule cells at 21 d ays post-lesion. N ew ly form ed d end ritic spines on 21d ay-old granule cells w ere present both in the outer m olecular layer (OML, top row) and in the inner m olecular layer (IML, bottom row). H ow ever, few er d end ritic spines form ed in the d enervated region (top row, middle panel) as com pared to the contralateral control (top row, left panel). In contrast, new born granule cells in the d eafferented d entate gyrus d eveloped m ore d end ritic spines in the inner m olecular layer (IML, bottom row, middle panel) com pared to the contralateral sid e (bottom row, left panel). Spine density in the d eafferented OML w as significantly low er than control (top row, right panel, p < 0.05), but significantly higher than control in the IML (bottom row, right panel, p < 0.01). (B) Mature granule cells at 21 d ays post-lesion. Distal dend ritic spines on m ature granule cells w ere d enervated by the lesion and w ere d ecreased in num ber (top row, middle panel) relative to the nonlesioned contralateral control (top row, left panel). H ow ever, unlike new born granule cells, the num ber of d end ritic spines on m ature granule cells in the IML w as not affected by the lesion (bottom row). Mature granule cells had low er d end ritic spine d ensity in the d eafferented region (top row, right panel, p < 0.01) than those in the control hem isphere, but no change w as observed in the inner m olecular layer (bottom row, right panel, N .S.). OML = outer m olecular layer, IML = inner m olecular layer, * = p < 0.05, ** = p < 0.01. Scale bar = 5 m . 49 Figure 11 – Perforant path lesion decreased the number of presynaptic terminals and intact synapses in the denervated zone. (legend on next page) 50 Figure 11 – Perforant path lesion decreased the number of presynaptic terminals and intact synapses in the denervated zone. (A) Top row: Electron micrographs of the non -d enervated inner m olecular layer (IML) at 21 d ays post-lesion, show ed norm al tissue structure in the control (left) and lesioned (right) d entate gyrus. Both d entate gyri had synaptic vesicles/ axon terminals (*) at intact synapses (arrow ) w ith postsynaptic d ensities (#). Bottom row: Electron micrographs of the d enervated outer m olecular layer (OML, right panel) as com pared w ith the outer m olecular layer on the contralateral control hem isphere (left panel). In contrast to the control, the d enervated region w as devoid of presynaptic term inals (*) and instead show ed electron d ense d egenerating axons (d t) apposed to postsynaptic d ensities (#). Insets show higher m agnification exam ples. (B) The synaptic com position in the inner and outer m olecular layers w as quantified by ind epend ently counting the num ber of postsynaptic d ensities (PSDs, [#]), the num ber of axon term inals (as ind icated by presence of vesicles, [*]), and the num ber of intact synapses (arrow s). At 21 d ays post-lesion, the num ber of postsynaptic d ensities w as unchanged in either layer, how ever presynaptic term inals and intact synapses w ere substantially d ecreased in the d enervated OML (p < 0.001), but unchanged in the non-d enervated IML (N .S.). (C) Low -m agnification micrograph (6800x) exem plifying reactive astrogliosis in the d enervated outer m olecular layer. Reactive astrocytes could occupy as m uch as 50% of the most severely affected regions (shad ed green). d = d end rite, s = d end ritic spine, dt = d egenerating axon term inal, a = astrocyte. Scale bars = 500nm . *** = p < 0.001. 51 Figure 12 – Perforant path lesion did not preclude formation of post-synaptic specializations on new born granule cell dendrites in the deafferented zone. (A) Flat em bed s of im m unostained EM tissue at 21 d ays post-lesion, 40 m sections. N ew born neurons w ere labeled w ith retroviral injection on the d ay of the lesion, thus labeling granule cells that d eveloped in the absence of perforant path input. Labeled new born neurons w ere present in the control (left panel) and lesioned (right panel) d entate gyrus. Black squares outline d ensely im m unolabeled regions of the suprapyram id al blad es that w ere chosen for thin-sectioning and subsequent analysis. White squares indicate approxim ate regions pictured in B. (B) Left panel: Im m uno-electron micrograph of the outer m olecular layer in the control d entate gyrus, 75nm section. Labeled d end rite (d ) of a 21-d ay-old control new born granule cell had d end ritic spines (s), a d eveloping postsynaptic d ensity (#), and initial features of a synapse form ing w ith an axon term inal (*). Left panel inset: close-up of another new ly form ed synapse (arrow ) betw een an axon term inal (*) and a labeled 21-d ay-old new born granule cell w ith a postsynaptic d ensity (#). Right panel: Im m uno-electron m icrograph of the denervated outer m olecular layer in the lesioned hemisphere, 75nm section. A prom inently labeled 21-d ay-old new granule cell had a postsynaptic d ensity (#), w hich w as apposed only by a d egenerated axon term inal (d t). Right panel inset: another exam ple of a labeled d end ritic spine w ith a post -synaptic d ensity (#) apposed by a d egenerating term inal (dt). Post -synaptic d ensities in the d enervated zone often w ere curvilinear, rather than the straight PSD characte ristic of the control. Scale bar in B = 500nm . 52 CHAPTER 1 – AD D EN D UM M ECHAN ISMS OF LESION -IN D UCED PROLIFERATION AN D D ISPERSION OF N EWBORN GRAN ULE CELLS IN THE D ENTATE GYRUS Possible mechanisms of injury-induced proliferation and dispersion of new born granule cells in the dentate gyrus Lesion-induced proliferation of newborn granule cells. In the ad ult d entate gyrus neurogenesis occurs throughout life, suggesting that the ad ult brain has at least a lim ited capacity to regenerate follow ing injury. Proliferation of new born neurons can be upregulated follow ing injury and their functional integration and survival can im pact the d egree of recovery. Generally, neurogenesis is stim ulated by increases in netw ork activity, such as w ould occur d uring seizure (Overstreet-Wadiche et al., 2006; Parent, 2007). H ow ever, proliferation of new born neurons is surprisingly also stim ulated by lesion of the perforant path (Figures 6 and 7), w hich effectively d ecreases overall netw ork activity (Figure 5). Consistent w ith such a d ecrease in excitatory activity, w e saw d eficits in d end ritic arborization of new born neurons follow ing lesion. H ow ever, w e hypothesized that the d ecline in glutamatergic innervation m ay be preced ed by a burst of activity triggered by axon transection, w hich may trigger proliferation of new born neurons. To ad d ress this possibility w e used a m arker of netw ork activity, the im m ediate early gene c-fos. We measured c-fos im m unoreactivity in the d entate gyrus at 2 and 6 hours after a unilateral perforant path lesion. As show n in Figure 13A, lesion of the perforant path triggered a d ram atic increase in c-fos expression in the d eafferented d entate gyrus at 2 hours post-lesion (right panel, red stain). This increase in activity w as lim ited to m ature granule cells, w hich make synaptic contacts w ith the perforant path. N ew born granule cells, labeled here w ith POMC-EGFP (green), d o not yet receive perforant path 53 input and therefore d o not show an increase in c-fos expression follow ing lesion. The increase in c-fos exp ression appears to be transient, as it w as back to baseline in the lesioned hemisphere at 6 hours post-lesion (d ata not show n). These results are consistent w ith a burst of activity triggered by m ass glutam ate release from cut perforant path axons and the subsequent absence of excitatory activity as these axons d egenerate. Because excitatory transm ission at these synapses is partially m ed iated by N MDA receptors, w e w ere able to block the increase in c-fos imm unoreactivity by pretreating the animal w ith the N MDA-receptor antagonist MK-801 20 m inutes prior to the lesion proced ure (injected intraperitoneally; Figure 13B, right panel). Because MK-801 elim inated the lesion-ind uced burst of activity, w e reasoned that it may be able to prevent proliferation of new b orn granule cells that w as observed at 14 d ays post-lesion. H ow ever, d espite pretreatm ent w ith MK-801, w e saw no d ifferences in proliferation or d ispersion of new born granule cells at 14 d ays post-lesion (Figure 13C, right panel; com pare w ith Figure 6B). Thus, although aberrant excitatory activity m ay contribute to new born granule cell proliferation, other injury-ind uced signaling cues, such as BDN F or inflam m atory cytokines, are likely to be the primary trigger (Suh et al., 2009). Lesion-induced dispersion of newborn granule cells. In ad d ition to increased granule cell proliferation follow ing unilateral lesion of the perforant path, w e observed increased d ispersion of new born neurons w ithin the granule cell layer, as m easured by the increase in the d ista nce betw een cell bod ies and the hilus bord er (Figure 6). Aberrant neuronal migration is a com plex phenom enon observed in several d evelopm ental pathologies, such as lissencephaly, epilepsy, and autism (Fatemi, 2005). Thus, w e hypothesized that som e of the u nd erlying causes of these pathologies, 54 includ ing m olecular cues such as reelin expression (Tissir and Goffinet, 2003), m ay also be altered in this lesion m od el. To test this hypothesis, w e im m unostained for reelin at d ifferent tim e points follow ing perforant path lesion (Figure 14). At all time points tested , the unlesioned contralateral control show ed slightly d enser staining surround ing the hippocam pal fissure (Figure 14A-D, left panels), the typical location of the GABAergic interneurons that secrete reelin in the ad ult hippocam pus (Pesold et al., 1998). H ow ever, reelin expression follow ing lesion of the perforant path increased in the outer m olecular layer as early as 1 d ay post-lesion (Figure 14A) and rem ained elevated for at least 14 d ays (Figure 14E). The staining pattern in the d eafferented d entate gyrus w as also m ore d iffuse than in the contralateral control, w ith signal d etected in the d eafferented zone (solid yellow lines). Although the sm all number of anim als preclud ed a quantitative analysis of this d ataset, som e speculation regard ing the im plications of these find ings is in ord er. The increased staining in this region m ay d epict lesion -ind uced ectopic m igration of reelin secreting interneurons into the d eafferented zone or m ay sim ply represent the form ation of a reelin grad ient spanning the m olecular layer (Figure 14E, inverted w hite triangle). These results are in conflict w ith an earlier stud y that found no changes in reelin m RNA expression follow ing lesion of the perforant path (H aas et al., 2000), and are perhaps contrad ictory to the d ogm a that a reduction in reelin expression lead s to aberrant neuronal m igration (Katsuyam a and Terashim a, 2009; H aas and Frotscher, 2010). H ow ever, new roles for reelin signaling in the m ature brain are em erging , suggesting that it m ay have both positive and negative effects on neuronal migration and lam inar organization (Fatem i, 2005). In ad d ition, the increase in reelin signaling w e observed follow ing lesion is 55 fairly minor and thus m ay have been m issed in the earlier stud y, as no form al quantitation of reelin m RN A expression w as d one. Sim ilarly, the d ifferences may be attributable to the m ethod of detection, i.e. the lesion m ay not have an effect on reelin m RN A expression, but m ay trigger increased translation of the protein. Although our results contrad ict earlier stud ies, the observed increase is theoretically possible, as lamina -specific increases in other m atrix proteins, includ ing tenascin -C and neurocan have been observed follow ing perforant path lesion (Deller et al., 1997; H aas et al., 1999). Although further experim ents are necessary to verify the specificity of our antibod y and the id entity of the labeled cells in the d eafferented zone, our results suggest a positive role for reelin in lesion -ind uced m igration of neuronal precursors. For exam ple, H ack et al. (2002) proposed that reelin is a d etachm ent signal for m igrating neuronal precursors in olfactory neurogenesis in the ad ult brain. In this slice culture experim ent, ad d ition of reelin triggered d etachm ent of tangentially migrating neuronal precursors from their chains, thus stim ulating their subsequent ind ivid ual rad ial m igration. H ow ever, if reelin w as m utated , precursor cells rem ained in clusters and failed to rad ially migrate, suggesting that reelin is required for d etachm ent from the m igration chains. Although in the d entate gyrus rad ial glia aid granule cell migration, upregulation of reelin m ay stim ulate d etachment of new neurons from their neurogenic niche in the subgranular zone, thus increasing their outw ard m igration into the granule cell layer. Interestingly, seizure stud ies, in w hich a red uction in reelin signaling is associated w ith granule cell d ispersion (H einrich et al., 2006), suggest that reelin is also im portant in the m aintenance of a com pact granule cell layer in the ad ult d entate gyrus. As such, aberrant granule cell m igration follow ing perforant path lesion m ay also be controlled by increased reelin 56 signaling, thus preclud ing ectopic localization of these cells. To ad d ress these questions, future experim ents w ill need to cond itionally m anipulate reelin activity follow ing lesion using either a tam oxifen -ind ucible reelin m utation or in situ viral knockd ow ns of this protein. Unfortunately, it is unlikely that reeler m ice w ill be useful in such experim ents because of their severe deficit in hippocam pal cellular organization (Stanfield and Cow an, 1979). Such aberrant m orphology w ould likely d isrupt the lam ination pattern and cell-cell interactions im portant for analysis of post-lesion circuit reorganization. During d evelopm ent, new ly generated granule cells m igrate along rad ial glia to take their final positions w ithin the granule cell layer (Rickm ann et al., 1987). In the ad ult d entate gyrus, rad ial glia continue to provid e a m igrator y scaffold for new ly generated neurons (Förster et al., 2002; Weiss et al., 2003) and serve as a pool of granule cell precursors (Seri et al., 2001). Thus, if the radial glial population is affected follow ing perforant path lesion, it m ay help to explain the increased proliferation and outw ard m igration of new born neurons observed follow ing perforant path lesion. In our prelim inary experim ents, w e saw an increase in the expression of the rad ial glial m arker nestin at 14 d ays post-lesion (Figure 15), w ith m any stained radial processes aligned w ithin the granule cell layer (Figure 15A, arrow head s). Cell bod ies of new born neurons seem ed to be in close proxim ity w ith the nestin -positive rad ial fibers (Figure 15B), how ever, further investigation is necessary to confirm these find ings. Interestingly, the rad ial glial scaffold fails to form in the absence of reelin (Weiss et al., 2003), but is rescued if reeler brain slices are treated w ith recombinant reelin (Zhao et al., 2004). A particularly interesting find ing from the latter stud y is that reelin m ust be in a specific topographical location relative to the glial scaffold in ord er to help establish its polarity. In the ad ult 57 hippocam pus, reelin is synthesized in the outer m olecular layer, w ith a grad ient reaching across the m olecular layer to interact w ith the radial glial population near the granule cell layer. Thus, perforant path lesion may trigger a com plex sequence of increased reelin expression in the d eafferented zone, subsequent enhancem ent of rad ial glial scaffolding, and aberrant granule cell m igration, that in turn m ay be kept w ithin the granule cell layer by increased reelin signaling. Aberrant granule cell m igration follow ing lesion has several im plications for post injury circuit reorganization. For exam ple, the strict lam ination pattern in the d entate gyrus provid es separation betw een d end rites and axons of the m ature granule cells, thus segregating incom ing and outgoing inform ation. The im portance of this segregation is evid ent follow ing seizure, w h en lam ination of granule cell bodies is perturbed . In this context, m ossy fiber collaterals can aberrantly innervate granule cell d end rites, form ing excitation loops that m ay trigger recurrent seizures (Wenzel et al., 2001). H ow ever, unlike in the seizure m od el, perforant path lesion d oes not trigger d ispersion of new born granule cells outside of the granule cell layer and new born granule cells seem to properly establish their d end ritic and axonal connections. Thus, proliferation of new born neurons in this context m ay be an ad aptive process, provid ing a highly plastic postsynaptic target for the sprouting axons in the inner m olecular layer. 58 CHAPTER 1 – AD D EN D UM : FIGURES Figure 13 – Perforant path lesion stimulates a burst of activity, but cause for proliferation is more complex. Single-layer snapshots. (A) Transection of perforant path axons triggers w id espread expression of the im m ediate early gene c-fos (right panel, red) at 2 hours follow ing injury, ind icating an increase in netw ork activity. Upregula tion of c-fos expression is no longer d etected at 6 hours post-lesion (not show n), suggesting that the activity burst is transient and associated w ith axon transection. N ote the absence of c-fos im m unoreactivity from 14 d ay-old neurons (POMC-EGFP) that d o not yet receive perforant path input. (B) The postsynaptic response to the transient burst of activity can be d am pened by injecting the anim al w ith MK-801, a noncom petitive N MDA antagonist, 20 m inutes prior to the lesion. 59 H ow ever, transiently blocking netw ork activity does not prevent lesion -ind uced proliferation of new born neurons (POMC-EGFP, right panel in (C)). 60 Figure 14 – Perforant path lesion may lead to an upregulation in reelin signaling in the deafferented zone. (legend on next page) 61 Figure 14 – Perforant path lesion may lead to an upregulation in reelin signaling in the deafferented zone. During d evelopm ent, reelin is secreted from Cajal-Retzius cells residing in the outerm ost m olecular layer in the d entate gyrus. H ow ever, in the ad ult brain r eelin is secreted from GABAergic interneurons, but these cells resid e in approxim ately the sam e region. (A) Snapshots of reelin im munostain in the lesioned (right panel) and contralateral control (left panel) d entate gyrus at 1 d ay post-lesion. In both hem ispheres at this tim e point, the d ensest staining surround s the source cells in the outer m olecular layer. (B) At 2 d ays post-lesion, reelin expression is increased in the outer m olecular layer (right panel) relative to the contralateral control (left panel) and labeled astrocyte-like cells can be seen throughout the m olecular layer. The actual id entity of these cells has not been d eterm ined and it is possible that labeling of these cells may be a consequence of non -specific staining or autofluorescence. (C,D ) The increase in reelin expression in the outer m olecular layer is sustained through 4- (C) and 7- (D) d ays post-lesion relative to the contralateral hem isphere, how ever a lam ina-specific increase in labeling should be noted in the d eafferented zone (solid yellow line, right panels). (E) H igher m agnification im age of the d orsal blad e but at 14 days post-lesion. Increased lam ina-specific reelin labeling can be observed throughout the d enervated region (solid yellow line, right panel), but m ay form a d ecreasing grad ient spanning the m olecular layer (inverted w hite triangle, right panel) betw een reelin-secreting cells and the granule cell layer. N ote the relatively stable population of new born neurons (POMC-EGFP, green cells) throughout first w eek postlesion. 62 Figure 15 – Lesion triggers an increase in nestin-expressing radial glia. (A) 14 d ays post-lesion: 50 m z-stack projection of the d orsal dentate gyrus stained for nestin, a marker for rad ial glial cells. N ote the increase in radial fibers (arrow head s) throughout the granule cell layer (GCL) in the lesioned hem isphere (right panel) as w ell as an increase in reactive glia in the m olecular layer (ML). (B) Green channel overlay of the sam e sections show n in A. Labeled cells are 14-day-old granule cells from POMC-EGFP transgenic mice. N ote the typical increase in proliferation of new born neurons observed at 14 d ays post-lesion (right panel). The granule cell layer (GCL) is d elineated by the dashed w hite line, beyond w hich is the m olecular layer (ML). 63 CHAPTER 2 ROLE OF EXTRACELLULAR EN VIRON MENT IN POST-LESION CIRCUIT REORGAN IZATION Laminar borders and axonal sprouting follow ing lesion. Although lesion of the perforant path triggers a variety of injury -ind uced responses, all circuit components – includ ing reactive gliosis, axonal sprouting, and d end ritic restructuring – und ergo reorganization w ithin their respective lam inae. In the case of reactive gliosis, lam inar segregation can be considered ad aptive, as glia play an instrum ental role in clearing axonal d ebris and maintaining the ion balance of the surround ing environm ent. H ow ever, in the case of neural circuit reorganization, lam inar bord ers m ay prevent translaminar sprouting of intact axons, thus possibly d im inishing the d egree of post-lesion functional recovery. Interestingly, lam inar specificity follow ing lesion is rem iniscent of the m olecular profile d uring hippocam pal developm ent, suggesting that som e d evelopm ental processes of lam inar assembly and maintenance can be, at least partially, recap itulated follow ing injury. Insights from the d eveloping hippocam pus m ay provid e clues as to the m echanism s of post -lesion circuit reorganization and m ay thus aid in local experim ental manipulation of the d eafferented region. Lamination in the developing dentate gyrus Lam inar bord ers in the dentate gyrus are d efined early in d evelopm ent, w ith excitatory afferents segregating into their respective layers as early as embryogenesis (Frotscher et al., 2007). For exam ple, perforant path axons from the entorhinal cortex reach 64 the d entate gyrus at em bryonic d ay 19 (E19) and occupy pred om inantly the outer m olecular layer. On the other hand , com m issural/ associational axons first appear at post natal day 2 (P2) and specifically populate the inner m olecular layer (Supe r and Soriano, 1994; Förster et al., 2006). Interestingly, these afferents show the sam e laminar preference even if the ord er of innervation is experim entally reversed (Frotscher and H eim rich, 1993), thus im plicating lam ina-specific cues in axonal guid ance d uring d evelopm ent. Such cues m ay arise in part from cell-cell interactions, w ith pioneer or established neurons provid ing a scaffold for innervation. For exam ple, early in developm ent, Cajal-Retzius cells populate the d eveloping outer m olecular layer and provid e a tem porary postsynaptic target for the incoming perforant path axons, prior to the availability of granule cell d end rites (Rihn and Claiborne, 1990; Ceranik et al., 2000). Thus, these “placehold er” cells attract perforant path fibers, resulting in lam inar specificity (Supèr et al., 1998). Once perforant path axons are in place, m ost Cajal-Retzius cells d egenerate, allow ing synaptogenesis w ith the now sufficiently m atured granule cell d end rites (Soriano and Del Rio, 2005). If the function of Cajal-Retzius cells is perturbed , perforant path fibers lack lam inar specificity and term inate throughout the m olecular layer (Del Rio et al., 1997). H ow ever, m utation of Cajal-Retzius cells d oes not affect the com m issural/ associational axons. These axons arrive around P2, after granule cell d end rites have m atured , suggesting that axon guid ance cues can also be provided by the postsynaptic targets them selves. Ind eed , com m issural/ associational axons lose their laminar preference if migration of their targets, the new ly generated granule cells, is perturbed (Gebhard t et al., 2002; Zhao et al., 2003). Furtherm ore, this effect is reversed if proper granule cell migration is experim entally restored (Förster et al., 2006). 65 Pre- and postsynaptic components of lesion-induced circuit reorganization Many com ponents of the d eveloping d entate gyrus are retained in the ad ult brain, suggesting the theoretical possibility of lamina -specific reinnervation follow ing lesion. Specifically, d espite the absence of placehold er cells in the ad ult d entate gyrus, d end rites of m ature granule cells as w ell as the nonlesioned fiber tracts m ay be able to synthesize guid ance cues that d irect sprouting axons tow ard the vacated d end ritic spines. In our prelim inary assessm ent of the interplay betw een pre- and postsynaptic com ponents follow ing lesion, w e assessed the tim e course of axonal d egeneration in parallel w ith the post-lesion response of dend ritic spines. We used the vesicular glutamate transporter vGlut1 as a marker of excitatory axons and a postsynaptic d ensity (PSD95) m arker to label d end ritic spines. Figure 16A show s the expression of these tw o proteins at baseline as w ell as in the contralateral d entate gyrus at all post-lesion time points tested . Diffuse vGlut1 staining (left panel, red) w as visible throughout the d entate m olecular layer, w ith a slight increase in intensity closer to the granule cell layer. Staining for PSD95 (right panel, blue) show ed a similar expression pattern, but ind icated a higher spine d ensity in the inner m olecular layer. vGlut1 expression began to d ecrease in the d enervated region at 2 d ays post-lesion (Figure 16C, left panel) and w as nearly gone by 7 d ays post-lesion (Figure 16E, left panel). In contrast, PSD95 staining w as not affected by d eafferentation (Fig ure 16B-E). This d iscrepancy betw een pre- and postsynaptic m arker expression w as sustained for at least 21 d ays post-lesion (Figure 16F), ind icating that no excitatory reinnervation occurs in the d eafferented zone. Thus, w e conclud e that postsynaptic targets alone are not sufficient to stim ulate regenerative hom otypic sprouting follow ing lesion. Extracellular matrix in development and follow ing lesion 66 Role of the extracellular matrix in generation and maintenance of laminar borders. Axon guid ance and fasciculation d uring d evelopm ent can also be regulated by grad ients of attractive or repulsive extracellular matrix m olecules, includ ing cell-ad hesion m olecules such as L1 and N CAM, soluble factors such as the sem aphorins and netrins, ephrin receptors and their ligand s, as w ell as ECM proteins, such as the chond roitin sulfate proteoglycans (CSPGs, Margolis and Margolis, 1997; Skutella and N itsch, 2001). Extracellular m atrix proteins, particularly CSPGs, are expressed throughout life, although their com position and spatiotem poral expression varies w ith age. These proteins assist in pattern cell m igration and axonal outgrow th, regulate availability of grow th factors and guid ance m olecules, and are involved in brain repair follow ing injury (Carulli et al., 2005; Kw ok et al., 2008). CSPGs are expressed d uring developm ent and have been suggested to regulate lam inar specificity of incoming perforant path axons (Pearlm an and Sheppard , 1996; Raugh, 1997; Wilson and Snow , 2000; Kw ok et al., 2012). For exam ple, layer specificity of entorhinal afferents, but not of comm issural/ associational afferents, is lost if the extracellular m atrix m olecule hyaluronan is perturbed (Förster et al., 2001; Zhao et al., 2003), suggesting that the extracellular m atrix is required for proper lam ination. In ad d ition to their role in d evelopm ent, extracellular m atrix proteins play a m ajor role in brain plasticity follow ing injury. Proteoglycans, for exam ple, are secreted by reactive astrocytes and can contribute to form ation an im penetrable b arrier to axonal grow th (Matsui and Oohira, 2004). Interestingly, lam ina-specific reactive astrogliosis is a prom inent feature of the hippocam pal response to lesion of the perforant path, suggesting that secretion of extracellular m atrix proteins may be in creased in the d eafferented region. Therefore, reactive gliosis, alongsid e the subsequent increase in proteoglycan expression, 67 m ay prevent translam inar sprouting by reintrod ucing som e of the d evelopm ental signals for lam inar segregation. Figure 17 show s th e tim e course of m icroglia (w hite) and astrocyte (red) activation follow ing lesion. The molecular layer is outlined w ith d ashed lines and the d eafferented zone is ind icated w ith a solid yellow line. At baseline, astrocytes had few branches and m icroglia w ere hard ly d etectable (Figure 17A). This section is representative of the contralateral control at all tim e points tested . At 1 d ay postlesion, gliosis w as m inimal, as circuit reorganization is just beginning. At 2 d ays post lesion, m icroglia in the deafferented zone began to increase in num ber and to acquire reactive m orphology (Figure 17C, arrow head s), w hereas astroglia rem ained at baseline. Within the next several days post-lesion, the m icroglial reaction peaked , w ith w hite clusters visible throughout th e d enervated region (Figure 17D-F). Reactive astrogliosis w as d elayed relative to the microglial response, w ith the first signs of astrocyte hypertrophy evid ent at 4 d ays post-lesion (Figure 17D) and the peak of reactivity around 7 d ays (Figure 17E). Although microglia returned to baseline shortly thereafter, astroglial hypertrophy w as prolonged and w as still evid ent at 1 m onth post-lesion (Figure 17F). H ow ever, note that reactive astrocytes rem ained w ithin their d om ains and did not form an im penetrable glial scar, suggesting that astrogliosis in this context is an ad aptive response to injury (Sofroniew , 2009). Although the sm all num ber of anim als used for each tim e point preclud ed statistical analysis, these d ata reflect previously published w ork on the sub ject (H ailer et al., 1999). The lam inar specificity of the glial response to perforant path lesion reflects the location of d egenerating axons, but also the stringency of laminar barriers in the ad ult brain. Reactive gliosis can contain the d eafferented area, thus preserving incom ing axons 68 in the inner m olecular layer. H ow ever, reactive gliosis m ay also play a role in preventing translaminar sprouting during post-lesion circuit reorganization. For example, reactive astrocytes secrete extracellular m atrix proteins (Fitch and Silver, 2008). As extracellular m atrix proteins, such as proteoglycans, guid e the lam ination process of afferent fibers d uring d evelopm ent, their lesion -ind uced increase in the ad ult brain can reintrod uce lam ina-specific guid ance cues for sprouting axons of uninjured afferents. Sim ilarly, changes in the extracellular m atrix com position m ay im pact d end ritic outgrow th of new born granule cells that m ature in the w eeks follow ing lesion. We began to test these hypotheses by looking at the tim e course of CSPG expression after unilateral transection of the perforant path. Und er norm al cond itions and in the contralateral control, CSPG expression w as largely diffuse, w ith slightly d enser staining in som e regions (Figure 18A). H ow ever, the density of CSPG staining w as noticeably increased by 4 d ays post-lesion (Figure 18D) and this effect w as sustained for at least tw o w eeks thereafter (Figure 18F). As expected , the increase in CSPG expression correspond ed w ith the lesion -ind uced activation of astrocytes and , like astrogliosis, w as lim ited to the d eafferented zone (Figure 18F, solid yellow line; note the sustained baseline expression of CSPG in the inner m olecular layer). Although our d ata w ere not quantified because of the sm all num ber of anim als used for these experim ents, these results reflect the know n responses of the extracellular m atrix to lesion of the perforant path. For exam ple, the proteoglycans brevican and neurocan, as w ell as tenascin -C, are upregulated in the d enervated zone w ith a tim e-course consistent w ith their potential role in neurite outgrow th (Deller et al., 1997; H aas et al., 1999; Thon et al., 2000). Because these m olecules are active d uring form ation of the d entate gyrus, their re-expression follow ing injury suggest that post-lesion circuit reorganization m ay be a recapitulation of d evelopm ent. 69 M anipulation of the post-lesion extracellular environment Increased expression of chond roitin sulfate proteoglycans (CSPG) is w elld ocum ented in the context of spinal cord injury, w her e it is a m ajor com ponent of the im penetrable glial scar (Bartus et al., 2012). Various attem pts have been mad e to aid regeneration past the glial scar by using enzym es that d egrad e the proteoglycans. For exam ple, injection of chond roitinase ABC (chABC) near the site of the lesion red uces CSPG inhibition follow ing spinal cord injury, thereby facilitating axonal regeneration and aid ing com pensatory plasticity of uninjured axons (Bradbury and Carter, 2011). In our prelim inary experim ents, w e saw an increase in CSPG expression in the denervated zone follow ing perforant path lesion, suggesting that the outer m olecular layer m ay have an inhibitory effect on translam inar axonal sprouting as w ell as on d end ritic outgrow th of new born granule cells. Thus, blocking th e CSPG response m ay reverse these d eficits in the reorganizing circuit. To test this hypothesis, w e stereotaxically injected chABC into the d orsal d entate gyrus in the lesioned hemisphere, w ith the contralateral d entate gyrus used as an unm anipulated control (no lesion, no chABC). Figure 19 (A-C) show s im ages of injection protocol optim ization. As show n in Figure 19A, the d orsal hippocam pus w as unilaterally injected w ith 0.2 l of chABC (yellow need le, right sid e). Im munostaining for the d igestion fragm ent C4S (red ) show s the approxim ate spread of enzym atic activity (d ashed yellow line). Although som e C4S expression w as d etected in the uninjected contralateral d entate gyrus, it w as lim ited to the m ed ial corner of the granule cell layer and thus d id not im pact subsequent analysis of the d orsal blad e. In future experim ents, sm aller volum es of the enzym e should be used to prevent contamination of neighboring areas. Figure 19(B,C) show s CSPG expression after treatm ent w ith chABC. Correctly 70 tim ing the chABC injection relative to the time of lesion w as im portant in elim inating lesion-ind uced CSPG activity. If chABC w as injected on the d ay of the lesion and tissue w as exam ined at 14 d ays post-lesion, elevated CSPG expression w as still detected in the d eafferented d entate gyrus (Figure 19B, right panel). H ow ever, if the chABC injection coincid ed w ith the peak of CSPG expression (at 7 d ays post-lesion, see Figure 18E), lesion ind uced activation of CSPG w as blocked (Figure 19C, right panel). Thus, to evaluate post lesion circuit reorganization and neurogenesis, chABC w as injected at 7 days post -lesion. We also used an alternative approach to block CSPG expression follow ing lesion – intraperitoneal (IP) injections of Xylosid e, injected tw ice d aily from 3 to 7 days post -lesion. Whereas chABC d egrad es alread y expressed CSPGs, Xylosid e prevents CSPG synthesis. Treatm ent w ith Xylosid e w as d elayed relative to the lesion because CSPGs w ere show n to have a beneficial effect w ithin the first tw o d ays after an injury (Rolls et al., 2008). Unfortunately, Xylosid e injections failed to block the increase in CSPG expression in the d entate gyrus w hen tissue w as exam ined at 14 d ays post-lesion (Figure 19D, n=4); therefore, no further analysis w as d one. In the original stud y (Rolls et al., 2008), xylosid e w as used follow ing spinal cord injury, w hich, unlike the perforant path lesion, is consid ered a m alad aptive response to injury. Therefore, the param eters of effective xylosid e injections m ay d iffer betw een the tw o mod els. Future experim ents w ill need to ad d ress the tim ing of xylosid e d elivery as w ell as the concentrations necessary to achieve optim al block of CSPG synthesis in the d entate gyrus follow ing lesion. Dendritic outgrowth and spine density following digestion of CSPG. Because of their role in inhibiting axonal regrow th follow ing spinal cord injury, CSPGs have been stud ied pred om inantly w ith regard to axons, but evid ence for the role of 71 CSPGs in d end ritic outgrow th is scarce. H ow ever, a recent stud y looking at em bryonic cortical neu rons in vitro found that CSPGs counteract the increase in d endritic spines follow ing treatm ent w ith BDN F, resulting in an overall d ecrease in spine d ensity relative to the untreated control (Kurihara and Yam ashita, 2012). Sim ilarly, See et al. (2010) saw a significant d ecrease in neurite outgrow th of neural progenitors cultured in inhibitory concentrations of CSPG, although survival and d ifferentiation of these cells w as not affected . In our experim ents, d end rites of new born granule cells w ere shorter in th e d eafferented d entate gyrus and had d ecreased com plexity in the d enervated region (Figure 8). Interestingly, this region also contains increased expression of CSPGs, suggesting that changes in the com position of the extracellular m atrix m ay im pact the d evelopm ent of d end rites and d end ritic spines. We tested this possibility in vivo by analyzing d end ritic arbors and spine d ensities of new born and m ature granule cells after a com bination of unilateral lesion + chABC. chABC w as injected at 7 d ays post -lesion (Figure 19A,C) and tissue w as exam ined either 7 or 14 d ays later (i.e. at 14 and 21 days post-lesion). As in our initial arborization and spine d ensity experim ents (Figures 8 and 10), the post-lesion tim e interval allow ed exam ination of granule cells that w ere d ividing at the tim e of the lesion. Dend ritic spines w ere counted in the inner and outer m olecular layers for m ature- and 21-d ay-old granule cells at 21 d ays post-lesion. All sections used for analysis w ere stained for the CSPG d igestion prod uct C4S (Figure 19A) to ensure specific d elivery of the enzym e only to the d eafferented dentate gyrus. If the d igestion prod uct w as d etected in the contralateral hem isphere, control d end rite m easurem ents w ere collected only from granule cells outsid e the C4S-stained zone. chABC treatm ent alone had no effect on d end ritic arborization relative to the contralateral control. It is im portant to note that d end ritic arbors of these cells w ere subject to the sam e post -lesion changes 72 d escribed in Chapter 1. Thus, chABC treatm ent w as em ployed to test w hether CSPG upregulation follow ing lesion und erlies som e of these changes. Out of all granule cells exam ined , only the 14-d ay-old neurons in the d eafferented d entate gyrus exhibited any im provem ents in their d end ritic arbors fo llow ing chABC treatm ent (d ata not show n). Although new born neurons still had a lesion -ind uced d ecrease in the num ber of branches (n=6, p < 0.001, t-test), they no longer show ed a d eficit in their total d end ritic length relative to the contralateral control (n=6, N . S., t-test). These results suggest that red ucing post-lesion CSPG expression in the d eafferented zone can create a m ore favorable environm ent for d end ritic outgrow th, though excitatory innervation by the perforant path is still required for branching. H ow ever, chABC treatm ent did not am eliorate the lesion effects on d end ritic spine d ensity in 21-d ay-old and m ature granule cells. Sim ilar to our earlier d ataset (Figure 10), w e observed a d ecrease in spine d ensities on d istal d end ritic segm ents of m a ture and 21-d ay-old granule cells, as w ell as an increase in spine d ensity on proxim al dend ritic segm ents in 21-day-old granule cells only, w ith these segm ents correspond ing to the d enervated and intact/ sprouting m olecular layers, respectively. Tw o reasons m ay explain the lack of a phenotype in old er granule cells. First, chABC activity is transient and thus requires optim ization of d elivery tim ing (Figure 19B). In our experim ents, 14-day-old granule cells w ere analyzed at a shorter interval after chABC treatm ent than old er cells (7 d ays vs. 14 d ays), raising the possibility that one chABC injection at 7 d ays post-lesion w as insufficient to span the entire experim ental period . Therefore, d end ritic arborization and spine density of m ature and 21-d ay-old granu le cells should be re-tested at 21 d ays post-lesion after tw o injections of chABC at 7- and 14- days post-lesion. To avoid the necessity of repeated injections, in 73 m ay be preferable to use a chABC-expressing viral vector (Zhao et al., 2011). Second , CSPGs are only one of many types of extracellular m atrix proteins that are im pacted follow ing lesion, suggesting that other inhibitors of d end ritic outgrow th are present in the d eafferented zone. Furtherm ore, CSPGs are only partially broken d ow n by chABC, leavin g behind intact core proteins that m ay them selves create an unfavorable environm ent for grow th (Oohira et al., 1991; Bukhari et al., 2011). As such, future stud ies should explore the effects of other locally injected enzym es, such as hyaluronid ase, that d e grad e either the entire CSPG com plex or other m atrix proteins elevated in this region. Effective d elivery of these enzym es, as w ell as m ore stringent regulation of their spread in the tissue, m ay be instrum ental in und erstand ing the role of the extracellular m atrix in lam inar specificity, d end ritic outgrow th, axonal sprouting, and functional recovery. 74 CHAPTER 2: FIGURES Figure 16 – Lesion alters vGlut1 expression in the deafferented zone, but not PSD 95 Post-lesion tim e course of pre- and postsynaptic reorganization. vGlut1 (red ) w as used as a m arker of excitatory axons and PSD95 (blue) w as used as a m arker of the postsynapse. Im ages in A-E are d epictions of d ifferent channels from the sam e brain slice, separated for clarity. (A) Baseline expression of excitatory axons and postsynaptic d ensities in the control/ contralateral adult hippocam pus. Both m arkers show diffuse staining throughout the m olecular layer, but PSD expression is slightly elevated in the inner molecular layer (IML). (B-E) Deafferentation of the dentate gyrus by the perforant path lesion (solid yellow line) triggers a slow d egeneration of excitatory axons in the m id d le and outer m olecular layers, first d etected at 2 d ays post-lesion (C, left panel) and alm ost entirely gone by 7 d ays post-lesion (E, left panel). N ote also the increase in vGlut1 staining in the inner m olecular layer starting at d ay 2 post-lesion, d epicting sprouting of the uninjured afferents. Postsynaptic d ensities w ere not affected by the lesion (B-E, right panels). The lack of excitatory innervation persists at least through 21 d ays post-lesion (F, left panel). vGlut1 staining w as also absent in 30 d ays post-lesion animals (not show n), suggesting no excitatory reinnervation occurred in the d enervated zone. 75 Figure 17 – Lamina-specific reactive gliosis in the denervated dentate gyrus. Lesion of the perforant path triggers lam ina-specific activation of m icroglia (CD68, w hite clusters) and astrocytes (GFAP, red ). Dashed w hite lines outline the m olecular layer, w hereas the solid yellow line d efines the d eafferented region. (A) Representative section of the non-lesioned d entate gyrus for all tim e points post-lesion. Astrocytes have few branches and m icroglia are hard ly noticeable. (B) Dentate gyrus at 1 d ay post-lesion. At this tim e point, circuit reorganization is just beginning to occur and changes in microglia (arrow head ) or astrocytes are not yet detectable. Deafferented region is marked by the solid w hite line on the right. (C) At 2 d ays post-lesion, microglia are in cluster form ation, ind icative of their activation (arrow head s). (D ) By 4 d ays post-lesion, m icroglia are still active (arrow head s), but astrocytes in the d eafferented zone (yellow bar) also begin to hypertrophy. This trend is also observed at 7 days post -lesion (E). (F) By 1 m onth postlesion, m icroglia have returned to their baseline expression level (not show n), how ever astrocytes rem ain active at least this long. Scale bar in E applies also to A -D. 76 Figure 18 – Lamina-specific upregulation of CSPG follow ing perforant path lesion. Tim e course of CSPG expression follow ing lesion. Dashed w hite line lies on the hippocam pal fissure and d epicts the bord er of the d entate m olecular layer. The solid yellow line ind icates approxim ate zone d eafferented by the injury. (A) Representative exam ple of baseline CSPG expression that can be observed in the contralateral control at all tim e points tested . The signal is largely diffuse, w ith som e ind ication of m ore d ensely stained areas. (B-F) An increase in CSPG expression first becom es apparent at 4 d ays postlesion (D) w ith d ense cloud s becom ing m ore prom inent. CSPG expression continues to increase, peaking at 7 d ays post-lesion (E) and persisting longer than tw o w eeks (F). Attem pts to eliminate injury-ind uced upregulation of CSPG m ust consid er the tim ing of its expression as w ell as the half-life of enzym atic activity. 77 78 Figure 19 Figure 19 – CSPG upregulation follow ing lesion can be knocked dow n by enzyme treatment, but timing is crucial. (A) Chond roitinase ABC (chABC) w as stereotaxically injected into the d eafferented d entate gyrus. The approxim ate spread of enzym atic activity is outlined w ith a d ashed yellow line. Although som e crossover can be observed , the area of interest (the d orsal blad e of the contralateral d entate gyrus) w as typically not affected by the injection. Enzym atic activity w as determ ined by staining for C4S (red ), the prod uct of CSPG d igestion by chABC. (B) Im m unostain of und igested CSPG (red) at 14 d ays post-lesion. chABC w as injected on the d ay of the lesion (0d pl), prior to the peak of CSPG expression (see Fig. 5). CSPG expression w as elevated in the lesioned hem isphere (right panel), d espite injection of the enzym e, suggesting that CSPG expression outlasted enzym atic activity in the region. (C) Im m unostain for und igested CSPG (red ) at 14 d ays post-lesion. chABC w as injected at 7 d ays post-lesion, d uring the peak of injury-ind uced CSPG expression (see Fig. 5). CSPG expression in the deafferented d entate gyrus w as at baseline, suggesting that chABC prevented the increase in CSPG (right panel). N ote the increased proliferation of new born granule cells (POMC-EGFP) d espite enzym atic acitivity. (D ) At alternative approach to decreasing CSPG expression follow ing injury. Xylosid e w as injected intraperitoneally (IP), tw ice daily, for 5 consecutive d ays, starting on the 3 rd d ay post-lesion. Tissue show n here w as stained for intact CSPG at 14 d ays post -lesion (7 d ays after the last Xylosid e injection). A lamina-specific increase in CSPG expression is still observed in the d eafferented d entate gyrus (right panel). 79 G EN ERAL D ISCUSSION LIMITS OF PLASTICITY The perforant path m od el serves as an exam ple of CN S plasticity that incorporates m any features of the injury response. N europlasticity in the ad ult brain is a com plex process that involves all aspects of the neural circuit – axonal sprouting and term inal bouton turnover, reorganization of d end rites and spines, activity -d epend ent m od ulation of synaptic strength, as w ell as ad ult neurogenesis. The d ynam ic natur e of the ad ult brain gives hope for end ogenous repair follow ing injury, how ever, limits of neuroplasticity m ust be recognized in ord er to optim ize potential m ed ical treatm ents. Follow ing perforant path lesion, new born neurons show a greater d egree of structural plasticity than m ature granule cells by accom mod ating sprouting axons in the inner m olecular layer. H ow ever, circuit-appropriate reinnervation of d enervated targets is essential for functional recovery, and this aspect of recovery has yet to be fully explored . For exam ple, follow ing ischem ic lesions, new born neurons from the expand ed ipsilateral SVZ can replenish cells lost in the striatum by m igrating in chains tow ard the site of infarction, w here they d ifferentiate into m ed ium spiny neurons (Arvid sson et al., 2002; Parent et al., 2002). Interestingly, migration of these cells can persist for at least one year after stroke (Kokaia et al., 2006), suggesting that repair m echanism s can rem ain active long after the insult . Som e evid ence show s that new ly d ifferentiated neurons in the striatum grow d end rites, form synapses, and have spontaneous post-synaptic activity, indicative of functional integration (H ou et al., 2008). H ow ever, w hether these cells receive appropriate inputs is unknow n (Burns et al., 2009). The im portance of appropriate reinnervation is perhaps best exem plified by stem cell therapy follow ing spinal cord injury. Although prom ising (Coutts and Keirstead , 2007; Bareyre, 2008), grafting of neural progenitor cells around the lesion site can tr igger 80 aberrant axonal sprouting and subsequent pain hypersensitivity in the forepaw (H ofstetter et al., 2005). This issue potentially m ay be resolved by creating a m ore favorable environm ent for stem cell m aturation and functional integration, includ ing axon guid ance m olecules, grow th factors, and , if necessary, im mune suppressors (Liu et al., 2003; William s and Lavik, 2009). The lam ina-specific reorganization follow ing perforant path lesion suggests that effective circuit regeneration and functional recove ry w ill also require a rebalancing of the glial response and the extracellular environment that w ill allow appropriate synaptogenesis betw een new axons and d end rites. 81 S UMMARY AN D CON CLUSION S It is w ell-established that ad ult-generated new born neurons can navigate the ad ult environm ent and integrate into neural circuits, but the response of these neurons to in vivo d enervation has not been d ocum ented . In our experim ents, the net effect of the d enervating injury on n ew born neurons w as to prom ote proliferation, consistent w ith the potentially positive influences of the injured environm ent on neural regeneration and repair. H ow ever, perforant path lesion also hind ered d end ritic d evelopm ent of new born neurons, suggesting that excitatory activity is necessary for elaboration of outgrow ing d end rites. Although this effect w as perhaps expected in 21-d ay-old granule cells, it w as surprising to see d ecreased d end ritic outgrow th in 14-d ay-old granule cells, w hich d o not yet m ake contacts w ith the perforant path. The r easons for the lesion effect on younger neurons are not yet clear, but likely involve changes in the extracellular environm ent or altered signaling of interneurons, w hich also may be affected by the injury. N ew ly prod uced granule cells also show ed enhanced structural plasticity. Specifically, new born granule cells had an altered d istribution of d end ritic spines postlesion, presum ably to accom m od ate sprouting of intact com m issural/ associational fibers in the inner m olecular layer (Matthew s et al., 1976b). The increase in spine d ensity in the inner m olecular layer m ay reflect the enhanced synaptic plasticity of new born neurons relative to mature granule cells. For exam ple, imm ature granule cells in the norm al d entate gyrus exhibit d ecreased LTP ind uction thr eshold s at tw o-three w eeks and increased LTP am plitud es at four-six w eeks, w hich can be observed even w ith sparse glutam atergic innervation (Schm id t-H ieber et al., 2004; Ge et al., 2007; Lemaire et al., 2012). Thus, new born neurons m ay out-recruit m ature cells for sprouting collaterals and m ay 82 thus have an ad vantage in de novo synaptogenesis and circuit reorganization. Sim ilarly, such post-lesion innervation of new d end rites by sprouting hom otypic axons m ay provid e a sufficient am ount of excitatory input to ensure functional integration and survival of new born granule cells, thus com pensating for the d egenerated perforant path. Although lesion of the perforant path stim ulates reorganization of the d entate gyrus, recovery is limited by lam inar barriers. Lam inar specificity is partially regulated by the injury-ind uced glial response that occurs in the d enervated zone, as w ell as changes in the com position of the extracellular m atrix, including upregulation of CSPGs and m atrix m etalloproteases. Such changes m ay im pact axonal as w ell as d end ritic reorganization follow ing perforant path lesion and limit reinnervation of the d eafferented d entate gyrus, especially w ith regard to hom otypic sprouting and functional recovery. Thus, future experim ents need to ad dress th e extracellular aspects of the post-lesion response by m anipulating the local environm ent surround ing d end ritic outgrow th and synaptogenesis. 83 REFEREN CES Abrous, D. N ., Koehl, M., and Le Moal, M. (2005), Ad ult neurogenesis: from precursors to netw ork and physiology. Physiol. Rev. 85(2), 523-69. Akbik, F., Cafferty, W.B., and Strittmatter, S.M. (2012). Myelin associated inhibitors: a link betw een injury-ind uced and experience-d epend ent plasticity. Exp. N eurol. 235, 43-52. Am brogini, P., Lattanzi, D., Ciuffoli, S., Agostini, D., Bertini, L., Stocchi, V., Santi, S., Cuppini, R. (2004). Morpho-functional characterization of neuronal cells at d ifferent stages of m aturation in granule cell layer of ad ult rat d entate gyrus. Brain Res. 1017(1-2), 21-31. Arvid sson, A., Collin, T., Kirik, D., Kokaia, Z., Lind vall, O. (2002). N euronal replacem ent from end ogenous precursors in the ad ult brain after stroke. N at. M ed. 8(9), 963-70. Balu, D. T., and Lucki, I. (2009). Ad ult hippocam pal neurogenesis: regulation, functional im plications, and contribution to d isease pathology. Cereb. Cortex 19, 241-248. Bareyre, F. M. (2008). N euronal repair and replacem ent in spinal cord injury. J. N eurol. Sci. 265(1-2), 63-72. Bartus, K., James, N . D., Bosch, K. D., Brad bury, E. J. (2012). Chond roitin sulphate proteoglycans: key m odulators of spinal cord and brain plasticity. Exp. N eurol. 235(1), 5-17. Bechm ann, I., and N itsch, R. (2000). Involvement of non -neuronal cells in entorhinalhippocam pal reorganization follow ing lesions. A nn. N . Y . A cad. Sci. 911, 192-206. Ben-Ari, Y. (2002), Excitatory actions of GABA d uring d evelopm ent: the nature of the nurture. N at. Rev. N eurosci. 3(9), 728-39. Ben-Ari, Y., and Gho, M. (1988). Long-lasting m od ification of the synaptic properties of rat CA3 hippocam pal neurones ind uced by kainic acid . J. Physiol. 404, 365-84. Birks, R., Katz, B., and Miled i, R. (1960). Physiological and structural changes at the am phibian m yoneural junction, in the course of nerve d egeneration. J. Physiol. 150, 145-68. Bosse, F. (2012). Extrinsic cellular and m olecular m ed iators of peripheral axonal regeneration. Cell Tissue Res. 349(1), 5-14. Boulland , J. L., Jenstad , M., Boekel, A. J., Wouterlood , F. G., Ed w ard s, R. H ., Storm Mathisen, J., Chaud hry, F. A. (2009). Vesicular glutam ate and GABA transporters sort to d istinct sets of vesicles in a population of presynaptic term inals. Cereb. Cortex 19, 241-248. Bovolenta, P., and Fernaud -Espinosa, I. (2000). N ervous system proteoglycans as m od ulators of neurite outgrow th. Prog. N eurobiol. 61(2), 113–132 84 Bozd agi, O., N agy, V., Kw ei, K. T., H untley, G. W. (2007). In vivo roles for m atrix m etalloproteinase-9 in mature hippocam pal synaptic physiology and plasticity. J. N europhysiol. 98, 334–344. Brad bury, E. J., and Carter, L. M. (2011). Manipulating the glial scar: chond roitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 84(4-5), 306-16. Bram billa, R., Bracchi-Ricard , V., H u, W. H ., Fryd el, B., Bram w ell, A., Karm ally, S., Green, E. J., Bethea, J. R. (2005). Inhibition of astroglial nuclear factor kB red uces inflam m ation and im proves functional recovery after spinal cord injury. J. Exp. M ed. 202, 145–156. Brow n, C. E., Boyd, J. D., and Murphy, T. H . (2010). Longitud inal in vivo im aging reveals balanced and branch -specific rem od eling of m ature cortical pyramid al d end ritic arbors after stroke. J. Cereb. Blood Flow M etab. 30(4), 783-91. Brow n, C. E., Wong, C., and Murphy, T. H . (2008) Rapid m orphologic plasticity of periinfarct d end ritic spines after focal ischemic stroke. Stroke 39(4), 1286–1291. Buckm aster, P. S., and Lew , F. H . (2011). Rapam ycin suppresses m ossy fiber sprouting but not seizure frequency in a m ouse m od el of tem poral lobe epilepsy. J. N eurosci. 31(6), 233747. Bukhari, N ., Torres, L., Robinson, J. K., Tsirka, S. E. (2011). Axonal regrow th after spinal cord injury via chond roitinase and the tissue plasm inogen activator (tPA)/ plasm in system . J. N eurosci. 31(42), 14931-43. Burns, T. C., Verfaillie, C. M., and Low , W. C. (2009). Stem cells for ischemic brain injury: a critical review . J. Comp. N eurol. 515(1), 125-44. Caceres, A., and Stew ard, O. (1983). Dend ritic reorganization in the d enervated d entate gyrus of the rat follow ing entorhinal cortical lesions: a Golgi and electron m icroscopic analysis. J. Comp. N eurol. 214, 387-403. Cam eron, H . A., McEw en, B. S., and Gould , E. (1995). Regulation of ad ult neurogenesis by excitatory input and N MDA receptor activation in the d entate gyrus. J. N eurosci. 15(6), 4687-92. Cam eron, H . A., and McKay, R. D. (2001). Ad ult neurogenesis prod uces a large pool of new granule cells in the d entate gyrus. J. Comp. N eurol. 435, 406–417. Carulli, D., Laabs, T., Geller, H . M., Faw cett, J. W. (2005). Chond roitin sulfate proteoglycans in neural d evelopm ent and regeneration. Curr. Opin. N eurobiol. 15(1), 11620. Ceranik, K., Zhao, S., and Frotscher, M. (2000). Developm ent of the entorhinohippocam pal projection: Guid ance by Cajal-Retzius cell axons. A nn. N ew Y ork A cad. Sci. 911, 43-54. 85 Chao, D. L., Ma, L., and Shen, K. (2009). Transient cell-cell interactions in neural circuit form ation. N at. Rev. N eurosci. 10(4), 262-71. Chen, Z. L., Yu, W. M., and Strickland , S. (2007). Peripheral regeneration. A nnu. Rev. N eurosci. 30, 209-33. Christopherson, K. S., Ullian, E. M., Stokes, C. C., Mullow ney, C. E., H ell, J. W., Agah, A., Law ler, J., Mosher, D. F., Bornstein, P., and Barres, B. A. (2005). Throm bospond ins are astrocyte-secreted proteins that prom ote CN S synaptogenesis. Cell 120, 421–433. Clusm ann, H ., N itsch, R., and H einem ann, U. (1994). Long lasting functional alterations in the rat d entate gyrus follow ing entorhinal cortex lesion: a current source density analysis. N euroscience 61(4), 805-15. Collazos-Castro, J. E., and N ieto-Sam ped ro, M. (2001). Developm ental and reactive grow th of d entate gyrus afferents: cellular and m olecular interactions. Restor. N eurol. N eurosci. 19(3-4), 169-87. Cotm an, C., Gentry, C., and Stew ard , O. (1977). Synaptic replacem ent in the d entate gyrus after unilateral entorhinal lesion: electron microscopic analysis of the exten t of replacem ent of synapses by the rem aining entorhinal cortex. J. N eurocytol. 6(4), 455-64. Coutts, M., and Keirstead , H . S. (2008). Stem cells for the treatm ent of spinal cord injury. Exp. N eurol. 209(2), 368-77. Dash, P. K., Mach, S. A., and Moore, A. N . (2001). Enhanced neurogenesis in the rod ent hippocam pus follow ing traum atic brain injury. J. N eurosci. Res. 63(4), 313-9. Dayer, A. G., Ford , A. A., Cleaver, K. M., Yassaee, M., Cam eron, H . A. (2003). Short-term and long-term survival of new neurons in the rat d entate gyrus. J. Comp. N eurol. 460(4), 563-72. DeCarolis, N . A., and Eisch, A. J. (2010). H ippocam pal neurogenesis as a target for the treatm ent of m ental illness: a critical evaluation. N europharmacology 58(6), 884-93. Del Río JA, H eim rich B, Borrell V, Förster E, Drakew A, Alcántara S, N akajim a K, Miyata T, Ogaw a M, Mikoshiba K, Derer P, Frotscher M, Soriano E. (1997). A role for Cajal– Retzius cells and reelin in the d evelopm ent of hippocam pal connections. N ature 385, 70–74. Del Turco, D., Wood s, A. G., Gebhard t, C., Phinney, A. L., Jucker, M., Frotscher, M., Deller, T. (2003). Com parison of com m issural sprouting in the m ouse and rat fascia d entata after entorhinal cortex lesion. Hippocampus 13(6), 685-99. Deller, T., Del Turco, D., Rappert, A., Bechm ann, I. (2007). Structural reorganization of the d entate gyrus follow ing entorhinal d enervation: species d ifferences betw een rat and m ouse. Prog. Brain Res. 163, 501-28. 86 Deller, T., Frotscher, M. (1997). Lesion-ind uced plasticity of central neurons: sprouting of single fibres in the rat hippocam pus after unilateral entorhinal cortex lesion. Prog. N eurobiol. 53(6), 687-727. Deller, T., Frotscher, M., and N itsch, R. (1995). Morphological evid ence for the sprouting of inhibitory com missural fibers in resp onse to the lesion of the excitatory entorhinal input to the rat d entate gyrus. J. N eurosci. 15(10), 6868-78. Deller, T., Frotscher, M., and N itsch, R. (1996a). Sprouting of crossed entorhinod entate fibers after a unilateral entorhinal lesion: anterograd e tracing of fiber reorganization w ith Phaseolus vulgaris-leucoagglutinin (PH AL). J. Comp. N eurol. 365(1), 42-55. Deller, T., H aas, C. A., and Frotscher, M. (2001). Sprouting in the hippocam pus after entorhinal cortex lesion is layer- specific but not translaminar: w hich m olecules m ay be involved ? Restor. N eurol. N eurosci. 19(3-4), 159-67. Deller, T., H aas, C. A., Naum ann, T., Joester, A., Faissner, A., Frotscer, M. (1997). Upregulation of astrocyte-d erived tenascin -C correlates w ith neurite outgrow th in the rat d entate gyrus after unilateral entorhinal cortex lesion. N euroscience 81, 829–846. Deller, T., N itsch, R., and Frotscher, M. (1996b). Layer-specific sprouting of com m issural fibres to the rat fascia d entata after unilateral entorhinal cortex lesion: a Phaseolus vulgaris leucoagglutinin tracing stud y. N euroscience 71(3), 651-60. Diekm ann, S., Ohm , T. G., and N itsch, R. (1996). Long-lasting transneuronal changes in rat d entate granule cell d end rites after entorhinal cortex lesion. A combined intracellular injection and electron microscopy stud y. Brain Pathol. 6(3), 205-15. Dissing-Olesen, L., Lad eby, R., N ielsen, H . H ., Toft-H ansen, H ., Dalm au , I., Finsen, B. (2007). Axonal lesion -induced m icroglial proliferation and m icroglial cluster form ation in the m ou se. N euroscience 149(1), 112-22. Dityatev, A., and Fellin, T. (2008). Extracellular matrix in plasticity and epileptogenesis. N euron Glia Biol. 4, 235–247. Dityatev, A., Frischknecht, R., and Seid enbecher, C. I. (2006). Extracellular m atrix and synaptic functions. Results Probl. Cell Differ. 43, 69–97. Dityatev, A., Schachner, M., and Sond eregger, P. (2010a). The d ual role of the extracellular m atrix in synaptic plasticity and hom eostasis. N at. Rev. N eurosci. 11, 735–746. Dityatev, A., Seidenbecher, C. I., and Schachner, M. (2010b). Com partm entalization from the outsid e: The extracellular m atrix and functional m icrod om ains in the brain. Trends N eurosci. 33, 503–512. Drakew , A., Müller, M., Gähw iler, B. H ., Thom pson, S. M., Frotscher, M. (1996). Spine loss in experim ental epilepsy: quantitative light and electron m icroscopic analysis of 87 intracellularly stained CA3 pyram id al cells in hippocam pal slice cultures. N euroscience 70(1), 31-45. Eroglu, C. (2009). The role of astrocyte-secreted matricellular proteins in central nervous system d evelopm ent and function. J. Cell Comm. Sig. 3, 167–176. Falo, M. C., Fillm ore, H . L., Reeves, T. M., Phillips, L. L. (2006). Matrix m etalloproteinase -3 expression profile d ifferentiates ad aptive and m alad aptive synaptic plasticity ind uced by traum atic brain injury. J. N eurosci. Res. 84(4), 768-81. Fatem i, S. H . (2005). Reelin glycoprotein: structure, biology and roles in health and disease. M ol. Psych. 10, 251–257. Fitch, M. T., and Silver, J. (2008). CN S injury, glial scars, and inflam m ation: Inhibitory extracellular m atrices and regeneration failure. Exp. N eurol. 209(2), 294-301. Frischknecht, R., and Gund elfinger, E. (2012). The brain's extracellular m atrix and its role in synaptic plasticity. A dv. Exp. M ed. Biol. 970, 153-71. Förster, E., Tielsch, A., Saum , B., Weiss, K.H ., Johanssen, C., Graus-Porta, D., Müller, U. & Frotscher, M. (2002) Reelin, Disabled 1, and beta 1 integrins are required for the form ation of the radial glial scaffold in the hippocam pus. Proc. N atl A cad. Sci. USA , 99, 13178–13183. Förster, E., Zhao, S., and Frotscher, M. (2001). H yaluronan-associated adhesive cues control fiber segregation in the hippocam pus. Development 128, 3029-3039. Förster, E., Zhao, S., and Frotscher, M. (2006). Lam inating the hippocam pus. N at. Rev. N eurosci. 7, 259-268. Frotscher, M., and H eimrich, B. (1993). Form ation of layer-specific fiber projections to the hippocam pus in vitro. Proc. N atl A cad. Sci. USA 90, 10400–10403. Frotscher, M., Zhao, S., and Förster, E. (2007). Developm ent of cell and fiber layers in the d entate gyrus. Prog. Brain Res. 163, 133-42. Gage, F. H ., Coates, P. W., Palmer, T. D., Kuhn, H . G., Fisher, L. J., Suhonen, J. O., Peterson, D. A., Suhr, S. T., Ray, J. (1995). Survival and differentiation of ad ult neuronal progenitor cells transplanted to the ad ult brain. Proc. N atl. A cad. Sci. U S A 92(25), 1187983. Gage, F. H ., Olejniczak, P., and Arm strong, D. M. (1988). Astrocytes are important for sprouting in the septohippocam pal circuit. Exp. N eurol. 102(1), 2-13. Gall, C., and Lynch, G. (1981). Fiber architecture of the dentate gyrus follow ing ablation of the entorhinal cortex in rats of d ifferent ages: evidence for tw o form s of axon sprouting in the im mature brain. N euroscience 6(5), 903-10. 88 Galtrey, C. M., and Faw cett, J. W. (2007). The role of chond roitin sulfate proteoglycans in regeneration and plasticity in the central nervous system . Brain Res. Rev. 54(1), 1-18. Ge, S., Sailor, K. A., Ming, G. L., Song, H . (2008). Synaptic integration and plasticity of new neurons in the ad ult hippocam pus. J. Physiol. 586(16), 3759-65. Ge, S., Yang, C. H ., H su, K. S., Ming, G. L., Song, H . (2007). A critical period for enhanced synaptic plasticity in new ly generated neurons of the ad ult brain. N euron 54(4), 559-66. Gebhard t, C., Del Turco, D., Drakew , A., Tielsch, A., H erz, J., Frotscher, M., Deller T. (2002). Abnorm al positioning of granule cells alters afferent fiber d istribution in the m ouse fascia d entata: m orphologic evid ence from reeler, apolipoprotein E receptor 2-, and very low d ensity lipoprotein receptor knockout mice. J. Comp. N eurol. 445(3), 278-92. Gehrm ann, J., Schoen, S. W., and Kreutzberg, G. W. (1991). Lesion of the rat entorhinal cortex lead s to a rapid microglial reaction in the dentate gyrus. A cta N europathol. 82, 442– 455. Giger, R. J., H ollis, E. R. II, and Tuszynski, M. H . (2010). Guidance m olecules in axon regeneration. Cold Spring Harb. Perspect. Biol. 2(7), a001867. Góm ez-Pinilla, F., Lee, J. W., Cotm an, C. W. (1992). Basic FGF in ad ult rat brain: cellular d istribution and response to entorhinal lesion and fim bria -fornix transection. J. N eurosci. 12(1), 345-55. Gottlieb, D. I., and Cow an, W. M. (1973). Autoradiographic stud ies of the com m issural and ipsilateral association connection of the hippocam pus and d et entate gyrus of the rat. I. The com m issural connections. J. Comp. N eurol. 149(4), 393-422. Gould , E., and Tanapat, P. (1997). Lesion-ind uced proliferation of neuronal progenitors in the d entate gyrus of the ad ult rat. N euroscience 80(2), 427-36. Gould , E., Tanapat, P. (1999). Stress and hippocam pal neurogenesis. Biol. Psychiatry 46(11), 1472-9. H aas, C. A., Deller, T., Krsnik, Z., Tielsch, A., Wood s, A., Frotscher, M. (2000). Entorhinal cortex lesion d oes not alter reelin m essenger RN A expression in the d en tate gyrus of young and ad ult rats. N euroscience 97(1), 25-31. H aas, C. A., and Frotscher, M. (2010). Reelin d eficiency causes granule cell d ispersion in epilepsy. Exp. Brain Res. 200(2), 141-9. H aas, C. A., Frotscher, M., and Deller, T. (1999). Differential ind uction of c-Fos, c-Jun and Jun B in the rat central nervous system follow ing unilateral entorhinal cortex lesion. N euroscience 90(1), 41-51. 89 H aas, C. A., Rauch, U., Thon, N ., Merten, T., Deller, T. (1999). Entorhinal cortex lesion in ad ult rats ind uces the expression of the neuronal chond roitin sulfate proteoglycan neurocan in reactive astrocytes. J. N eurosci. 19, 9953–9963. H ack, I., Bancila, M., Loulier, K., Carroll, P., Cremer, H . (2002). Reelin is a d etachm ent signal in tangential chain -m igration d uring postnatal neurogenesis. N at. N eurosci. 5(10), 939-45. H ailer, N . P., Gram pp, A., and N itsch, R. (1999). Proliferation of m icroglia and astrocytes in the d entate gyrus follow ing entorhinal cortex lesion: a quantitative bromod eoxyurid ine labelling stu d y. Eur. J. N eurosci. 11(9), 3359-64. H am by, M. E., and Sofroniew , M. V. (2010). Reactive astrocytes as therapeutic targets for CN S d isord ers. N eurotherapeutics 7, 494–506. H egarty, D. M., Tonsfeldt, K., H erm es, S. M., H elfand , H ., Aicher, S. A. (2010). Differential localization of vesicular glutam ate transporters and peptid es in corneal afferents to trigem inal nu cleus caudalis. J. Comp. N eurol. 518(17), 3557-69. H einrich, C., N itta, N ., Flu bacher, A., Müller, M., Fahrner, A., Kirsch, M., Freim an, T., Suzuki, F., Depaulis, A., Frotscher, M., H aas, C. A. (2006). Reelin d eficiency and d isplacem ent of m ature neurons, but not neurogenesis, und erlie the formation of granule cell d ispersion in the epileptic hippocam pus. J. N eurosci. 26(17), 4701-13. H ickm ott, P. W., and Steen, P. A. (2005). Large-scale changes in d end ritic structure d uring reorganization of ad ult som atosensory cortex. N at. N eurosci. 8(2), 140-2. H irano, A., and Dem bitzer, H . M. (1973). Cerebellar alterations in the w eaver m ouse. J. Cell Biol. 56, 478-486. H ill, C. E., Beattie, M. S., and Bresnahan, J. C. (2001). Degeneration and sprouting of id entified descend ing supraspinal axons after contusive spinal cord injury in the rat. Exp. N eurol. 171(1), 153-69. H jorth-Sim onsen, A. (1972). Projection of the lateral part of the entorhinal area to the hippocam pus and fascia d entata. J. Comp. N eurol. 146(2), 219-32. H jorth-Sim onsen, A., and Jeune, B. (1972). Origin and term ination of the hippocam pal perforant path in the rat stud ied by silver im pregnation . J. Comp. N eurol. 144(2), 215-32. H ofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T., H übener, M. (2006). Lifelong learning: ocular d om inance plasticity in m ouse visual cortex. Curr. Opin. N eurobiol. 16(4), 451-9. H ofstetter, C. P., H olm ström , N . A., Lilja, J. A., Schw einhard t, P., H ao, J., Spenger, C., Wiesenfeld -H allin, Z., Kurpad , S. N ., Frisén, J., Olson, L. (2005). Allod ynia lim its the usefulness of intraspinal neural stem cell grafts; directed d ifferentiation improves outcom e. N at. N eurosci. 8(3), 346-53. 90 H osp, J. A., and Luft, A. R. (2011). Cortical plasticity d uring m otor learning and recovery after ischemic stroke. N eural Plast. 2011, 871296. H ou, S. W., Wang, Y. Q., Xu, M., Shen, D. H ., Wang, J. J., H uang, F., Yu, Z., Sun, F. Y. (2008). Functional integration of new ly generated neurons into striatum after cerebral ischem ia in the ad ult rat brain. Stroke 39(10), 2837-44. H untley, G. W. (2012). Synaptic circuit rem od elling by m atrix m etalloproteinases in health and d isease. N at. Rev. N eurosci. 13(11), 743-57. Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gund ersen, G. A., Benveniste, H ., Vates, G. E., Deane, R., Gold m an, S. A., N agelhus, E. A., N ed ergaard , M. (2012). A paravascular pathw ay facilitates CSF flow through the brain parenchym a and th e clearance of interstitial solutes, includ ing am yloid β. Sci. Transl. M ed. 4(147), 147ra111. Jansen, L. A., Uhlm ann, E. J., Crino, P. B., Gutm ann, D. H ., Wong, M. (2005). Epileptogenesis and reduced inw ard rectifier potassium current in tuberous sclerosis com plex-1-deficient astrocytes. Epilepsia 46, 1871–1880. Jessberger, S., Röm er, B., Babu, H ., Kem perm ann, G. (2005). Seizures ind uce proliferation and d ispersion of d oublecortin -positive hippocampal progenitor cells. Exp. N eurol. 196(2), 342-51. Katsuyam a. Y., and Terashim a, T. (2009). Developm ental anatom y of reeler m utant m ouse. Dev. Growth Differ. 51(3), 271-86. Katz, B., and Miled i, R. (1959). Spontaneous subthreshold activity at d enervated am phibian end -plates. J. Physiol. 146, 44-45P. Kernie, S. G., and Parent, J. M. (2010). Forebrain neurogenesis after focal ischem ic and traum atic brain injury. N eurobiol. Dis. 37(2), 267-74. Kim , B. G., Dai, H . N ., McAtee, M., Vicini, S., Bregm an, B. S. (2006). Rem odeling of synaptic structures in the m otor cortex follow ing spinal cord injury. Exp. N eurol. 198(2), 401-15. Know les, W. D. (1992). Norm al anatom y and neurophysiology of the hippocam pal form ation. J. Clin. N europhysiol. 9(2), 252-63. Koistinaho, M., Lin, S., Wu, X., Esterm an, M., Koger, D., H anson, J., H iggs, R., Liu, F., Malkani, S., Bales, K. R., Paul, S. M. (2004). Apolipoprotein E prom otes astrocyte colocalization and d egrad ation of d eposited am yloid -beta peptid es. N at. M ed. 10, 719–726. Kokaia, Z., Thored , P., Arvid sson, A., Lind vall, O. (2006). Regulation of stroke-ind uced neurogenesis in ad ult brain —recent scientific progress. Cereb. Cortex 16(suppl 1), i162–i167. 91 Kuhn, H . G., Biebl, M., Wilhelm , D., Li, M., Fried land er, R. M., Winkler, J. (2005). Increased generation of granule cells in ad ult Bcl-2-overexpressing mice: a role for cell d eath d uring continued hippocam pal neurogenesis. Eur. J. N eurosci. 22(8), 1907-15. Kurihara, D., and Yamashita, T. (2012). Chond roitin sulfate proteoglycans d ow n -regulate spine form ation in cortical neurons by targeting tropom yosin-related kinase B (TrkB) protein. J. Biol. Chem. 287(17), 13822-8. Kw ok, J. C., Afshari, F., García-Alías, G., Faw cett, J. W. (2008). Proteoglycans in the central nervous system : plasticity, regeneration and their stim ulation w ith chond roitinase ABC. Restor. N eurol. N eurosci. 26(2-3), 131-45. Kw ok, J. C., Warren, P., and Faw cett, J. W. (2012). Chond roitin sulfate: a key m olecule in the brain m atrix. Int. J. Biochem. Cell Biol. 44(4), 582-6. Laud er, J. M. (1993). N eurotransm itters as grow th regulatory signals: role of receptors and second m essengers. Trends N eurosci. 16(6), 233-40. Lem aire, V., Tronel, S., Montaron, M. F., Fabre, A., Dugast, E., Abrous, D. N . (2012). Long lasting plasticity of hippocam pal ad ult-born neurons. J. N eurosci. 32(9), 3101-8. Leranth, C., and H ajszan, T. (2007). Extrinsic afferent systems to the d entate gyrus. Prog. Brain Res. 163, 63-84. Lew is, P. F., and Emerman, M. (1994). Passage through m itosis is required for oncoretroviruses but not for the hum an im m unodeficiency vir us. J. V irol. 68(1), 510-6. Lichtenw alner, R. J., and Parent, J. M. (2006). Ad ult neurogenesis and the ischem ic forebrain. J. Cereb. Blood Flow M etab. 26(1), 1-20. Liu, C. Y., Apuzzo, M. L., and Tirrell, D. A. (2003). Engineering of the extracellular m atrix: w orking tow ard neural stem cell program ming and neurorestoration --concept and progress report. N eurosurgery 52(5), 1154-65. Liu, J., Solw ay, K., Messing, R. O., Sharp, F. R. (1998). Increased neurogenesis in the d entate gyrus after transient global isch em ia in gerbils. J. N eurosci. 18(19), 7768-78. Lois, C., H ong, E. J., Pease, S., Brow n, E. J., Baltimore, D. (2002). Germ line transm ission and tissue-specific expression of transgenes d elivered by lentiviral vectors. Science 295, 868-872. Liu, J., Solw ay, K., Messing, R. O., Sharp, F. R. (1998). Increased neurogenesis in the d entate gyrus after transient global ischem ia in gerbils. J. N eurosci. 18, 7768-78. Liu, K., Ted eschi, A., Park, K. K., H e Z. (2011). N euronal intrinsic m echanism s of axon regeneration. A nnu. Rev. N eurosci. 34, 131-52. 92 Luikart, B. W., Schnell, E., Washburn, E. K., Bensen, A. L., Tovar, K. R., Westbrook, G. L. (2011a). Pten knockd ow n in vivo increases excitatory d rive onto d entate granule cells. J. N eurosci. 31(11), 4345-54. Luikart, B. W., Pered eriy, J. V., Westbrook, G. L. (2011b). Dentate gyrus neurogenesis, integration and microRN As. Behav. Brain Res. 227(2), 348-55. Lynch, G., Matthew s, D. A., Mosko, S., Parks, T., Cotm an, C. (1972). Ind uced acetylcholinesterase-rich layer in rat d entate gyrus follow ing entorhinal lesions. Brain Res. 42(2), 311-8. Maier, I. C., and Schw ab, M. E. (2006). Sprouting, regeneration and circuit form ation in the injured spinal cord : factors and activity. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361(1473), 1611-34. Margolis, R. U., and Margolis, R. K. (1997). Chond roitin sulfate proteoglycans as m ed iators of axon grow th and pathfinding. Cell Tissue Res. 290(2), 343-8. Marrone, D. F., LeBoutillier, J. C., and Petit, T. L. (2004b). Com parative analyses of synap tic d ensities d uring reactive synaptogenesis in the rat d entate gyrus. Brain Res. 996, 19-30. Matsui, F., and Oohira, A. (2004). Proteoglycans and injury of the central nervous system . Congenit. A nom. (Kyoto) 44, 181-188. Matthew s, D. A., Cotm an, C. and Lynch, G. (1976a). An electron m icroscopic stud y of lesion-ind uced synaptogenesis in the dentate gyrus of the ad ult rat. I. Magnitud e and tim e course of d egeneration. Brain Res. 115(1), 1-21. Matthew s, D. A., Cotm an, C., and Lynch, G. (1976b). An electron m icroscopic stud y of lesion-ind uced synaptogenesis in the dentate gyrus of the ad ult rat. II. Reappearance of m orphologically norm al synaptic contacts. Brain Res., 115, 23-41. Mayer, J., H am el, M. G., and Gottschall, P. E. (2005). Evid ence for proteolytic cleavage of brevican by the ADAMTSs in the d entate gyrus after excitotoxic lesion of the m ouse entorhinal cortex. BM C N eurosci. 6, 52. Miled i, R., and Slater, C. R. (1968). Electrophysiology and electron -m icroscopy of rat neurom uscular junctions after nerve d egeneration. Proc. R. Soc. Lond. B. Biol. Sci. 169(16), 289-306. Milligan, E. D., and Watkins, L. R. (2009). Pathological and protective roles of glia in chronic pain. N at. Rev. N eurosci. 10, 23-36. Ming, G., and Song, H . (2011). Ad ult neurogenesis in the m amm alian brain: significant answ ers and significant questions. N euron 70(4), 687-702. 93 Mitchell, B. D., Emsley, J. G., Magavi, S. S., Arlotta, P., Macklis, J. D. (2004). Constitutive and ind uced neurogenesis in the ad ult m am m alian brain: m anipulation of e nd ogenous precursors tow ard CN S repair. Dev. N eurosci. 26(2-4), 101-17. Möller, J. C., Klein, M. A., H aas, S., Jones, L. L., Kreutzberg, G. W., Raivich, G. (1996). Regulation of thrombospond in in the regenerating m ouse facial m otor nucleus. Glia 17(2), 121-32. Mostany, R., and Portera-Cailliau, C. (2011). Absence of large-scale d end ritic plasticity of layer 5 pyram id al neurons in peri-infarct cortex. J. N eurosci. 31(5), 1734-8. Mori, T., Buffo, A., and Götz, M. (2005). The novel roles of glial cells revisited : the contribution of rad ial glia and astrocytes to neurogenesis. Curr. Top. Dev. Biol. 69, 67-99. N ad ler, J. V., Cotm an, C. W., and Lynch, G. S. (1977a). H istochemical evidence of altered d evelopm ent of cholinergic fibers in the rat d entate gyrus follow ing lesions. I. Tim e course after com plete unilateral entorhinal lesion at various ages. J. Comp. N eurol. 171(4), 561-87. N ad ler, J. V., Cotm an, C. W., Paoletti, C., Lynch, G. S. (1977b). H istochem ical evidence of altered d evelopm ent of cholinergic fibers in the rat d entate gyrus follow ing lesions. II. Effects of partial entorhinal and sim ultaneous multiple lesions. J. Comp. N eurol. 171(4), 589604. N agy, V., Bozd agi, O., Matynia, A., Balcerzyk, M., Okulski, P., Dzw onek, J., Costa, R. M., Silva, A. J., Kaczm arek, L., H untley, G. W. (2006). Matrix m etalloproteinase-9 is required for hippocam pal late-phase long-term potentiation and m em ory. J. N eurosci. 26, 1923–1934. N eum ann, H ., Kotter, M. R., and Franklin, R. J. (2009). Debris clearance by m icroglia: an essential link betw een degeneration and regeneration. Brain 132(2), 288-95. N itsch, R., and Frotscher, M. (1992). Red uction of posttraum atic transneuronal "early gene" activation and d end ritic atrophy by the N -m ethyl-D-aspartate receptor antagonist MK801. Proc. N atl. A cad. Sci. USA 89(11), 5197-200. Oohira, A., Matsui, F., and Katoh-Sem ba, R. (1991). Inhibitory effects of brain chond roitin sulfate proteoglycans on neurite outgrow th from PC12D cells. J. N eurosci. 11(3), 822-7. Overstreet, L. S., H entges, S. T., Bum aschny, V. F., d e Souza, F. S., Sm art, J. L., Santangelo, A. M., Low , M. J., Westbrook, G. L., Rubinstein, M. (2004). A transgenic m arker for new ly born granule cells in d entate gyrus. J. N eurosci. 24(13), 3251-9. Overstreet-Wadiche, L., Brom berg, D. A., Bensen, A. L., Westbrook, G. L. (2005). GABAergic signaling to new born neurons in d entate gyrus. J. N europhysiol. 94(6), 4528-32. Overstreet-Wadiche, L. S., and Westbrook, G. L (2006). Functional maturation of ad ultgenerated granule cells. Hippocampus 16(3), 208-15. 94 Parent, J. M. (2003). Injury-ind uced neurogenesis in the ad ult m am malian brain. N euroscientist 9(4), 261-72. Parent, J. M. (2007). Ad ult neurogenesis in the intact and epileptic d entate gyrus. Prog. Brain Res. 163, 529-40. Parent, J. M., Elliott, R. C., Pleasure, S. J., Barbaro, N . M., Low enstein, D. H . (2006). Aberrant seizure-ind uced neurogenesis in experim ental tem poral lobe epilepsy. A nn. N eurol. 59(1), 81-91. Parent, J. M., and Murphy, G. G. (2008). Mechanism s and functional significance of aberrant seizure-ind uced hippocam pal neurogenesis. Epilepsia 49 Suppl 5, 19-25. Parent, J. M., Vexler, Z. S., Gong, C., Derugin, N ., Ferriero, D. M. (2002). Rat forebrain neurogenesis and striatal neuron replacem ent after focal stroke. A nn. N eurol. 52(6), 802-13. Parnavelas, J. G., Lynch, G., Brecha, N ., Cotm an, C. W., Globus, A. (1974). Spine loss and regrow th in hippocam pus follow ing d eafferentation. N ature 248(443), 71-3. Pearlm an, A. L., and Sheppard , A.M. (1996). Extracellular m atrix in early cortical d evelopm ent. Prog. Brain Res. 108, 117–134. Pered eriy, J. V., Luikart, B. W., Washburn, E. K., Schnell, E., Westbrook, G. L. (in revision). N eural injury triggers a biphasic response in ad ult-generated neurons in the d entate gyrus. J. N eurosci. Pesold , C., Im pagnatiello, F., Pisu, M.G., Uzunov, D.P., Costa, E., Guid otti, A., Caruncho, H .J., (1998). Reelin is preferentially expressed in neurons synthesizing gamm a am inobutyric acid in cortex and hippocam pus of ad ult rats. Proc. N atl. A cad. Sci. U. S. A . 95, 3221-3226. Peterson, G. M. (1994). Sprouting of central norad renergic fibers in the d entate gyrus follow ing com bined lesions of its entorhinal and septal afferents. Hippocampus 4(6), 635-48. Phinney, A. L., Calhoun, M. E., Wood s, A. G., Deller, T., Jucker, M. (2004). Stereological analysis of the reorganization of the d entate gyrus follow ing entorhinal cortex lesion in m ice. Eur. J. N eurosci. 19(7), 1731-40. Raineteau, O., and Schw ab, M. E. (2001). Plasticity of m otor system s after incom plete spinal cord injury. N at. Rev. N eurosci. 2(4), 263-73. Ram irez, J. J. (2001). The role of axonal sprouting in functional reorganization after CN S injury: lessons from the hippocam pal form ation. Restor. N eurol. N eurosci. 19(3-4), 237-62. Rauch, U. (1997). Mod eling an extracellular environm ent for axonal pathfind ing and fasciculation in the central nervous system . Cell Tissue Res. 290, 349–356. 95 Reeves, T. M., and Stew ard O. (1988). Changes in the firing properties of neurons in the d entate gyrus w ith d enervation and reinnervation: im plications for behavioral recovery. Exp. N eurol. 102(1), 37-49. Rickm ann, M., Amaral, D. G., and Cow an, W. M. (1987). Organization of rad ial glial cells d uring the d evelopm ent of the rat d entate gyrus. J. Comp. N eurol. 264, 449479. Rihn, L. L., and Claiborne, B. J. (1990). Dend ritic grow th and regression in rat d entate granule cells d uring late postnatal d evelopm ent. Dev. Brain Res. 54, 115-124. Rolls, A., Shechter, R., Lond on, A., Segev, Y., Jacob-H irsch, J., Am ariglio, N ., Rechavi, G., Schw artz, M. (2008). Tw o faces of chond roitin sulfate proteoglycan in spinal cord repair: a role in microglia/ m acrophage activation. PLoS M ed. 5(8), e171. Rothstein, J. D., Dykes-H oberg, M., Pard o, C. A., Bristol, L. A., Jin, L., Kunci, R. W., Kanai, Y., H ed iger, M. A., Wang, Y., Schielke, J. P., Welty, D. F. (1996). Knockout of glutam ate transporters reveals a m ajor role for astroglial transport in excitotoxicity and clearance of glutam ate. N euron 16, 675-686. Scharfm an, H . E., Goodm an, J. H ., and Sollas, A.L. (2000). Granule-like neurons at the hilar/ CA3 bord er after status epilepticus and their synchrony w ith area CA3 pyram id al cells: functional im plications of seizure-ind uced neurogenesis. J. N eurosci. 20, 6144-58. Schauw ecker, P. E., and McN eill, T. H . (1995). Enhanced but d elayed axonal sprouting of the com missural/ associational pathw ay follow ing a com bined entorhinal cortex/ fimbria fornix lesion. J. Comp. N eurol. 351(3), 453-64. Schauw ecker, P. E., and McN eill, T. H . (1996). Dend ritic rem od eling of dentate granule cells follow ing a combined entorhinal cortex/ fimbria fornix lesion. Exp. N eurol. 141(1), 145-53. Schm id t-H ieber, C., Jonas, P., and Bischofberger, J. (2004). Enhanced synaptic plasticity in new ly generated granule cells of the ad ult hippocam pus. N ature 429(6988), 184-7. See J, Bonner J, N euhuber B, Fischer I. (2010). N eurite outgrow th of neural progenitors in presence of inhibitory proteoglycans. J. N eurotrauma. 27(5), 951-7. Seri, B., Garcia-Verd ugo, J.M., McEw en, B.S., Alvarez-Buylla, A. (2001). Astrocytes give rise to new neurons in the ad ult m am m alian hippocam pus. J. N eurosci., 21, 7153-7160. Sierra, A., Encinas, J. M., Deud ero, J. J., Chancey, J. H ., Enikolopov, G., OverstreetWad iche, L. S., Tsirka, S. E., Maletic-Savatic, M. (2010). Microglia shape adult hippocam pal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7(4), 483-95. Silver, J., and Miller, J. H . (2004). Regeneration beyond the glial scar. N at. Rev. N eurosci. 5, 146–156. 96 Sim bürger, E., Plaschke, M., Fritschy, J. M., N itsch, R. (2001). Localization of tw o m ajor GABA(A) receptor subunits in the d entate gyrus of the rat and cell type-specific up regulation follow ing entorhinal cortex lesion. N euroscience 102(4), 789-803. Sim bürger, E., Plaschke, M., Kirsch, J., N itsch , R. (2000). Distribution of the receptoranchoring protein gephyrin in the rat d entate gyrus and changes follow ing entorhinal cortex lesion. Cereb. Cortex 10(4), 422-32. Skutella, T., and N itsch, R. (2001). N ew m olecules for hippocam pal d evelopm ent. Trends N eurosci. 24(2), 107-13. Sofroniew , M. V. (2009). Molecular d issection of reactive astrogliosis and glial scar form ation. Trends N eurosci. 32(12), 638-47. Sofroniew , M. V., and Vinters, H . V. (2010). Astrocytes: biology and pathology. A cta N europathol. 119(1), 7-35. Soriano, E. and Del Río, J. A. (2005). The cells of Cajal–Retzius: still a m ystery one century after. N euron 46, 389–394. Soriano, E., and Frotscher, M. (1994). Mossy cells of the rat fascia d entata are glutam ate im m unoreactive. Hippocampus 4(1), 65-9. Sotelo, C. (1990). Cerebellar synaptogenesis: w hat w e can learn from m utant m ice. J. Exp. Biol. 153, 225–279. Stanfield , B. B., and Cow an, W. M. (1979). The m orphology of the hippocam pus and d entate gyrus in norm al and reeler mice. J. Comp. N eurol. 185, 393-422. Stanfield , B. B., and Cow an, W. M. (1982). The sprouting of septal afferents to the d entate gyrus after lesions of the entorhinal cortex in ad ult rats. Brain Res. 232(1), 162-70. Stew ard , O. (1976). Reinnervation of d entate gyrus by hom ologous afferents follow ing entorhinal cortical lesions in ad ult rats. Science 194(4263), 426-8. Stew ard , O. (1992). Signals that ind uce sprouting in the central nervous system : sprouting is d elayed in a strain of m ouse exhibiting d elayed axonal d egeneration. Exp. N eurol. 118(3), 340-51. Stew ard , O., Cotm an, C. W., and Lynch, G. S. (1973). Re-establishment of electrophysiologically functional entorhinal cortical input to the dentate gyrus d eafferented by ipsilateral entorhinal lesions: innervation by the contr alateral entorhinal cortex. Exp. Brain Res. 18(4), 396-414. Stew ard , O., and J. A. Messenheim er (1978). H istochem ical evid ence for a postlesion reorganization of cholinergic afferents in the hippocam pal form ation of the m ature cat. J. Comp. N eurol. 178, 697-710. 97 Stew ard , O., and Vinsant, S. L. (1983). The process of reinnervation in the dentate gyrus of the ad ult rat: A quantitative electron m icroscopic analysis of term inal proliferation and reactive synaptogenesis. J. Comp. N eurol. 214(4), 370-386. Suh, H ., Deng, W., and Gage, F. H . (2009). Signaling in ad ult neurogenesis. A nnu. Rev. Cell Dev. Biol. 25, 253-75. Supèr, H ., Martínez, A., Del Río, J. A., and Soriano, E. (1998). Involvem ent of d istinct pioneer neurons in the form ation of layer-specific connections in the hippocam pus. J. N eurosci. 18, 4616-4626. Supèr, H ., and Soriano, E. (1994). The organization of the embryonic and early postnatal m urine hippocam pus. II. Developm ent of entorhinal, com m issural, and septal connections stud ied w ith the lipophilic tracer DiI. J. Comp. N eurol. 344(1), 101-20. Sutula, T. P., and Dud ek, F. E. (2007). Unm asking recurrent excitation generated by m ossy fiber sprouting in the epileptic d entate gyrus: an em ergent property of a com plex system . Prog. Brain Res. 163, 541-63. Sw anson, R. A., Ying, W., and Kauppinen, T. M. (2004). Astrocyte influences on ischem ic neuronal d eath. Curr. M ol. M ed. 4, 193-205. Takano, T., Kang, J., Jaisw al, J. K., Sim on, S. M., Lin, J. H ., Yu, Y., Li, Y., Yang, J., Dienel, G., Zielke, H . R., N ed ergaard , M. (2005). Receptor-med iated glutam ate release from volum e sensitive channels in astrocytes. Proc. N atl. A cad. Sci. USA 102, 16466-16471. Tashiro, A., Makino, H ., and Gage, F. H . (2007). Experience-specific functional m od ification of the d entate gyrus through ad ult neurogenesis: a critical period d uring an im m ature stage. J. N eurosci. 27(12), 3252-9. Tashiro, A., Sand ler, V. M., Toni, N ., Zhao, C., Gage, F. H . (2006). N MDA-receptorm ed iated , cell-specific integration of new neurons in ad ult d entate gyr us. N ature 442, 929933. Thon, N ., H aas, C. A., Rauch, U., Merten, T., Fässler, R., Frotscher, M., Deller T. (2000). The chond roitin sulphate proteoglycan brevican is upregulated by astrocytes after entorhinal cortex lesions in ad ult rats. Eur. J. N eurosci. 12(7), 2547-58. Tian, G. F., Azmi, H ., Takano, T., Xu, Q., Peng, W., Lin, J., Oberheim , N ., Lou, N ., Wang, X., Zielke, H . R., Kang, J., N ed ergaard , M. (2005). An astrocytic basis of epilepsy. N at. M ed. 11, 973–981. Tissir, F., and Goffinet, A.M. (2003). Reelin and brain d evelopm ent. N at. Rev. N eurosci. 4, 496–505. Tuszynski, M.H ., and Stew ard , O. (2012). Concepts and m ethod s for the stud y of axonal regeneration in the CN S. N euron 74, 777-791. 98 Ullian, E. M., Christopherson, K. S., and Barres, B. A. (2004). Role for glia in synaptogenesis. Glia 47(3), 209-16. van Groen, T., Kadish, I., Wyss, J. M. (2002). Species d ifferences in the projections from the entorhinal cortex to the hippocam pus. Brain Res. Bull. 57(3-4), 553-6. van Groen, T., Miettinen, P., and Kadish, I. (2003). The entorhinal cortex of the m ouse: organization of the projection to the hippocam pal form ation. Hippocampus 13(1), 133-49. van Praag, H ., Kem perm ann, G., and Gage, F. H . (1999). Running increases cell proliferation and neurogenesis in the ad ult m ouse d entate gyrus. N at. N eurosci. 2(3), 26670. van Praag, H ., Schind er, A. F., Christie, B. R., Toni, N ., Palm er, T. D., Gage, F. H . (2002). Functional neurogenesis in the ad ult hippocam pus. N ature 415(6875), 1030-4. Vargas, M. E., and Barres, B. A. (2007). Why is Wallerian d egeneration in the CN S so slow ? A nnu. Rev. N eurosci. 30, 153-79. Vlachos, A., Bas Orth, C., Schneid er, G., Deller, T. (2012a). Tim e-lapse im aging of granule cells in m ouse entorhino-hippocam pal slice cultures reveals changes in spine stability after entorhinal d enervation. J. Comp. N eurol. 520(9), 1891-902. Vuksic, M., Del Turco, D., Vlachos, A., Schuldt, G., Müller, C. M., Schneid er, G., Deller, T. (2011). Unilateral entorhinal d enervation lead s to long -lasting d end ritic alterations of m ouse hippocam pal granule cells. Exp. N eurol. 230(2), 176-85. Warren, K. M., Reeves, T. M., and Phillips, L. L. (2012). MT5-MMP, ADAM-10, and N cad herin act in concert to facilitate synapse reorganization after traum atic brain injury. J. N eurotrauma. 29(10), 1922-40. Weiss, K. H ., Johanssen, C., Tielsch, A., H erz, J., Deller, T., Frotscher, M., Förster, E. (2003). Malform ation of the rad ial glial scaffold in the d entate gyrus of reeler m ice, scram bler m ice, and ApoER2/ VLDLR-d eficient m ice. J. Comp. N eurol., 460, 56-65. Wenzel, H . J., Robbins, C. A., Tsai, L. H . & Schw artzkroin, P. A. (2001). Abnorm al m orphological and functional organization of the hippocam pus in a p35 mutant m od el of cortical displasia associated w ith spontaneous seizures. J. N eurosci. 21, 983-998. Wilhelm sson, U., Bushong, E. A., Price, D. L., Smarr, B. L., Phung, V., Terad a, M., Ellism an, M. H ., Pekny, M. (2006). Red efining the concept of reactive astrocytes as cells that rem ain w ithin their unique d om ains upon reaction to injury . Proc. N atl. A cad. Sci. USA 103, 17513-17518. William s, C. A., and Lavik, E. B. (2009). Engineering the CN S stem cell m icroenvironment. Regen. M ed. 4(6), 865-77. 99 Wilson, M. T., and Snow , D. M. (2000). Chond roitin sulfate proteoglycan expression pattern in hippocam pal d evelopm ent: potential regulation of axon tract form ation. J. Comp. N eurol. 424(3), 532-46. Xu, J., Xiao, N ., and Xia, J. (2010). Throm bospondin 1 accelerates synaptogenesis in hippocam pal neurons through neuroligin 1. N at. N eurosci. 13, 22-24. Zeng, L. H ., Xu, L., Rensing, N . R., Sinatra, P. M., Rothm an, S. M., Wong, M. (2007). Kainate seizures cause acute d end ritic injury and actin d epolym erization in vivo. J. N eurosci. 27(43), 11604-13. Zhao, S., Chai, X., Förster, E., Frotscher, M. (2004). Reelin is a positional signal for the lam ination of d entate granule cells. Development, 131, 5117-5125. Zhao, S., Förster, E., Chai, X., Frotscher, M. (2003).Different signals control lam inar specificity of com missural and entorhinal fibers to the d entate gyrus. J. N eurosci. 23, 73517357. Zhao, R. R., Muir, E. M., Alves, J. N ., Rickm an, H ., Allan, A. Y., Kw ok, J. C., Roet, K. C., Verhaagen, J., Schneid er, B. L., Bensad oun, J. C., Ahm ed, S. G., Yáñez-Muñoz, R. J., Keynes, R. J., Faw cett, J. W., Rogers, J. H . (2011). Lentiviral vectors express chond roitinase ABC in cortical projections and prom ote sprouting of injured corticospinal axons . J. N eurosci. M ethods. 201(1), 228-38. Zim m er, J., Laurberg, S., and Sund e, N . (1986). N on -cholinergic afferents determ ine the d istribution of the cholinergic septohippocam pal projection: a stud y of the AChE staining pattern in the rat fascia dentata and hippocam pus after lesions, X-irrad iation, and intracerebral grafting. Exp. Brain Res. 64(1), 158-68. Zuo, J., H ernandez, Y. J., and Muir, D. (1998). Chond roitin sulfate proteoglycan w ith neurite-inhibiting activity is up -regulated follow ing peripheral nerve injury. J. N eurobiol. 34(1), 41-54. 100