* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fluorescent in situ hybridization technique for cell type identification
Human brain wikipedia , lookup
Subventricular zone wikipedia , lookup
Neural engineering wikipedia , lookup
Electrophysiology wikipedia , lookup
Neuroeconomics wikipedia , lookup
Neuroplasticity wikipedia , lookup
Single-unit recording wikipedia , lookup
Aging brain wikipedia , lookup
Neurogenomics wikipedia , lookup
Multielectrode array wikipedia , lookup
Cortical cooling wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Synaptic gating wikipedia , lookup
Metastability in the brain wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cerebral cortex wikipedia , lookup
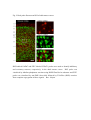
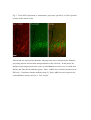
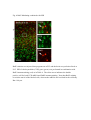
Fluorescent in situ hybridization technique for cell type identification and characterization in the central nervous system Akiya Watakabe*, a, b, Yusuke Komatsua, Sonoko Ohsawaa and Tetsuo Yamamoria, b a Division of Brain Biology, National Institute for Basic Biology, 38 Nishigonaka Myodaiji, Okazaki 444-8585, Japan b Department of Molecular Biomechanics, The Graduate University for Advanced Studies, 38 Nishigonaka Myodaiji, Okazaki 444-8585, Japan This is an author preprint version of the article published in “Methods”, by Elsevier. The article is available on line at Fluorescent In Situ Hybridization technique for cell type identification and characterization in the central nervous system Akiya Watakabe, Yusuke Komatsu, Sonoko Ohsawa and Tetsuo Yamamori Abstract Central nervous system consists of a myriad of cell types. In particular, many subtypes of neuronal cells, which are interconnected with each other, form the basis of functional circuits. With the advent of genomic era, there have been systematic efforts to map gene expression profiles by in situ hybridization (ISH) and enhancer-trapping strategy. To make full use of such information, it is important to correlate “cell types” to gene expression. Toward this end, we have developed highly sensitive method of fluorescent dual-probe ISH, which is essential to distinguish two cell types expressing distinct marker genes. Importantly, we were able to combine ISH with retrograde tracing and antibody staining including BrdU staining that enables birthdating. These techniques should prove useful in identifying and characterizing the cell types of the neural tissues. In this article, we describe the methodology of these techniques, taking examples from our analyses of the mammalian cerebral cortex. 1. Introduction Central nervous system consists of a myriad of cell types, including neurons, glias, endothelial cells, etc. On top of it, each cell type can be further subdivided into many different subtypes [4,27]. Considering that the neuronal circuit is an assembly of various neuronal types, the identification and characterization of each subtype is central to the understanding of the circuit [13]. Recently, systematic efforts to map gene expression in the brain, such as Allen Brain Atlas ([22]; http://www.brain-map.org/), GENSAT ([11]; http://www.gensat.org/index.html) and others (e.g., genepaint.org; http://www.genepaint.org/Frameset.html) have revealed many candidate marker genes for cell type identification. Obviously, certain genes are specifically expressed by particular subsets of neurons. But what are the common features of these neurons? How are they related to the classical neuronal subtypes defined by morphology, electrophysiological and pharmacological properties, antibody staining and connection specificity? What exactly is “cell type” of neurons? Our laboratory has been trying to identify the unique features of the primate neocortex using molecular biological techniques. Specifically, we have been searching for area- and/or layer-specific genes and using them as probes for comparative ISH analyses [37,41]. What we considered critical in these analyses was the identification of cell types, because, if we want to compare something across species, we need to compare the same thing. In the cerebral cortex, there are two fundamental cell types, excitatory and inhibitory neurons [23]. These two types can be unambiguously identified by expression of vesicular glutamate transporter 1 (VGluT1) and GABA or GABA synthesizing enzyme GAD, respectively [10,33]. The subtypes of inhibitory neurons can further be classified by expression of several well-known markers [4,7,17]. Because of such specific marker expression, antibody staining has been used extensively to histologically identify these neuronal subtypes. However, some proteins are not localized in the cell body (such as VGluT1) and difficult to be combined with ISH. Furthermore, there are many potentially good marker genes, whose expressions can be detected only by ISH due to lack of good antibodies. It is, therefore, desirable that we can perform dual-probe ISH, in which we can directly compare the mRNA expression of two genes simultaneously at cellular resolution. Conceptually, dual-probe ISH is similar to immunofluorescent double staining using two antibodies simultaneously. However, the former is often technically more demanding, because the copy number of mRNA molecules could be very low and often requires higher degree of amplification for visualization. The key for success depends on the method of signal amplification. Initially, the detection in ISH was done by using radioactive probes [25]. Then, non-radioactive method using haptens, such as biotin, digoxigenin (DIG), and fluorescein (FITC) for probe labeling became more popular. In a typical method, the hybridized DIG-labeled probe is detected by anti-DIG antibody conjugated with alkaline-phosphatase, which catalytically converts the hybridization signal to nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) precipitation. By using radioactive and non-radioactive probes for two genes, these methods can be combined for double labeling. Another way for dual probe ISH is to use different haptens to label two genes and detect them consecutively using the substrates with different colors for alkaline phosphatase reaction (e.g., see [21]). Although these and other methods of dual probe ISH have been used successfully for some purposes, most of the methods lacked the resolution and sensitivity comparable to the immunofluorescent double labeling. The only exceptions were those that used tyramide signal amplification (TSA) technique (e.g., [19,20,40]). TSA is one type of “CARD” or CAtalyzed Reporter Deposition technique [35], in which the horse radish peroxidase (HRP)-conjugated anti-hapten antibody catalyzes the deposition of another hapten, such as biotin, dinitrophenol (DNP), and various fluorescent moieties to its near vicinity. Once the hybridization signal is TSA-amplified, it can be converted to any fluorescent color (see Fig. 1). The fluorescent detection of alkaline-phosphatase activity using HNPP/Fast Red as substrate is also highly sensitive. Thus, at this point, we have several options to visualize the hybridization signals fluorescently. With such advancement at hand, ISH can now be combined with various other histological techniques. In this paper, we describe the TSA-based dual probe ISH method, which is useful to visualize diverse cell populations in the cerebral cortex and other brain regions. We also describe the method to combine fluorescent ISH with retrograde tracing, antibody staining and BrdU labeling. The identification of neuronal subtype is often enigmatic because of diversity of neuronal phenotypes. It is also often the case that a particular phonotype is not necessarily an all-or-none property and is a spectrum between 0 and 1. Thus, to identify and characterize neuronal subtypes, it is essential to define properties that are central to the “identity” of each neuron. The ISH-based characterization, combined with various other techniques, has a promise to clarify the complex issue of “cell type”. The protocols described here can be found in our past studies [19,38] and are also available at our website (http://www.nibb.ac.jp/brish/indexE.html). 2. Description of method 2.1 Overview There are many variations of the ISH protocol. The implementation of the fluorescent detection method described herein, however, should be applicable to most protocols. In this paper, we first describe the methodology of free-floating dual probe ISH, in which free floating sections are used for hybridization (chapter 2.2). We then describe an alternative protocol, in which cryostat sections on a slideglass are used (chapter 2.3). These two protocols use different procedures before the fluorescent detection steps, and are suitable for different types of tissues. But the fluorescent detection steps are essentially the same. In chapter 2.3, we also describe modification of the basic two-color ISH to triple ISH. In chapter 2.4, we will describe how we can combine the retrograde tracing with fluorescent ISH. We will explain the choice of the tracer for ISH and show typical results obtained by this method. Depending on the antibodies and antigens, it is also possible to combine ISH with immunohistochemsitry. In chapter 2.5, we will explain some of the successful examples, among which we will describe the method to combine BrdU birthdating with ISH. The reagents used in these protocols are listed in Table 5. 2.2 Free-floating dual probe ISH with fluorescent detection The original protocol for our free-floating ISH method came from the study by Liang et al. [24]. In this protocol, the perfusion fixed brain tissue is sliced to 15-50 µm sections. The sections are postfixed with paraformaldehyde, digested with proteinase K for probe penetration, acetylated and hybridized with DIG-labeled antisense RNA probe. After RNase treatment to remove non-specifically bound probes, followed by several washes, the hybridized DIG-probe is detected by anti-DIG antibody conjugated with alkaline-phosphatase, and visualized by NBT/BCIP color reaction. The free-floating sections are mounted on a slideglass for observation, after all these procedures. Generally speaking, free-floating method gives us better ISH images than the ISH done on slideglasses. But the latter method also provides nice results and will be described below. To perform dual probe ISH, FITC-labeled antisense RNA probe is used simultaneously with DIG-labeled probe in the hybridization solution. Instead of NBT/BCIP color reaction, DIG and FITC probes are fluorescently detected in the following manner (Fig. l). First, the FITC probe is detected by HRP-conjugated anti-FITC antibody for TSA-DNP reaction followed by detection with anti-DNP antibody conjugated with Alexa 488. Second, DIG probe is detected by alkaline-phosphatase conjugated anti-DIG antibody, in the same manner as single ISH, but we use HNPP/Fast Red as the substrate for red fluorescence. The step-by-step protocol for this dual probe ISH method is presented in Table 1. Depending on the abundance of the transcripts, and the cell-type specificity of mRNA expression, it can rival the immunofluorescent double labeling (see Fig. 2 for an example of dual probe ISH). Key parameters for fluorescent detection The key point of this protocol is the use of TSA. There are now many variations of TSA system, by which various haptens and fluorophores can be deposited. In our hands, TSA-Plus (DNP) system worked most consistently and sensitively for ISH. For abundant mRNAs, we had good results with TSA-biotin (use streptavidin-Cy2 for detection). In both systems, the extent of signal amplification is superb. However, because of the high degree of amplification, the background noises (scattered granular speckles) tend to be amplified to obscure the true hybridization signals. To avoid this, the quality of anti-FITC HRP antibody, as well as its titration is most critical. The HRP antibody of some vendors did not work well even after titration. 1/4000 dilution of the Jackson lab antibody (Table 5) generally gives good results. But when granular backgrounds are high, the titration of the HRP antibody needs to be empirically determined. The time for antibody binding also requires caution. 30 min incubation time recommended in the manufacturer’s protocol is too short to provide good signal-to-noise (S/N) ratio, even when the HRP antibody is titrated. The incubation time for TSA reaction also affects the result. Generally speaking, if the true hybridization signals are as strong as the background noises, the S/N ratio would be dramatically improved by changing the above mentioned parameters. When we perform dual probe ISH, there is a choice of which genes to label with DIG and FITC. Also, although the protocol uses anti-FITC-HRP for TSA reaction and anti-DIG alkaline phosphatase for HNPP/Fast Red, we can also use anti-DIG-HRP for TSA and anti-FITC alkaline phosphatase for HNPP/Fast Red reaction. The choice depends on the abundance of the genes to be tested. When the mRNA is abundant, TSA-labeling provides an image with higher S/N contrast than HNPP/Fast Red. However, due to granular nature of hybridization signals of TSA-staining, we feel that the overall sensitivity of detection is better for HNPP/FastRed staining than TSA-staining. As to the probe labeling, DIG label is, somehow, always better than FITC label. So, depending on the combination of the genes, we need to switch DIG/FITC label and TSA/HNPP Fast Red detection to get optimal staining. Regarding the HNPP/Fast Red staining, it is useful to keep in mind that the reaction products of HNPP/Fast Red easily get diffused. The mounting media is quite critical. CC/Mount (Diagnostic Biosystems, India) that we are using is compatible with HNPP/Fast Red, but we had bad luck with other mounting media including Fluoromount from the same manufacturer. Even when we use CC/Mount, we occasionally find the HNPP/Fast Red signals to diffuse for some unknown reason. In such cases, we repeat the reaction on the slideglass. Once we get good preparation, the red stain is stable for months, when stored frozen. Nevertheless, we recommend taking photos as soon as possible. Compared with TSA-staining, HNPP/Fast red staining exhibits uniform but high tissue background and requires thin section (15-20 µm) for good images. Confocal imaging will be required to process thicker sections. Key parameters of in situ hybridization in general The success of the dual-probe ISH, of course, depends on the quality of the ISH itself. Here we explain some of the important parameters for ISH in general. First, the quality of the tissue sections for ISH is important. Good perfusion fix with 4 % paraformaldehyde is required for optimal signals. Uneven perfusion due to clumsy manipulation during cardiac infusion can potentially result in uneven staining. The fixation solution could also contain picric acid and/or glutaraldehyde. We had good result with a brain fixed in 4% paraformaldehyde, 0.2% picric acid, 0.1% glutaraldehyde in 0.1M PB. The over-fixation, however, decreases the signals. Regarding the ISH conditions, there are two important parameters that need to be determined empirically. First, the concentration of the proteinase K greatly affects the strength of the ISH signals. Generally speaking, the higher, the concentration, the stronger, the signals. However, because the sections become very fragile after proteinase K treatment, we must determine the optimal condition that gives strong enough signals, but retains the integrity of the tissue sections. We routinely use 0.5 µg/ml proteinase K for adult mouse brain and 5 µg/ml for adult monkey brain, but this must be empirically determined. Second, the hybridization temperature needs to be adjusted for each probe. Practically speaking, most probes work nicely with 60 ℃ hybridization. If the probe is GC-rich and/or contain some unusual sequences, high background noise may appear. In such cases, raising the hybridization temperature to 65, 68 and 72 ℃ would help. About the probe We make antisense RNA probes by standard in vitro transcription using T3/T7/SP6 RNA polymerase. The standard length of the cDNA used for probe production is 500-1000 base pair. Although some other protocols suggest short RNA probes (~100 nt) for ISH, the penetration into the tissue section seems to be efficient up to 1000 nt in our protocol. However, long probes (>2000 nt) need to be degraded by alkaline hydrolysis for efficient hybridization in the floating in situ method (for ISH on slideglass, it is not necessary). Because the sensitivity of ISH increases with the length of the probe, we routinely make multiple probes covering different regions of the gene and use them mixed. When using multiple probes, it is necessary to confirm that each probe exhibits the same hybridization profile. Actually, this is a good method to confirm the specificity of hybridization. The specificity of hybridization is always a matter of concern in the ISH experiments. Non-specific hybridization could occur in the GC-rich region. There could also be cross-hybridization to similar sequences. Perform BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search at NCBI to check for cross hybridization with the family genes. Based on ISH experiments using mouse and monkey specific probes for the same gene, we think that sequence identity of 80 % is a reasonable line to judge the possibility of cross hybridization. It must be kept in mind, however, that cross-hybridization is not completely predictable. Control experiments by sense probe, comparison of multiple probes, and changing hybridization temperature are all useful to judge the credibility of the hybridization specificity. 2.3 Alternative protocol In this section, we cover two topics. First, we describe an alternative protocol for ISH, which is performed on a slideglass. Second, we describe TSA-biotin system, which can serve as an alternative to TSA-DNP system or can be used to add one more color to achieve triple ISH. ISH on a slideglass Although free-floating ISH provides excellent images for relatively “hard” adult brains, it is not suitable for “soft” brains of embryos and neonates. It is also not suitable to make serial sections of olfactory bulb, spinal cord and cerebellum, because of uniformity and small size. When the tissue sections are prepared on a slideglass by a cryostat, we use the protocol by Schaeren-Wiemers et al. [34] with slight modifications. Typically, the fresh dissected brain tissue is quick-frozen in Tissue-Tek OCT compound 4583 (Sakura Finetechnical Co. Ltd. Tokyo, Japan) using liquid nitrogen cooled 2-methylbutane isopentan and 10-20 µm sections are collected on a slideglass using cryostat. The tissue sections on a slideglass are postfixed in 4% paraformaldehyde/PBS, acetylated and hybridized with the antisense RNA probe at 72 ℃ overnight. After hybridization, the slideglasse is washed three times in 0.2xSSC at 72 ℃, blocked in 1 % blocking buffer and processed for DIG/FITC detection as in the floating method. The step-by-step protocol for this slideglass method is presented in Table 2. A characteristic feature of this method is that high S/N ratio and probe penetration is achieved by hybridization at high temperature (72 ℃). The procedure is simple and the sensitivity is good. Unless the tissue is “hard”, neither proteinase K nor RNAse treatment is required. Despite such simplicity, slideglass method provides excellent images of fluorescent dual probe ISH, although the probe does not penetrate as deep as the floating method and may be less sensitive. Using TSA-biotin system Although we had best results with TSA-Plus (DNP) system, the kit is very expensive. A more economical alternative is TSA-biotin system, in which biotin is deposited to the tissues. It can be used in place of TSA-Plus (DNP) and fluorescent detection can be done by incubation with streptavidin-Cy2 or other fluorescent reagents. TSA-biotin system can be easily made in the lab (see, for example [14]). Although TSA-biotin system is less sensitive and finicky compared with TSA-DNP system, we had good results for abundant transcript using the in-house reagents. Using different kinds of TSA-system, it is possible to perform triple-probe ISH [30,38]. Briefly, three genes are labeled with DIG, FITC and biotin and used for simultaneous hybridization. First, the FITC signal is converted to DNP by TSA-DNP as in dual-probe ISH. Subsequently, the bound antibody is removed by 0.1 M glycine-HCl (pH 2.2), 0.1 % Tween 20 as in [21]. The sections are blocked again in 0.5 % TNB, which is the blocking reagent that comes together with the TSA-DNP kit. The biotin-labeled probe is, then, detected by anti-biotin antibody, conjugated with HRP, and processed for TSA-biotin reaction. In the final visualization step, anti-DIG-alkaline phosphatase, anti-DNP-Alexa488 and streptavidin-Alexa350 are incubated with the sections and processed for HNPP/Fast Red reaction. For some unknown reason, the biotin-label detection sometimes fails. But if the protocol works, the co-expression of three genes can be determined. 2.4 Tracer ISH Invention of anterograde and retrograde tracers for tract-tracing greatly contributed to the elucidation of neural connectivity [18]. The retrograde tracer, in particular, provides an important link between cell type and connections. For example, if you inject a retrograde tracer into thalamus, a subset of neurons in layer 6 of the cerebral cortex are lit up (Fig. 3). These thalamic projecting neurons take up the tracer at their nerve terminals in the thalamus, and the tracer is transported back to the cell body. In the cerebral cortex, the projection specificity is a key property of a distinct class of neurons. For example, layer 5 neurons projecting to subcortical nuclei and contralateral cortex have been long recognized to exhibit differential morphology and electrophysiological properties [15,26]. Similarly, layer 6 neurons projecting to thalamus and claustrum have differential morphology and potentially distinct physiological properties [6,16,43]. These studies suggest that the projection specificity may be a key “identifier” of cortical cell type. Indeed, there is now evidence to believe that expression of certain genes is correlated with projection specificity [1,2,12,38,42]. The flourish of molecular information is now available in the literature and in public database. Of particular note are the large-scale ISH database of Allen Brain Institute (http://www.brain-map.org/), and BAC-transgenic database of GENSAT (http://www.gensat.org/index.html). Due to these systematic efforts and other molecular biological works (e.g., [27,29]), there are many candidate marker genes for cell-type identity. It is thus quite important that we can combine retrograde tracing with ISH. The success of tracer-ISH experiment depends on whether the tracer can withstand the rather harsh treatment of ISH or not. In our previous work, we have shown that FastBlue can be used for tracer-ISH experiment [38]. Because FastBlue shows blue fluorescence, it can be combined with single or double ISH without any changes in the protocol. However, the fluorescence of FastBlue diminishes considerably during ISH. Other choice of tracer includes FluoroGold and cholera toxin B fragment (CTB) conjugated with Alexa dyes. Not only can they withstand ISH treatment, their signals can be enhanced by antibody against FluoroGold or Alexa dyes after ISH. In Table 3, we show a model protocol for FluoroGold-ISH. In this protocol, the FluoroGold signal is enhanced by antibody detection and ISH signal is visualized by HNPP/Fast Red. The ISH detection step can be modified to provide green color by using TSA, if desired. In Fig. 3, we show an example of such tracer-ISH experiment. In this example, lack of Nurr1 mRNA in the corticothalamic layer 6 neurons is clearly demonstrated, consistent with the previous tracer-immunofluorescence study [2]. We believe that this kind of analyses is important for integrative understandings of the “cell type” in the cerebral cortex. 2.5 Combination with antibody staining (BrdU birthdating) There are many good antibodies that provide useful information about the anatomical structure of the neural tissues. Despite rather harsh tissue treatment of the ISH procedure, it is often possible to combine antibody staining with ISH. Although many good monoclonal antibodies (such as SMI32) were not suitable for immunofluorescence-ISH, we had success with some polyclonal antibodies such as those against paravalbumin and calbindin, among others. The success seems to depend on the nature of the antibody and there is not much we can control to enable immunostaining of the ISH processed samples. One of the few parameters that can be changed is the selection of the blocking reagent. 1 % Blocking Reagent (Roche Diagnostics) used for ISH is often too strong: skim milk or other reagents used for normal immunohistochemistry should provide better results for immunostaining. If the antibody of interest is not compatible with ISH, one possibility is to produce a compatible antibody de novo. Interestingly, Nakamura et al. succeeded in producing the anti-GFP antibody suitable for double staining with ISH by modifying the antigen preparation step for immunization [28]. Their method is worth considering if combining immunostaining with ISH is required. As a successful example of combining immunostaining with ISH, here we provide a protocol to combine BrdU birthdating with ISH. Because the specification of cortical neurons occurs according to the developmental timetable, labeling neurons in their final cell division at a particular embryonic period has been a useful technique to investigate corticogenesis. Originally, it was done by injecting 3H-thimidine to the pregnant animal and observing the incorporation of the radioactivity in the nuclei of the cortical neurons that were born at the time of injections [5,31]. Recently, injection of thymidine analog, BrdU (bromodeoxyuridine), followed by anti-BrdU immunostaining [9] has become a popular alternative. The same technique is used to examine the neurogenesis in the adult brain [3,9]. To combine BrdU birthdating with ISH, one obstacle was the denaturation step before anti-BrdU immunostaining, which is required to expose the BrdU incorporated into the chromosomal DNA. After this step, the hybridized probe and/or bound antibodies are released and lost from the site. To overcome this obstacle, we used the TSA system to convert the DIG hybridization signal to DNP, before the BrdU immunodetection step (Table 4). By this protocol, we succeeded in combining BrdU labeling with ISH (Fig. 4). It is also possible to perform the double staining by first colorizing the ISH signal to NBT/BCIP as in normal ISH and then perform BrdU staining. Either way, the correlation of birthdate and gene expression profile is an important information to consider “cell type” of the cerebral cortex. 3. Concluding remarks In the field of developmental neuroscience, it has been considered that the fate of cortical neurons is specified early during development to later acquire characteristic features of each “cell type” (e.g., [8,32,36,39]). Although recent studies are rapidly unraveling the molecular genetic mechanism of cortical cell type specification (for a review, [27]), the significance of each cell type within the cortical circuit still remains elusive. In a way, we still do not know what “cell type” exactly means [4,26,27,29,37]. By using the fluorescent ISH techniques that we described in this article, we will better understand the physiological meaning of gene expression, which will, in turn, help to elucidate normal neurological function. Acknowledgements We thank Drs. Fengyi Liang and Tsutomu Hashikawa for teaching us their floating in situ methods when we started our study. We also thank Dr. Takashi Kitsukawa for instructing the slideglass method. We thank helpful information of Drs. Hiroyuki Hioki and Takeshi Kaneko as well as Drs. Noritaka Ichinohe and Kathleen Rockland. Supported by the grant from the JSPS (KAKENHI19500304 and 22500300). Table 1: protocol for free-floating dual probe ISH <DAY1> Cut the section and postfix in 4% paraformaldehyde/0.1MPB 4℃ overnight <DAY2> 0.1M PB 10 min x 2 0.75% Glycine/0.1M PB 15 min x 2 0.3% Triton X100/0.1M PB 20 min 0.1M PB 5 min Proteinase K (0.5-5 µg/ml) in PK buffer 37℃ 30 min Acetylation Buffer 10 min 0.1M PB 10 min x 2 Hybridization sol. Hybridization sol.+RNA probes (0.5~1 µg/ml total) Denature the probe at 80 ℃ for 5 min before adding to Hybridization sol. 60℃ 1 hour 60℃ overnight <DAY3> 2xSSC/50% formamide/0.1% N-lauroilsarcosine (NLS) 60℃ 15~20 min x 2 RNase buffer 5 min RNase A (20 µg/ml) treatment in RNase buffer 37℃ 30 min 2xSSC /0.1% NLS 37℃ 15~20 min x 2 0.2xSSC/0.1% NLS 37℃ 15~20 min x 2 TS7.5 5 min ***fluorescent detection*** 1% Blocking reagent/TS7.5 30 min~1 hour 1/4000 anti-FITC-HRP in 1% Blocking reagent/TS7.5 2~5 hours or 4℃ overnight TNT 15 min x 3 TSA-Plus (DNP) 30 min TNT 10 min x 3 1/1000 anti-DIG-AP, 1/500 anti-DNP Alexa488 in 1% Blocking reagent/TS7.5 2~5 hours or 4℃ overnight <DAY4> TNT 15 min x 3 TS8.0 10 min HNPP/Fast Red in TS8.0 20~30min PBS-EDTA 3~5 minx2 Hoechst 33342 (1µg/ml) in PBS-EDTA 5 min PBS-EDTA 3~5 min x 2 Mount on slideglass and envelop in CC/Mount Table 2: protocol for dual probe ISH on a slideglass (Schaeren-Wiemers et al. 1993 [34]) <DAY1> Cut the section and save on a slideglass Can be stored at –80 ℃ 4% paraformaldehyde 10 min PBS 3 min x 3 (Proteinase K (0.5~5 µg/ml) in PK buffer)# 37 ℃ 30 min (4% paraformaldehyde)# 5 min For fixed tissue (PBS)# 3 min x 3 For fixed tissue Acetylation buffer 10 min (0.3% Triton X100/0.1M PB)# 20 min PBS 5 min x 3 SW Hybridization sol. Room temp briefly SW Hybridization sol.+RNA probe (0.5~1 µg/ml total) 72 ℃ overnight For fixed tissue For fixed tissue <DAY2> 0.2 x SSC 72 ℃ 30 min x 3 TS7.5 5 min ***fluorescent detection*** 1% Blocking reagent/TS7.5 1/4000 anti-FITC-HRP in 1% Blocking reagent/TS7.5 1hour 2~5 hours or 4℃ overnight TNT 10 min x 3 TSA-Plus (DNP) $ 3~10 min TNT 5 min x 3 1/1000 anti-DIG-AP, 1/500 anti-DNP-Alexa488 in 1% Blocking reagent/TS7.5 2~5 hours or 4℃ overnight <DAY4> TNT 10 min x 3 TS8.0 5 min HNPP/Fast Red in TS8.0 20~40min PBS-EDTA Hoechst 33342 (1µg/ml) in PBS-EDTA 3~5 minx2 PBS-EDTA 3~5 min x 2 5 min Envelop in CC/Mount # skip these procedures for non-fixed tissues. $ Dilute the already diluted TSA reagents two fold with distilled water. Don’t dry Table 3: protocol for Tracer-ISH (Fluoro Gold) <DAY1~3> Same as normal ISH before blocking The following is the protocol for DIG-labeled probe ***fluorescent detection*** 2% skim milk/TS7.5 1/500 anti-FluoroGold in 2% skim milk/TS7.5 30 min 2~5 hours or 4℃ overnight TNT 1/1000 anti-DIG-AP, 1/500 anti-rabbit-Cy2 in 1% Blocking reagent/TS7.5 15 min x 3 2~5 hours or 4℃ overnight <DAY4> TNT 15 min x 3 TS8.0 10 min HNPP/Fast Red in TS8.0 20~40min PBS-EDTA 3~5 minx2 Hoechst 33342 (1µg/ml) in PBS-EDTA 5 min PBS-EDTA 3~5 min x 2 Mount on slideglass and envelop in CC/Mount Table 4: protocol for BrdU-ISH <DAY1~3> Injection of BrdU solution into pregnant animals (50 mg/kg, i.p.) Same as normal ISH before blocking The following is the protocol for DIG-labeled probe ***fluorescent detection*** 1% Blocking reagent/TS7.5 1/2000 anti-DIG-HRP in 1% Blocking reagent/TS7.5 30 min-1hour 2~5 hours or 4℃ overnight TNT 15 min x 3 TSA-Plus (DNP) 30 min TNT 10 min x 3 1.5N HCl (for denaturation) 37℃ 30 min TNT 5 min x2 5% skim milk/PBST 20 min 1/75 anti-BrdU in 5% skim milk/PBST 4℃ overnight <DAY4> PBS 1/500 anti-rat-biotin in 5% skim milk/PBST 10min x 3 2~5 hours or 4℃ overnight TNT 10 min x 3 ABC kit (sol A:1/100, sol B:1/100, in TNT) 30 min TNT 10 min x3 2~5 hours or 4℃ overnight 1/500 Streptavidin-Cy3, 1/500 anti-DNP-Alexa488 in 5% skim milk/PBST TNT Mount on slideglass and envelop in CC/Mount 10 min x 3 Table 5: Reagents list <Buffers> PK buffer 0.1 M Tris.HCl (pH8.0), 50 mM EDTA Acetylation Buffer 0.1 M triethanolamine 169.7 ml +0.3 ml HCl, Add 10 µl of Acetic anhydride per 4 ml just before use Hybridization sol. Mix the following solutions: 20xSSC; 12.5 ml, 10 % Blocking reagent; 10 ml, Formamide; 25 ml, 2% N-lauroylsarcosine; 2.5 ml, 10 % SDS; 0.5 ml SW Hybridization sol. 5xSSC, 50 % Formamide, 5xDenhardt’s, 250 µg/ml yeast tRNA, 500 µg/ml salmon sperm DNA RNase buffer 10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.5 M NaCl TS7.5 0.1 M Tris-HCl, pH7.5, 0.15 M NaCl TS8.0 0.1 M Tris-HCl. PH 8.0, 0.1 M NaCl , 10 mM MgCl2 TNT TS7.5, 0.05% Tween20 PBS-EDTA PBS with 10 mM EDTA BrdU solution (20 mg/ml) BrdU (sigma#B5002) in 0.007 N NaOH, 0.9% NaCl PBST PBS with 0.2 % TritonX100 <Reagents> Proteinase K Roche Diagnostics: Proteinase K #3115887 Blocking Reagent Roche Diagnostics: Blocking Reagent #11 096 176 001 anti-FITC-HRP Jackson ImmunoResearch laboratory: Peroxidase-IgG Fraction Monoclonal Mouse Anti-FITC #200-032-037 TSA-Plus (DNP) Perkin Elmer: #NEL747A: Make working solution as instructed HNPP/Fast Red Roche Diagnostics: #1 758 888: Make working solution as instructed anti-DIG-AP Roche Diagnostics: Anti-Digoxigenin-AP Fab fragments 11 093 274 910 anti-DNP Alexa488 Molecular Probe: #A-11097 Hoechst 33342 Dojindo: 346-07951 CC/Mount Diagnostic Biosystems: #K002 FluoroGold Biotium, Inc: #80014 Anti-FluoroGold Chemicon: AB153 rabbit anti-Fluorogold Polyclonal antibody Anti-BrdU Abcam: BrdU antibody [BU1/75 (ICR1)] Elite ABC kit Vectastain: PK-6100 Streptavidin-Cy3 Jackson ImmunoResearch laboratory: #016-160-084 Fig.1 The scheme for Fluorescent double ISH (Top panel) Digoxigenin (DIG) and FITC (fluorescein)-labeled antisense RNA is hybridized simultaneously to the target mRNAs. (Middle panel) FITC is recognized by anti-FITC antibody conjugated to HRP (horse radish peroxidase), which catalyze TSA reaction, in which the free radical form of DNP-Tyramide reacts to be deposited to the nearby tissue. (Bottom panel) DIG signal is converted to red fluorescence by alkaline phosphatase activity of the anti-DIG-AP antibody. FITC signal is converted to green fluorescence by anti-DNP antibody conjugated to Alexa 488. Fig. 2 Dual probe fluorescent ISH of adult mouse cortex DIG-labeled GAD67 and FITC-labeled VGluT1 probes were used to identify inhibitory and excitatory neurons, respectively, in the adult mouse cortex. DIG probe was visualized by alkaline phophatase reaction using HNPP/Fast Red as substrate and FITC probe was visualized by anti-DNP Alexa 488 followed by TSA-Plus (DNP) reaction. Note complete segregation of these signals. Bar: 100µm. Fig. 3 Tracer-ISH experiment to demonstrate projection specificity of Nurr1-positive neurons in the mouse cortex FluoroGold was injected into thalamus, taken up at the nerve teminals by the thalamic projecting neurons and was then transported back to the cell body. In this photo, the thalamic projecting neurons in the cortex (corticothalamic neurons) were revealed after ISH by anti-FluoroGold antibody (green). Nurr1 mRNA was visualized by fluorescent ISH (red). Consistent with the antibody study [2], Nurr1 mRNA was not expressed in corticothalamic neurons in layer 6. Bar: 100µm. Fig. 4 BrdU bithdating combined with ISH BrdU solution was injected into pregnant rat at E15 and the brain was perfusion-fixed at P15. ISH of cholecystokinin (CCK) gene (green) was performed in combination with BrdU immunostaining (red) as in Table 4. The white arrow indicates the double positive cell for both CCK mRNA and BrdU immunostaining. Note that BrdU staining reveals the nuclei of the labeled cells, whereas the mRNAs are localized in the cell body. Bar: 100 µm. References [1] Y. Arimatsu, M. Ishida, Distinct neuronal populations specified to form corticocortical and corticothalamic projections from layer VI of developing cerebral cortex, Neuroscience 114 (2002) 1033-1045. [2] Y. Arimatsu, M. Ishida, T. Kaneko, S. Ichinose, A. Omori, Organization and development of corticocortical associative neurons expressing the orphan nuclear receptor Nurr1, J Comp Neurol 466 (2003) 180-196. [3] P. Arlotta, S.S. Magavi, J.D. Macklis, Induction of adult neurogenesis: molecular manipulation of neural precursors in situ, Ann N Y Acad Sci 991 (2003) 229-236. [4] G.A. Ascoli, L. Alonso-Nanclares, S.A. Anderson, G. Barrionuevo, R. Benavides-Piccione, A. Burkhalter, G. Buzsaki, B. Cauli, J. Defelipe, A. Fairen, D. Feldmeyer, G. Fishell, Y. Fregnac, T.F. Freund, D. Gardner, E.P. Gardner, J.H. Goldberg, M. Helmstaedter, S. Hestrin, F. Karube, Z.F. Kisvarday, B. Lambolez, D.A. Lewis, O. Marin, H. Markram, A. Munoz, A. Packer, C.C. Petersen, K.S. Rockland, J. Rossier, B. Rudy, P. Somogyi, J.F. Staiger, G. Tamas, A.M. Thomson, M. Toledo-Rodriguez, Y. Wang, D.C. West, R. Yuste, Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex, Nat Rev Neurosci 9 (2008) 557-568. [5] S.A. Bayer, J. Altman, Neocortical Development. Raven Press, 1991 [6] F. Briggs, Organizing principles of cortical layer 6, Front Neural Circuits 4 (2010) 3. [7] A. Burkhalter, Many specialists for suppressing cortical excitation, Front Neurosci 2 (2008) 155-167. [8] F. Clasca, A. Angelucci, M. Sur, Layer-specific programs of development in neocortical projection neurons, Proc Natl Acad Sci U S A 92 (1995) 11145-11149. [9] P.S. Eriksson, E. Perfilieva, T. Bjork-Eriksson, A.M. Alborn, C. Nordborg, D.A. Peterson, F.H. Gage, Neurogenesis in the adult human hippocampus, Nat Med 4 (1998) 1313-1317. [10] F. Fujiyama, T. Furuta, T. Kaneko, Immunocytochemical localization of candidates for vesicular glutamate transporters in the rat cerebral cortex, J Comp Neurol 435 (2001) 379-387. [11] S. Gong, C. Zheng, M.L. Doughty, K. Losos, N. Didkovsky, U.B. Schambra, N.J. Nowak, A. Joyner, G. Leblanc, M.E. Hatten, N. Heintz, A gene expression atlas of the central nervous system based on bacterial artificial chromosomes, Nature 425 (2003) 917-925. [12] A. Groh, H.S. Meyer, E.F. Schmidt, N. Heintz, B. Sakmann, P. Krieger, Cell-type specific properties of pyramidal neurons in neocortex underlying a layout that is modifiable depending on the cortical area, Cereb Cortex 20 (2010) 826-836. [13] M. Helmstaedter, C.P. de Kock, D. Feldmeyer, R.M. Bruno, B. Sakmann, Reconstruction of an average cortical column in silico, Brain Res Rev 55 (2007) 193-203. [14] A.H. Hopman, F.C. Ramaekers, E.J. Speel, Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for In situ hybridization using CARD amplification, J Histochem Cytochem 46 (1998) 771-777. [15] E.M. Kasper, A.U. Larkman, J. Lubke, C. Blakemore, Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets, J Comp Neurol 339 (1994) 459-474. [16] L.C. Katz, Local circuitry of identified projection neurons in cat visual cortex brain slices, J Neurosci 7 (1987) 1223-1249. [17] Y. Kawaguchi, Y. Kubota, GABAergic cell subtypes and their synaptic connections in rat frontal cortex, Cereb Cortex 7 (1997) 476-486. [18] C. Kobbert, R. Apps, I. Bechmann, J.L. Lanciego, J. Mey, S. Thanos, Current concepts in neuroanatomical tracing, Prog Neurobiol 62 (2000) 327-351. [19] Y. Komatsu, A. Watakabe, T. Hashikawa, S. Tochitani, T. Yamamori, Retinol-binding protein gene is highly expressed in higher-order association areas of the primate neocortex, Cereb Cortex 15 (2005) 96-108. [20] D. Kosman, C.M. Mizutani, D. Lemons, W.G. Cox, W. McGinnis, E. Bier, Multiplex detection of RNA expression in Drosophila embryos, Science 305 (2004) 846. [21] D. Kosman, S. Small, Concentration-dependent patterning by an ectopic expression domain of the Drosophila gap gene knirps, Development 124 (1997) 1343-1354. [22] E.S. Lein, M.J. Hawrylycz, N. Ao, M. Ayres, A. Bensinger, A. Bernard, A.F. Boe, M.S. Boguski, K.S. Brockway, E.J. Byrnes, L. Chen, L. Chen, T.M. Chen, M.C. Chin, J. Chong, B.E. Crook, A. Czaplinska, C.N. Dang, S. Datta, N.R. Dee, A.L. Desaki, T. Desta, E. Diep, T.A. Dolbeare, M.J. Donelan, H.W. Dong, J.G. Dougherty, B.J. Duncan, A.J. Ebbert, G. Eichele, L.K. Estin, C. Faber, B.A. Facer, R. Fields, S.R. Fischer, T.P. Fliss, C. Frensley, S.N. Gates, K.J. Glattfelder, K.R. Halverson, M.R. Hart, J.G. Hohmann, M.P. Howell, D.P. Jeung, R.A. Johnson, P.T. Karr, R. Kawal, J.M. Kidney, R.H. Knapik, C.L. Kuan, J.H. Lake, A.R. Laramee, K.D. Larsen, C. Lau, T.A. Lemon, A.J. Liang, Y. Liu, L.T. Luong, J. Michaels, J.J. Morgan, R.J. Morgan, M.T. Mortrud, N.F. Mosqueda, L.L. Ng, R. Ng, G.J. Orta, C.C. Overly, T.H. Pak, S.E. Parry, S.D. Pathak, O.C. Pearson, R.B. Puchalski, Z.L. Riley, H.R. Rockett, S.A. Rowland, J.J. Royall, M.J. Ruiz, N.R. Sarno, K. Schaffnit, N.V. Shapovalova, T. Sivisay, C.R. Slaughterbeck, S.C. Smith, K.A. Smith, B.I. Smith, A.J. Sodt, N.N. Stewart, K.R. Stumpf, S.M. Sunkin, M. Sutram, A. Tam, C.D. Teemer, C. Thaller, C.L. Thompson, L.R. Varnam, A. Visel, R.M. Whitlock, P.E. Wohnoutka, C.K. Wolkey, V.Y. Wong, M. Wood, M.B. Yaylaoglu, R.C. Young, B.L. Youngstrom, X.F. Yuan, B. Zhang, T.A. Zwingman, A.R. Jones, Genome-wide atlas of gene expression in the adult mouse brain, Nature 445 (2007) 168-176. [23] S. LeVay, Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations, J Comp Neurol 150 (1973) 53-85. [24] F. Liang, Y. Hatanaka, H. Saito, T. Yamamori, T. Hashikawa, Differential expression of gamma-aminobutyric acid type B receptor-1a and -1b mRNA variants in GABA and non-GABAergic neurons of the rat brain, J Comp Neurol 416 (2000) 475-495. [25] M.C. Loni, M. Green, Detection of viral DNA sequences in adenovirus-transformed cells by in situ hybridization, J Virol 12 (1973) 1288-1292. [26] Z. Molnar, A.F. Cheung, Towards the classification of subpopulations of layer V pyramidal projection neurons, Neurosci Res 55 (2006) 105-115. [27] B.J. Molyneaux, P. Arlotta, J.R. Menezes, J.D. Macklis, Neuronal subtype specification in the cerebral cortex, Nat Rev Neurosci 8 (2007) 427-437. [28] K.C. Nakamura, H. Kameda, Y. Koshimizu, Y. Yanagawa, T. Kaneko, Production and histological application of affinity-purified antibodies to heat-denatured green fluorescent protein, J Histochem Cytochem 56 (2008) 647-657. [29] S.B. Nelson, K. Sugino, C.M. Hempel, The problem of neuronal cell types: a physiological genomics approach, Trends Neurosci 29 (2006) 339-345. [30] M.V. Puig, A. Watakabe, M. Ushimaru, T. Yamamori, Y. Kawaguchi, Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors, J Neurosci 30 (2010) 2211-2222. [31] P. Rakic, Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition, Science 183 (1974) 425-427. [32] P. Rakic, A.E. Ayoub, J.J. Breunig, M.H. Dominguez, Decision by division: making cortical maps, Trends Neurosci 32 (2009) 291-301. [33] C.E. Ribak, Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase, J Neurocytol 7 (1978) 461-478. [34] N. Schaeren-Wiemers, A. Gerfin-Moser, A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes, Histochemistry 100 (1993) 431-440. [35] E.J. Speel, A.H. Hopman, P. Komminoth, Amplification methods to increase the sensitivity of in situ hybridization: play card(s), J Histochem Cytochem 47 (1999) 281-288. [36] Y. Tanabe, T.M. Jessell, Diversity and pattern in the developing spinal cord, Science 274 (1996) 1115-1123. [37] A. Watakabe, Comparative molecular neuroanatomy of mammalian neocortex: what can gene expression tell us about areas and layers?, Dev Growth Differ 51 (2009) 343-354. [38] A. Watakabe, N. Ichinohe, S. Ohsawa, T. Hashikawa, Y. Komatsu, K.S. Rockland, T. Yamamori, Comparative analysis of layer-specific genes in Mammalian neocortex, Cereb Cortex 17 (2007) 1918-1933. [39] J.M. Weimann, Y.A. Zhang, M.E. Levin, W.P. Devine, P. Brulet, S.K. McConnell, Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets, Neuron 24 (1999) 819-831. [40] M. Yamagata, J.A. Weiner, J.R. Sanes, Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina, Cell 110 (2002) 649-660. [41] T. Yamamori, K.S. Rockland, Neocortical areas, layers, connections, and gene expression, Neurosci Res 55 (2006) 11-27. [42] H. Yoneshima, S. Yamasaki, C.C. Voelker, Z. Molnar, E. Christophe, E. Audinat, M. Takemoto, M. Nishiwaki, S. Tsuji, I. Fujita, N. Yamamoto, Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices, Neuroscience 137 (2006) 401-412. [43] Y. Zhou, B.H. Liu, G.K. Wu, Y.J. Kim, Z. Xiao, H.W. Tao, L.I. Zhang, Preceding inhibition silences layer 6 neurons in auditory cortex, Neuron 65 (2010) 706-717.