* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download onion cell (before)
Chromatophore wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell membrane wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Programmed cell death wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
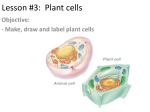
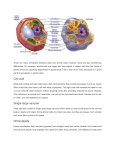
ONION OSMOSIS LAB Water passes through aquaporins in cell membranes from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration). This process is called osmosis. It requires no cellular energy to be used, and occurs due to the random, continuous motion of all molecules. If a cell is placed in an environment in which the concentration of water is less / concentration of solutes is greater than in the cell (hypertonic), water will flow from the cytoplasm and/or water vacuole through the membrane into the environment. (Some solute moves into the cell but this does not account for the dramatic changes that you see or weigh) The cytoplasm and/or central vacuole of the cell shrinks. In an animal cell, the entire cell shrivels. In a plant cell, the central cacuole shrinks away from the cell wall. A cell placed in a hypotonic environment, with a higher concentration of water / lower concentration of solutes, will gain water by osmosis. A cell placed in an isotonic environment, containing the same concentration of water and solute as in the cell, will not gain weight, as water moves equally in both directions. Purpose: to observe the process of osmosis in living cells. Materials: red onion cell, slides, coverslip, compound microscope, 4% salt solution, distilled water, Procedure: 1. Make a wet mount of a small, thin piece of purple epidermis from a red onion bulb's leaf scales. 2. Photograph and label what you observe under high power. Be sure to include: cell wall (note:cell membrane not visible since it adheres tightly to cell wall), nucleus, central vacuole (takes up most of the space in the cell and contains water and a reddish/ purple pigment called anthocyanin, central vacuole membrane - will only be visible after adding salt). 3. Lift off the coverslip and place 2 drops of 4% or 8% salt solution directly on the sample. 4. Photograph your cells. Note the color and position/shape of the vacuole. Label central vacuole membrane. 5. Lift the coverslip and add 3-5 drops of distilled water to the slide. 6. Photograph the cells. Again, note the color and shape of the vacuole. 7. Watch onion cell plasmolysis at: https://www.youtube.com/watch?v=rMtaqq2bmFU Results: onion cell (before) onion cell – after % salt / % water onion cell – after distilled water (100% water) Image Analysis: 1. Download the software program Image J at: http://rsb.info.nih.gov/ij/download.html Download ImageJ for Mac OS X from the Download page. The ZIP file you download (Image1.xx.zip) should automatically expand to a folder named "ImageJ". Copy this folder to the Applications folder, and open it. The first time you run ImageJ you may get get an "ImageJ can't be opened because it is from an unidentified developer" message, which can usually be bypassed by right clicking on ImageJ.app and selecting "Open" from the drop down menu. if that doesn't work, open the "Security & Privacy" panel in System Preferences and change "Allow apps downloaded from:" to "Anywhere". You can switch back to the original setting once ImageJ is running. 2. In Image J, open your microscope photo of the red onion cell "before". Convert your image to a black and white image: Image / Type / 32 bit. 3. Pick 3 representative cells and measure the average pixel (color) value in those cells by using the freehand tool to outline the central vacuole. Select Analyze / Measure and read the mean pixel value. (lighter = higher number; darker = lower number range = 0-256) freehand tool outlined central vacuole mean pixel value nucleus central vacuole filled with water and pigment cell wall 4. Open your red onion "after" image. Convert it to a 32-bit black and white image. Measure the area of 3 cells by outlining each with the freehand drawing tool. Measure along the inner portion of the cell wall. We are assuming that the central vacuole has almost the same area as the entire cell. Analyze / Measure freehand tool yellow outlined cell 5. Measure the area of the central vacuole of those same 3 cells using the freehand selection tool. Analyze / Measure 6. Measure the average pixel value of the central vacuole in those cells by reading the Mean value in the results window. Table 1 Data and statistics for onion osmosis in X % salt solution % salt average pixel average pixel area value of central value of cell/central vacuole before central vacuole before vacuole after cell 1 cell 2 cell 3 avg stand.dev 95% CI area central vacuole after % change in area 7. Get the results from another group that used a different % salt than your group. Table 2 Data and statistics for onion osmosis in X % salt solution % salt average pixel average pixel area value of central value of cell/central vacuole before central vacuole before vacuole after cell 1 cell 2 cell 3 avg stand.dev 95% CI area central vacuole after t-test Was there a difference in the effects of the different salt solutions? Do a t-test to find out! null hypothesis = p value = Explain these results. % change in area Discussion Questions: 1. Under each picture in the caption, explain the changes in the size of the central vacuole and relative color of the pigment (mean pixel value) following the addition of the salt solution and then the distilled water. Use arrows and labels showing the movement of water. 2. Animal cells exposed to distilled water swell and burst but plant cells do not. Based on your knowledge of cell structure, why might this occur? 3. HONORS ONLY: How do organisms that live in fresh or salt water cope with an environment that differs from that of their cells? Use your class notes, textbook, Haiku Osmoregulation article and/or this website (or others) to help you answer this. http://en.wikipedia.org/wiki/Osmoregulation 4. The solute concentration of contact lens saline solutions must be isotonic to the cells of your eyes. Why? 5. How should sports drinks need to be formulated so that they are able to effectively rehydrate your cells? Why might Coke or lemonade not be good at quenching your thirst?