* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Role of adiponectin in the regulation of carbohydrate and lipid
Butyric acid wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene nomenclature wikipedia , lookup
Biochemical cascade wikipedia , lookup
Gene expression wikipedia , lookup
Point mutation wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Paracrine signalling wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Gene regulatory network wikipedia , lookup
Lipid signaling wikipedia , lookup
Phosphorylation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Signal transduction wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Expression vector wikipedia , lookup
Biochemistry wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
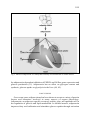
JOURNAL OF PHYSIOLOGY AND PHARMACOLOGY 2006, 57, Supp 6, 103113 www.jpp.krakow.pl J. KARBOWSKA, Z. KOCHAN ROLE OF ADIPONECTIN IN THE REGULATION OF CARBOHYDRATE AND LIPID METABOLISM Department of Biochemistry, Medical University of Gdansk, Gdansk, Poland Adiponectin, an adipocyte-derived plasma protein, has been shown to play an important role in the regulation of fatty acid and glucose metabolism. Adiponectin enhances fatty acid oxidation both in skeletal and cardiac muscle as well as in the liver, thus reducing triglyceride content in these tissues. Moreover, it stimulates glucose uptake by skeletal and cardiac muscle, and inhibits glucose production by the liver; consequently decreasing blood glucose levels. This review focuses on the molecular mechanisms underlying adiponectin effects on carbohydrate and lipid metabolism in skeletal muscle, cardiac muscle and liver. Key w o r d s : adiponectin, adiponectin receptor, glucose metabolism, fatty acid oxidation, muscle, heart, liver ADIPONECTIN, THE ADIPOCYTE-DERIVED HORMONE Adiponectin is an adipocyte-derived plasma protein which was identified by four research groups independently in the mid-1990s and was named AdipoQ, apM1 - adipose most abundant gene transcript 1, GBP28 - gelatin-binding protein, or Acrp30 - adipocyte complement-related protein 30 (1 - 4). Human adiponectin is encoded by the ADIPOQ gene (previously named APM1 or ACDC), which spans 17 kb on chromosome locus 3q27 (5, 6). Interestingly, human chromosome 3q27 has been identified as a region carrying susceptibility genes for type 2 diabetes and metabolic syndrome (7, 8). The gene for human adiponectin contains three exons, with the start codon in exon 2 and stop codon in exon 3 (5, 6). Adiponectin nevertheless, its is expressed expression and and secreted serum predominantly levels decrease by with adipose obesity tissue; and are 104 positively associated with whole-body insulin sensitivity (1, 9, 10). Moreover, weight loss significantly elevates plasma adiponectin levels (10). In the plasma of non-obese subjects adiponectin levels lie in the range from 2 to 17 µg/ml (9). Plasma adiponectin levels in men are significantly lower than in women among non-obese and obese subjects (9). Although the ADIPOQ gene is expressed mainly in adipocytes, recent studies have found that adiponectin gene expression can be induced in hepatocytes (11), in myotubes (12), and in skeletal muscle (13). Moreover, adiponectin expression occurs in bone-forming cells (14) and cardiomyocytes (15). REGULATION OF ADIPONECTIN GENE EXPRESSION Regulation of adiponectin gene expression remains to be elucidated. Several studies have shown that adiponectin is induced during adipocyte differentiation and its secretion is stimulated by insulin (1, 4, 16). It has also been observed that IGF-1 up-regulates adiponectin gene, whereas TNF- α and glucocorticoids decrease adiponectin gene transcription (16 - 18). Recently, a functional PPARresponsive element (PPRE) in the promoter region of the human adiponectin gene has been identified (19). Moreover, proliferator-activated receptor γ γ it has been shown that peroxisome (PPAR ), which is a well-known transcriptional activator of many adipocyte-specific genes, is required for adiponectin gene γ induction (19). Up-regulation of PPAR accompanied by an increase in gene expression in rat adipose tissue is adiponectin gene expression (20, 21). γ activators, thiazolidinediones (TZD), which are widely used Furthermore, PPAR to ameliorate insulin sensitivity and glucose tolerance in type 2 diabetes, increase adiponectin expression and secretion by adipocytes, elevating plasma adiponectin γ plays a levels (17, 22, 23). These experimental observations suggest that PPAR significant role in the transcriptional activation of adiponectin gene expression via the PPRE in its promoter; however, the exact mechanism of adiponectin gene induction needs further investigation. PROTEIN STRUCTURE OF ADIPONECTIN Secreted region adiponectin followed by a consists conserved of an N-terminal collagenous species-specific domain highly variable homologous in sequence to collagen VIII and collagen X, and a C-terminal globular domain which shows significant sequence similarity with the complement factor C1q (2, 24). Circulating adiponectin forms a wide range of multimers, including trimers, hexamers and high molecular weight (HMW) multimers (25, 26). The globular domains of adiponectin form homotrimers (24, 25). The crystal structure of the α adiponectin globular trimer reveals an unexpected homology to trimeric TNF- (24). Both proteins feature the ability to trimerise via key conserved hydrophobic residues. Higher order structures of adiponectin are formed through the collagen like region (24, 25). Non-reducing gel electrophoresis analysis of human plasma 105 demonstrated that HMW multimers of adiponectin are less abundant in male than in female subjects (25). These results suggest that not only the total adiponectin concentration but also multimer distribution are different in two genders. Moreover, Fruebis et al. showed the presence of a truncated form of adiponectin, containing only the globular head, in human plasma (27). Mutations in the ADIPOQ gene result in an impaired multimerization and/or impaired secretion of adiponectin from adipocytes, both linked to the development of insulin resistance and type 2 diabetes. ADIPONECTIN RECEPTORS A few years ago, Yamauchi et al. cloned two different isoforms of adiponectin receptor, AdipoR1 and AdipoR2 (28). Both isoforms are expressed in many cell types, including adipocytes (20, 28, 29). Since adiponectin receptors are expressed in fat cells, adiponectin may play an important role in the regulation of adipose tissue metabolism via autocrine and/or paracrine manner. In human tissues AdipoR1 is expressed mainly in skeletal muscle, whereas AdipoR2 is predominantly expressed in the liver (28). Moreover, it has been demonstrated that two types of adiponectin receptor have different binding affinity for globular and full-length adiponectin, AdipoR1 is a high-affinity receptor for globular adiponectin but a very low-affinity receptor for full-length adiponectin, whereas AdipoR2 is an intermediate affinity receptor for globular and full-length adiponectin (28). In vitro studies have revealed that both isoforms of adiponectin receptor can mediate increased AMP-activated protein kinase (AMPK) α activity by adiponectin binding, thus activating fatty phosphorylation and PPAR acid oxidation and glucose uptake (28). Transcriptional regulation of genes encoding adiponectin receptors has not yet been clarified. So far, it has been shown that in human macrophages adiponectin receptor gene expression is regulated by PPAR (30) and that the induction of AdipoR1 in adipose tissue is correlated with an increase in the expression of γ (20). These observations suggest that the AdipoR1 encoding gene may be PPAR regulated by PPARs. EFFECTS OF ADIPONECTIN ON CARBOHYDRATE AND LIPID METABOLISM IN SKELETAL MUSCLE Adiponectin has potent effects on carbohydrate and lipid metabolism in skeletal muscle (Fig. 1). Numerous studies have shown that treatment with the globular domain of adiponectin improves fatty acid utilization, both in isolated muscle as well as in cultured skeletal muscle cells (27, 31, 32). The action of adiponectin in muscle is mediated by adiponectin receptors, AdipoR1 and AdipoR2. In human skeletal muscle, AdipoR1 is expressed at the highest level, with lower levels of AdipoR2 (28). The binding of globular and full-length 106 Fig. 1. Effects of adiponectin on carbohydrate and fatty acid metabolism in skeletal muscle. α adiponectin to adiponectin receptors increased PPAR activity and stimulated glucose uptake and fatty acid oxidation in myocytes (28). The molecular mechanisms underlying adiponectin-dependent increase in muscle fatty acid oxidation include up-regulation of several genes involved in muscle lipid metabolism, such as fatty acid translocase (FAT/CD36); acyl-CoA oxidase (ACO), the rate-limiting enzyme of the β-oxidation pathway in peroxisomes (33); and mitochondrial uncoupling protein 2 (UCP2), accompanied α gene expression and increase in PPARα activity (28, α is required for transcription of many genes by the induction of PPAR 32). The nuclear receptor PPAR involved in fatty acid oxidation pathway (34), thus its activation by adiponectin may improve fatty acid utilization in muscle. Additional effect of adiponectin on skeletal muscle is an increased phosphorylation of AMP-activated protein kinase (AMPK) (28, 31). Moreover, activation of AMPK has been shown to be 107 necessary for adiponectin effects on fatty acid oxidation in skeletal muscle cells (27, 31, 35). AMPK activation triggers many metabolic changes that act to restore energy balance in muscle cells, such as increased glucose uptake and metabolism, and increased oxidation of fatty acids (36). Regulation of fatty acid oxidation pathway by AMPK involves phosphorylation of acetyl-CoA carboxylase (ACC), which leads to the inhibition of ACC activity followed by a decrease in malonylCoA levels (36). Adiponectin-dependent AMPK activation in skeletal muscle was associated with an increase in ACC phosphorylation and a decrease in the concentration of malonyl-CoA (28, 31). Malonyl-CoA is an allosteric inhibitor of carnitine palmitoyl transferase 1 (CPT-1), an enzyme responsible for the transport of fatty acids into mitochondria, where fatty acid oxidation occurs (37). Thus a decrease in malonyl-CoA concentration after adiponectin treatment may be the reason for increased fatty acid oxidation in muscle (31). In skeletal muscle, the pathways of glucose metabolism include glycogen synthesis, glucose oxidation and lactate production. Globular adiponectin has been reported to stimulate glucose transport both in isolated skeletal muscle as well as in cultured myocytes (31, 35, 38). Ceddia et al. have recently demonstrated that globular adiponectin increases glucose uptake into skeletal muscle cells via enhanced translocation of glucose transporter 4 (GLUT4) molecules to the cell membrane (39). Adiponectin also reduced the basal and insulin-stimulated rates of glycogen synthesis in muscle cells (39). Since AMPK can directly phosphorylate and inactivate glycogen synthase (36), the reduction of glycogen synthesis upon adiponectin treatment may be mediated by activation of AMPK. Moreover, it has been demonstrated that in adiponectin-treated myocytes glucose metabolism is shifted toward lactate production (39). ADIPONECTIN IS INVOLVED IN CARDIAC CARBOHYDRATE AND FATTY ACID METABOLISM Both isoforms of adiponectin receptor, AdipoR1 and AdipoR2, are expressed in the myocardium (Fig. 2). In recent years, Furuhashi et al. have provided indirect evidence demonstrating for that the serum binding of adiponectin adiponectin levels in to the these receptors coronary sinus by are significantly lower than in the aortic root (40). The gradient of adiponectin concentrations existing across the heart indicates that adiponectin binds to its receptors in the myocardium and/or accumulates in vascular walls. This finding, together with the observation that adiponectin receptors are expressed in the myocardium, suggests specific effects of adiponectin in the heart. Indeed, it has recently been reported that adiponectin treatment significantly enhances glucose and fatty acid uptake by cardiomyocytes (15). Moreover, adiponectin induces phosphorylation of AMPK in cultured cardiac myocytes (15, 41). Since AMPK is known to stimulate glucose uptake and translocation of the cardiomyocyte glucose transporter GLUT4 to the cell surface (42), it seems possible that the 108 Fig. 2. The expression of both isoforms of adiponectin receptor in rat myocardium. AdipoR1 and AdipoR2 gene expression was measured by the real-time RT-PCR in the myocardium of control (n=8) and clenbuterol-treated male Lewis rats (cardiac hypertrophy, n=10). adiponectin-dependent increase in glucose uptake in cardiomyocytes is mediated by activation of AMPK impairment of glucose exacerbation of heart (Fig. 3). Adiponectin metabolism, failure (43). deficiency insulin is resistance Interestingly, associated and AdipoR1, the with subsequent predominant isoform of adiponectin receptor in the myocardium, is induced during cardiac hypertrophy (Fig. 2); suggesting increased adiponectin signalling in this condition. Adiponectin is also involved in cardiac fatty acid metabolism. Treatment with the globular domain of adiponectin significantly increases fatty acid oxidation in the heart (26). It has been proposed that this effect is independent of AMPK activation (26); however, the exact mechanism by which adiponectin increases fatty acid oxidation in cardiac muscle remains to be elucidated. ADIPONECTIN MODULATES CARBOHYDRATE AND LIPID METABOLISM IN THE LIVER The liver, where adiponectin receptor AdipoR2 is expressed, is one of the adiponectin modulates target hepatic organs (28). carbohydrate Several and studies lipid have shown metabolism that (Fig. 4). adiponectin Long-term treatment with adiponectin improved insulin sensitivity and reduced triglyceride content in the liver (32). Adiponectin suppresses hepatic glucose production by down-regulation of phosphoenolpyruvate carboxykinase (PEPCK) and glucose6-phosphatase (G6Pase) gene expression, thus decreasing plasma glucose levels (35, 44, 45). An inhibitory effect of adiponectin on gluconeogenesis is probably mediated by AMPK phosphorylation. In the liver, AMPK activation is necessary 109 Fig. 3. Effects of adiponectin on carbohydrate and fatty acid metabolism in cardiac muscle. for adiponectin-dependent inhibition of PEPCK and G6Pase gene expression and glucose production (35). Adiponectin has no effect on glycogen content and synthesis, glucose uptake or glycolysis in the liver (44, 45). CONCLUSIONS Over recent years, adipose tissue has been shown to secrete a variety of protein factors and hormones involved in many aspects of organs physiology. Adiponectin, an adipocyte-specific secretory protein, plays an important role in the regulation of glucose and lipid metabolism. In skeletal muscle, adiponectin improves fatty acid utilization and stimulates glucose uptake through activation 110 Fig. 4. Effects of adiponectin on carbohydrate and lipid metabolism in the liver. of AMPK (27, 28, 31, 32). Since skeletal muscle accounts for 80-90% of the insulin-stimulated glucose disposal (46), adiponectin-induced increase in glucose uptake may be beneficial in subjects with type 2 diabetes. Adiponectin has also been shown to enhance glucose and fatty acid uptake by cardiomyocytes, and to increase fatty acid oxidation in the heart (15, 26). Moreover, in various animal models adiponectin inhibits hepatic glucose production (35, 44, 45). In general, adiponectin increases insulin sensitivity and improves glucose tolerance (27, 44). In humans, plasma adiponectin concentrations are positively correlated with whole-body insulin sensitivity (9). Recently, an association between adiponectin concentrations in plasma and risk of type 2 diabetes in apparently healthy individuals has been reported (47). Moreover, there is increasing evidence that genetic variants in the adiponectin gene itself and/or in genes encoding 111 adiponectin regulatory proteins, such γ as PPAR , are associated with hypoadiponectinaemia, insulin resistance and type 2 diabetes. These observations suggest that adiponectin is probably a major insulin-sensitising hormone secreted by adipose tissue and may play an important role in the prevention and treatment of diabetes through modulation of insulin sensitivity and direct regulation of lipid and glucose metabolism. Acknowledgements: This work was supported by the State Committee for Scientific Research Grant 3 P05A 098 23 and ST-41. REFERENCES 1. Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996; 271: 10697-10703. 2. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). Biochem Biophys Res Commun 1996; 221: 286-289. 3. Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 1996; 120: 803-812. 4. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270: 26746-26749. 5. Saito K, Tobe T, Minoshima S, et al. Organization of the gene for gelatin-binding protein (GBP28). Gene 1999; 229: 67-73. 6. Takahashi M, Arita Y, Yamagata K, et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes Relat Metab Disord 2000; 24: 861-868. 7. Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci USA 2000; 97: 14478-14483. 8. Vionnet N, Hani EH, Dupont S, et al. Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet 2000; 67: 1470-1480. 9. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999; 257: 79-83. 10. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000; 20: 1595-1599. 11. Yoda-Murakami M, Taniguchi M, Takahashi K, et al. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem Biophys Res Commun 2001; 285: 372-377. 12. Staiger H, Kausch C, Guirguis A, et al. Induction of adiponectin gene expression in human myotubes by an adiponectin-containing HEK293 cell culture supernatant. Diabetologia 2003; 46: 956-960. 13. Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology 2004; 145: 5589-5597. 14. Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in boneforming cells. Bone 2004; 35: 842-849. 112 15. Pineiro R, Iglesias MJ, Gallego R, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 2005; 579: 5163-5169. 16. Halleux CM, Takahashi M, Delporte ML, et al. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun 2001; 288: 1102-1107. 17. Maeda N, Takahashi M, Funahashi T, et al. PPAR gamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001; 50: 2094-2099. 18. Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2002; 290: 1084-1089. 19. Iwaki M, Matsuda M, Maeda N, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 2003; 52: 1655-1663. 20. Karbowska J, Kochan Z. Effect of DHEA on endocrine functions of adipose tissue, the γ involvement of PPAR . Biochem Pharmacol 2005; 70: 249-257. 21. Karbowska J, Kochan Z. Weight cycling increases adiponectin and resistin gene expression in adipose tissue: the effect of nuclear receptors. Eur J Biochem 2004; 271 (Suppl 1): 233. 22. Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002; 51: 2968-2974. 23. Combs TP, Wagner JA, Berger J, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPAR γ agonists: a potential mechanism of insulin sensitization. Endocrinology 2002; 143: 998-1007. 24. Shapiro L, Scherer PE. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol 1998; 8: 335-338. 25. Waki H, Yamauchi T, Kamon J, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 2003; 278: 40352-40363. 26. Onay-Besikci A, Altarejos JY, Lopaschuk GD. gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem 2004; 279: 44320-44326. 27. Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 2001; 98: 2005-2010. 28. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003; 423: 762-769. 29. Fasshauer M, Klein J, Kralisch S, et al. Growth hormone is a positive regulator of adiponectin receptor 2 in 3T3-L1 adipocytes. FEBS Lett 2004; 558: 27-32. 30. Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPAR α, γ PPAR , and LXR. Biochem Biophys Res Commun 2004; 314: 151-158. 31. Tomas E, Tsao TS, Saha AK, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 2002; 99: 16309-16313. 32. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7: 941-946. 33. Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5 flanking sequence of the rat acyl CoA oxidase gene. EMBO J 1992; 11: 433-439. 34. Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARs) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta 1996; 1302: 93-109. 113 35. Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fattyacid oxidation by activating AMP-activated protein kinase. Nat Med 2002; 8: 1288-1295. 36. Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1: 15-25. 37. Roepstorff C, Halberg N, Hillig T, et al. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab 2005; 288: E133-E142. 38. Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med 2002; 8: 731-737. 39. Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 2005; 48: 132-139. 40. Furuhashi M, Ura N, Moniwa N, et al. Possible impairment of transcardiac utilization of adiponectin in patients with type 2 diabetes. Diabetes Care 2004; 27: 2217-2221. 41. Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 2004; 10: 1384-1389. 42. Li J, Hu X, Selvakumar P, et al. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab 2004; 287: E834- E841. 43. Liao Y, Takashima S, Maeda N, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res 2005; 67: 705-713. 44. Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 2001; 7: 947-953. 45. Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 2001; 108: 1875-1881. 46. DeFronzo RA, Gunnarsson peripheral and splanchnic R, Bjorkman glucose O, Olsson metabolism in M, Wahren J. Effects noninsulin-dependent of (type insulin II) on diabetes mellitus. J Clin Invest 1985; 76: 149-155. 47. Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003; 361: 226-228. R e c e i v e d : September 15, 2006 A c c e p t e d : October 2, 2006 Authors address: Dr. Zdzislaw Kochan, Department of Biochemistry, Medical University of Gdansk, ul. Debinki 1, 80-211 Gdansk, Poland. Phone/fax: +48583491465. E-mail: [email protected]