* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structural organization of the transfer RNA gene clusters of cholera
SNP genotyping wikipedia , lookup
Pathogenomics wikipedia , lookup
Nucleic acid double helix wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
DNA supercoil wikipedia , lookup
Oncogenomics wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Ridge (biology) wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Mitochondrial DNA wikipedia , lookup
Genetic engineering wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Nucleic acid tertiary structure wikipedia , lookup
DNA vaccination wikipedia , lookup
Epigenomics wikipedia , lookup
Point mutation wikipedia , lookup
Human genome wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genome (book) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Minimal genome wikipedia , lookup
History of RNA biology wikipedia , lookup
Non-coding DNA wikipedia , lookup
Molecular cloning wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Non-coding RNA wikipedia , lookup
Genome evolution wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Primary transcript wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Designer baby wikipedia , lookup
Genome editing wikipedia , lookup
Microevolution wikipedia , lookup
Helitron (biology) wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Epitranscriptome wikipedia , lookup
History of genetic engineering wikipedia , lookup
Expanded genetic code wikipedia , lookup
Genomic library wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
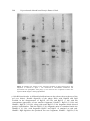
J. Biosci., Vol. 18, Number 2, June 1993, pp 195–205. © Printed in India. Structural organization of the transfer RNA gene clusters of cholera phage ϕ 149 NRIPENDRANATH MANUAL and RANAJIT KUMAR GHOSH* Indian Institute of Chemical Biology, 4, Raja S C Mullick Road, Jadavpur, Calcutta 700 032, India MS received 15 July 1991; revised 4 March 1993 Abstract. Vibrio cholerae phage φ l49 codes tRNAs specific for twelve different amino acids. These tRNA genes are contained in two different HindIII fragments 11 and 3·4 kb in size, of the phage genome. The 3·4 kb HindIII fragment was cloned in Escherichia coli using pBR328 as vector. The recombinant plasmid pNR347 produced nine of the twelve tRNA species (arginine, proline, serine, tyrosine, histidine, lysine, leucine, tryptophan and aspartic acid) encoded in the phage genome. Keywords. Phage φ149; phage coded tRNAs; tRNA-gene cloning. 1 Introduction Infections with bacteriophages bring about dramatic changes in the metabolic environment of the host bacteria. The major metabolic shift is directed towards the rapid macromolecular synthesis necessary for phage development. Translation of the phage messenger RNA takes place on the preexisting host ribosomes, using in most cases, host aminoacyl-tRNA synthetases and tRNAs. However, infections of Escherichia coli cells with T5 (Scherberg and Weiss 1970) and T-even phages (McClain et al 1972; Wilson et al 1972; Desai and Weiss 1977) induce the synthesis of a large number of tRNA species coded by the phage genome. A great deal of information is available regarding the characteristics of these tRNAs, structural organization of the tRNA genes on the phage genome (Hunt et al 1980; Velton and Abelson 1980) as well as the sequence organization and control of transcriptions of tRNA genes (Broida and Abelson 1985). Yet, it remained unanswered why a bacteriophage that infects a host having a complement of various tRNAs should direct the synthesis of an additional population of tRNAs. Since mutant phages that lacked tRNA genes are viable, it was concluded that these tRNAs are not essential for phage development. In the absence of any other host-phage system inducing the synthesis of phage coded tRNAs it was difficult to ascertain the universality of this conclusion. We had reported earlier that the infection of Vibrio cholerae classical strains with phage φ 149 induces the synthesis of specific tRNA molecules coded by the phage genome (Ghosh and Guhathakurta 1983). The genome of this phage encodes tRNAs specific for twelve different amino acids (Mandal and Ghosh 1988). Thus phage φ149 may be useful as an alternative system to understand the role of phage-coded tRNAs. For further analysis it is essential to get an idea about the structural organization of the tRNA genes on the phage genome. It is in this context, that we have constructed a physical map of the tRNA gene cluster of the *Corresponding author. 195 196 Nripendranath Mandal and Ranajit Kumar Ghosh phage φ 149 genome. The results presented here show that the tRNA genes are contained in two HindIII fragments, 11 and 3·4 kb in length. These two fragments do not bear any sequence homology and the smallest fragment alone carried the genes for tRNAs specific for nine different amino acids. Secondly, despite a great deal of advances in our understanding of tRNA biogenesis in prokaryotes (Carbon et al 1974; Ghosh and Deutscher 1978; Kole and Altman 1982), nuclease(s) processing multimeric tRNA precursors to monomeric ones is yet to be characterized. This was primarily due to the unavailability of adequate amount of the precursor for use as a substrate for enzyme purification. This necessitated the availability of enriched source of tRNA gene clusters which may be transcribed in vitro to generate large amount of multimeric tRNA precursors. Transfer RNA genes cloned into plasmids may be very useful for this purpose. Transfer RNA gene clusters of phage T4 could not be cloned due to the presence of some 'lethal genes' in the region (Fukuda et al 1980). The present paper also describes the cloning and expression of tRNA gene cluster of phage φ149 which may be useful in studying tRNA biogenesis. 2. Materials and methods 2.1 Bacteria and bacteriophage strains V. cholerae Ogawa 154 was used for phage propagation and as an indicator host, cholera phage φ149 was used in the study. Host and phage strains were propagated as described earlier (Ghosh and Guhathakurta 1983). 2.2 Isolation of φ 149 DNA Phage DNA was prepared from the high titre phage stock essentially according to the method of Ghosh and Guhathakurta (1983). 2.3 Isolation of tRNA and [ 32 P] tRNA [32P]tRNA was isolated from uninfected and φ 149 infected cells according to the method of Ghosh and Guhathakurta (1983). 2.4 Isolation of phage φ149 coded tRNAs from the clones This was done according to the method described by Daniel et al (1968). Briefly, 14 mg of denatured φ149 DNA was hybridized with 4 mg of tRNA isolated from recombinant clones carrying φ 149 tRNA genes in 6 × SSC at 70° C for 60 min. The mixture was then chilled to 0° C and was filtered through nitrocellulose membrane (BA 85, 82 mm. Schleicher and Schuell). About 1 mg of DNA was loaded on each filter paper which was then washed thoroughly with 6 × SSC. Both sides of the filters were then washed with 2 × SSC. To extract tRNA from RNA-DNA hybrid filters were suspended in 10 mM Tris-Cl, pH 7·4, for 90 min at 37° C. The combined extract was then concentrated with n-butanol and tRNA was precipitated with 2· Phage φ149 tRNA gene clusters 197 volumes of ethanol at –20° C overnight. tRNA was collected by centrifugation (8000 g for 10 min at 4° C) and was dissolved in 1 ml sterile water. 2.5 Restriction endonuclease digestion of the φ149 DNA DNA (1-2 μg) was used for each digest. Digestion was carried out according to the instructions of the manufacturer (New England Biolab). Restriction fragments were separated by electrophoresis (Ghosh et al 1985). Bands were visualized by staining with ethidium bromide (1 µg/ml) and photographed. In some experiments restriction fragments were end labelled according to Downing et al (1979) prior to electrophoresis. Gels were dried after electrophoresis and autoradiographed using Curix-100 (Agfa) X-ray film. 2.6 Fragment nomenclature and molecular size Unless otherwise mentioned, the fragments were designated by letters of the alphabet according to size, where A was the largest fragment. From their relative staining intensities in the gel, some of the fragments have been identified as doublets. The prefix designates which enzyme(s) was used to generate the specific fragment. The sizes of the various fragments were obtained from their relative mobilities on gel with those of λ DNA-HindIII fragments. 2.7 Southern blot analysis Briefly, phage φ 149 DNA was digested to completion with various restriction enzymes, singly or with respective double combinations, and the DNA fragments were separated by electrophoresis on vertical 1% agarose gel (as above). DNA fragments were then transferred onto nitrocellulose membrane (Schelicher and Schull, BA 85) according to the method described by Southern (1975). The filter was then baked at 80° C for 2 h under vacuum and the DNA fragments were hybridized with [32P] tRNA from either φ 149 infected or uninfected V. cholerae 154 cells under the conditions described by Gillespie and Spiegelman (1965). 2.8 Construction of the recombinant plasmids containing phage φ149 tRNA genes Plasmid pBR328 was digested with HindIII under the conditions described by the manufacturer (New England Biolab) and then treated with calf intestinal alkaline phosphatase to prevent recircularization. Phage φ 149 DNA was digested with HindIII to completion. The HindIII digested pBR328 and phage φ 149 DNA were ligated at 10° C for 12 h. The ligation product was then used to transform competent E. coli HB101 cells (Kushner 1978) and the desired recombinants were identified by colony hybridization (Grunstein and Hogness 1975) separately with nick translated (Rigby et al 1977) 11 and 3·4 kb HindIII fragments of phage φ 149 DNA as a probe. [32P] tRNA from phage infected cells was also used as a probe whenever necessary. 198 Nripendranath Mandal and Ranajit Kumar Ghosh 2.9 Aminoacylation Aminoacylation was carried out according to Mandal and Ghosh (1988) using [3H] aminoacid (sp. activity 70-100 Ci/mmol, Amersham), either singly or in combination. After incubation at 37° C for 30 min, aminoacyl-tRNA was precipitated with 3 ml of 10% trichloroacetic acid (TCA). After 30 min in ice, the precipitate was filtered through Whatman GF/C filters. Filters were washed three times with 2·5 % TCA containing 0·1% casamino acid and once with ethanol ether (1:1) . Filters were then counted for radioactivity using toluene based scintillation fluid in a LKB Rackbeta, Model 1211 liquid scintillation counter. 2.10 Isolation of aminoacyl-tRNA synthetases Aminoacyl-tRNA synthetases were prepared from both uninfected and φ 149 infected (prior to lysis) V. cholerae Ogawa 154 cells according to the procedure already described (Mandal and Ghosh 1988). 2.11 Hybridization Hybridization of [32P] tRNA or aminoacyl-tRNA with φ149 DNA was carried out according to the method of Weiss et al (1968). Denatured φ l49 DNA (100 µg) was immobilised on each nitrocellulose membrane (25 mm; 0·45 μ Millipore) and hybridized with tRNA in 2 × SSC containing 50% formamide at 30° C. 3. Results 3.1 Identification of phage DNA fragments containing the phage ϕ 149 tRNA genes Phage φ 149 was known to induce the synthesis of specific tRNA molecules coded by the phage genome, upon infecting V. cholerae classical strains (Ghosh and Guhathakurta 1983). In order to identify the DNA fragment(s) carrying the tRNA genes, phage φ 149 DNA was digested with various restriction enzymes, either singly or in combination, and the resulting fragments were separated by agarose gel electrophoresis. A large number of fragments were produced with most of the enzyme(s) (figure 1). The DNA fragments were denatured in situ and transferred onto nitrocellulose membrane (Schleicher and Schuell BA85) by the method of Southern (1975). Hybridization was carried out with [ 32 P] tRNA isolated from phage φ 149 infected cells. Of the large number of fragments produced by the various enzyme(s), [ 32 P] tRNA hybridised only to very few fragments (figure 2). These results indicate that the tRNA genes are clustered in limited regions on the phage genome. 3.2 Mapping of the phage φ149 tRNA gene clusters The map of the phage φ 149 tRNA gene clusters was constructed from Southern blot data as follows : The fragments described below refer only to bands hybridizing with [32P] tRNA from phage φ 149 infected cells. Phage φ 149 tRNA gene clusters 199 Figure 1. Restriction endonuclease digestion pattern of phage φ 149 DNA. Phage DNA was digested with various restriction endonucleases and the fragments were separated by electrophoresis on 1 % agarose gels. The bands were visualized by staining with ethidium bromide and photographed as described under §2. The position of λ-DNA-HindIII fragments are indicated at the left. Alphabets indicate the respective bands showing hybridization. Most significantly, [32P ] tRNA hybridized to two HindIII fragments. HindIII-A (11 kb) and HindIII-I (3·4 kb) (figure 2). Double digestion with HindIII and other enzymes revealed that the 3·4 kb HindIII fragment was resistant to these enzymes. The 11 kb HindIIIfragment (HindIII-A) on the other hand contained other restriction sites and thus produced smaller fragments showing positive hybridization reactions. Three BglI fragments, BgII-E (8·6 kb), –F (7 kb) and –1 (5 kb), hybridized with 200 Nripendranath Mandal and Ranajit Kumar Ghosh Figure 2. Southern blot analysis of the restriction fragments of phage ϕ149 DNA. The fragments as obtained in figure 1 was transferred to nitrocellulose membranes and hybridized with [32P]tRNA from phage φ 149 infected cells. Alphabets indicate the respective bands of figure 1 showing hybridization. φ 149 tRNA molecules. A diffused hybridization was also observed near the top of the gel (see below). Double digestion of the phage DNA with HindIII and BglI resulted in the disappearance of BglI-E (8·6 kb) and BglI-F (7 kb) with the concomitant appearance of two smaller fragments, HindIII + BglI-I (3·6 kb) and HindIII+ BglI-N (2·36 kb), along with usual BglI-I (5 kb) fragment which showed positive hybridization. Thus the BglI-I (5 kb) fragment must be contained inside the HindIII-A (11 kb), with fragments BglI-E and Bgll-F of attached at each end. HindIII+ BglI digestion also produced two new fragments, HindIII + BglI-D (5 kb, Phage φ149 tRNA gene clusters 201 double) and HindIII + BglI-E (4·6 kb) (figure 1) which did not hybridize with φ 149 tRNA and was a part of BglI-E (8·6 kb) and BglI-F (7 kb) respectively. Thus the arrangement of the BglI fragment in relation to HindIII-A (11 kb) fragment was as shown in figure 3. Figure 3. Restriction map of the DNA fragments carrying the tRNA genes of phage ϕ l49. (A) Fragment containing HindIII-A and (B) HindIII-I. Hatched region indicates the location of tRNA genes. Double digestion of the phage DNA with BamHI and BglI generated two new fragments BamHI + BglI-L (3·6 kb) and -M (1·4 kb) at the expense of BglI-I (5 kb). In addition to BamHI + BglI-G (8·6 kb) and BamHI + Bgll-I (7 kb) fragments, the newer fragment of also hybridized with phage φ 149 tRNA (figure 2). Thus the (5 kb BglI-I) fragment must have a single BamHI site which should generate two fragments from HindIII-A fragment. Indeed, double digestion of the phage DNA with HindIII and BamHI resulted in the disappearance of HindIII-A (11 kb) fragment generating two new fragments HindIII + BamHI-C (7·4 kb) and HindIII + BamHI-H (3·6 kb), each hybridizing with φ 149 tRNA (figure 2). Thus, the arrangements of various fragments carrying the tRNA genes in the HindIII-A (11 kb) fragment was as given in figure 3. This was also confirmed by reciprocal digestion of isolated HindIII-A (data not shown). Discrete fragments of BglI and BamHI + BglI digestion of phage φ l49 DNA hybridizing with phage φ 149 tRNA could be accounted for in terms of the HindIIIA fragment. Besides these discrete bands, diffused hybridization was obtained near the top of the gel for these two digests and may reflect the location of the 3·4 kb HindIII (Hind III-I) fragment. The genome of the phage φ 149 has been shown to be circularly permuted and terminally redundant (Sengupta et al 1985). It is probable that the 3·4 kb HindIII fragment carrying the tRNA genes is present near the top fragment is submolar proportions. This was confirmed by the observation that when the phage DNA was digested with BglI-HindIII, the diffused hybridization at the top disappeared completely giving rise to the HindIII + BglI-J (3·4 kb) fragment 202 Nripendranath Mandal and Ranajit Kumar Ghosh (figure 2). Thus, it is probable that the tRNA genes contained in this fragment are present in of submolar proportions in the terminally redundant region. 3.3 Construction of the recombinant plasmids containing the tRNA genes of phage ϕ149 In order to characterize the various tRNAs contained in the above two fragments, HindIII fragments of φ 149 DNA were cloned into pBR328 as described under §2. Seven clones hybridizing with [ 32P]tRNA from φ l49 infected cells were obtained The recombinant plasmids isolated from these clones produced two fragments; one corresponding to pBR328 and another 3·4 kb fragment. The latter hybridized well with [32P] tRNA from φ 149 infected cells. 3.4 Expression of the cloned tRNA genes The expression of the cloned tRNA genes of phage φ149 in Ε. coli HBlOl was investigated. [ 32 P]tRNA was isolated from the recombinant clones as described under §2. Various aliquots of these tRNAs were then allowed to hybridize with denatured phage φ 149 DNA immobilized onto nitrocellulose membranes. Amount of fixed radioactivity was measured in each case. As can be seen from figure 4, the Figure 4. Hybridization of [32P] tRNA isolated from various recombinant clones with phage φ149 DNA. 100 μg of phage φ149 DNA was immobilised to each filter and hybridized with varying amounts of [32P] tRNA as described under §2. Phage φ 149 tRNA gene clusters 203 clone pNR 347 showed maximum hybridization. Poorer level of expression in other clones may be due to wrong orientation of the gene clusters. tRNA from E. coli HB 101 cells containing pBR328 failed to show any appreciable hybridization. 3.5 Characterization of the tRNAs coded by the recombinant plasmid To characterize the various tRNA species expressed in the clone pNR347, total tRNA was isolated from the cells carrying pNR347. Phage specific tRNAs were then isolated from these preparations by selective hybridization with phage φ 149 DNA and dissociation of the DNA-RNA complex. The amino acid acceptor activities of these tRNAs were then determined by aminoacylation with individual [ 3 H ] - L amino acids using aminoacyl-tRNA synthetases from φ 149 infected V. cholerae, Ogawa 154 cells. Of the various amino acids tested, significant acid precipitable radioactivity was found only when charged with arginine, proline, serine, tyrosine, histidine, lysine, leucine, tryptophan and aspartic acid (table 1). Thus, the clone pNR 347 of carried the genes for tRNAs specific for nine out of twelve amino acids encoded in the phage φ 149 genome. tRNA isolated from Ε. coli HB 101 cells carrying pBR328 and treated under identical conditions failed to show any appreciable amount of fixed radioactivity. Table 1. Aminoacylation of plasmid pNR347 coded tRNAs after isolation from phage 149 DNA-tRNA hybrids. 4. Discussion The results presented above describe the identification and mapping of the tRNA clusters encoded in the phage φ 149 genome as well as cloning and expression of one of the gene clusters. Like phage T5, the tRNA genes in phage φ 149 are located in two different unlinked fragments. The 3·4 kb HindIII fragment alone contains genes for tRNAs specific for nine amino acids out of twelve encoded in the phage genome. The fact that the 11 kb HindIII fragment showed more intense hybridization with 204 Nripendranath Mandal and Ranajit Kumar Ghosh [32P]tRNA from phage φ 149 infected cell than the 3·4 kb fragment indicates that the large fragment probably contains genes for other isoacceptor tRNA species as well as yet unidentified tRNAs specific for other amino acids. However, the possibility of the presence of small stable RNA species resembling stable RNA of species I and II of T4 (Gold-farb and Daniel 1981) cannot be ruled out. Despite our repeated efforts, the tRNA gene cluster contained in the 11 kb HindIII fragment could not be cloned. It is probable that the gene may be adjacent to some lethal genes which make the clones nonviable. It has been reported that T4-tRNA gene clusters lie close to the lysozyme gene and thus could not be cloned in plasmid vectors (Fukuda et al 1980). Attempts are being made to clone various subfragments of the 11 kb HindIII fragment to characterize tRNAs contained in these fragments. Our present study indicates that phage φ 149 closely resembles T5 (Hunt et al 1980) in coding a large number of tRNAs clustered in various fragments. The complexity of tRNA species produced by T5 is somewhat surprising especially since the tRNA genes appear not to be required for phage growth. However, our preliminary result with phage φ 149 tRNAs showed that four of the phage coded tRNAs are aminoacylated 50-100-fold better by aminoacyl-tRNA synthetases from phage infected cells than the corresponding tRNAs from uninfected host (N Mondal and R Κ Ghosh, unpublished observation). This preferential aminoacylation suggests that the φ 149 encoded tRNAs may be essential for phage development. Thus the phage φ 149 system may provide a valuable mode in understanding the biological significance of phage coded tRNAs. Finally, the cloned tRNA gene cluster may provide a valuable tool in understanding the mechanism of tRNA biogenesis. Cloned tRNA gene cluster may be transcribed in vitro to generate a large amount multimeric tRNA precursor for identification of the enzyme involved in processing such precursors to monomeric ones. Acknowledgement NM is grateful to the Council of Scientific and Industrial Research, New Delhi, for a Senior Research Fellowship. References Broida J and Abelson J 1985 Sequence organization and control of transcription in bacteriophage T 4 tRNA region; J. Mol. Biol. 185 545-563 Carbon J, Chang S and Kirk L L 1974 Clustered tRNA genes in Escherichia coli: Transcription and processing; in Processing of RNA Brookhaven Symposia in Biology, Vol. 26, pp 26-36 Daniel V, Sarid S and Littauer U Ζ 1968 Amino acid acceptor activity of bacteriophage T4 transfer RNA; FEBS Lett. 2 39-41 Desai S Μ and Weiss S B 1977 Study of transfer RNA coded by T 2 , T 4 , T 6 bacteriophages; J. Biol. Chem. 252 4935-941 Downing R G, Duggleby C J, Villems R and Broda Ρ 1979 An endonuclease cleavage map of the plasmid pWWO-B, a derivative of the TOL Plasmid of Pseudomonas putida mt-2; Mol Gen. Genet. 168 97-99 Fukuda Κ, Gossens L and Abelson J 1980 The cloning of a T4 transfer RNA gene cluster; J. Mol. Biol 137 213–234 Phage φ149 tRNA gene clusters 205 Ghosh R Κ and Deutscher Μ Ρ 1978 Purification of a potential 3' processing nuclease using synthetic tRNA precursors; Nucleic Acids Res. 5 3831-3842 Ghosh R Κ and Guhathakurta I 1983 Synthesis of phage specific transfer RNA molecules by Vibriophage φ 149; FEBS Lett. 162 177–179 Ghosh R K, Siddiqui Κ Α Ι, Mukhopadhyay G and Ghosh A 1985 Evidence that a system similar to rec A system of Escherichia coli exists in Vibrio cholerae; Mol. Gen. Gent. 200 439-441 Gilliespie D and Spiegelman S 1965 A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane; J. Mol. Biol. 12 829-842 Goldfarb A and Daniel V 1981 Mapping of transcription units in the bacteriophage T 4 tRNA gene cluster; J. Mol. Biol. 146 393-412 Grünstein Μ and Hogness D S 1975 Colony hybridization : A method for the isolation of cloned DNAs that contain a specific gene; Proc. Natl. Acad. Sei. USA 72 3961-3965 Hunt C, Desai S Μ, Vaughan J and Weiss S Β 1980 Bacteriophage T5 transfer RNA : Isolation and characterization of tRNA species and refinement of the tRNA gene map; J. Biol. Chem. 255 31643173 Kole R and Altman S 1982 tRNA processing enzymes from Escherichia coli; in The enzymes (ed.) Ρ D Boyer (New York: Academic Press) vol. 24, pp 469-483 Kushner S R 1978 An improved method for transformation of Escherichia coli with Col El-derived plasmids; in Genetic engineering (ed.) Η Β Boyer and S Nicosia (Amsterdam: Elsevier/North Holland) PP 17-23 McClain W H, Guthrie C and Barrell Β G 1972 Eight transfer RNAs induced by infection of Escherichia coli with bacteriophage T4; Proc. Natl. Acad. Sci. USA 69 3703-3707 Mandal Ν and Ghosh R Κ 1988 Characterization of the phage specific transfer RNA molecules coded by cholera phage φ 149; Virology 166 583-585 Rigby Ρ W J, Dieckmann Μ, Rhodes C and Berg Ρ 1977 Labelling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I; J. Mol. Biol. 113 237–251 Scherberg Ν Η and Weiss S Β 1970 Detection of bacteriophage T 4 and T5 coded transfer RNAs; Proc. Natl. Acad. Sci. USA 67 1164–1171 Sengupta A, Ray Ρ and Das J 1985 Characterization and physical map of φl49 DNA; Virology 140 217229 Southern Ε 1975 Detection of specific sequences among DNA fragments separated by gel electrophoresis; J. Mol. Biol. 98 503–516 Velton J and Abelson J 1980 The generation and analysis of clones containing bacteriophage T4 DNA fragments; J. Mol. Biol. 137 235–248 Weiss S Β, Hsu W Tr, Foft J W and Scherberg Ν Η 1968 Transfer RNA coded by the T 4 bacteriophage genome; Proc. Natl. Acad. Sci. USA 61 114–121 Wilson J H, Kim J S and Abelson J Ν 1972 Bacteriophage T 4 transfer RNA : Clustering of the genes for T4 transfer RNAs; J. Mol. Biol. 71 541–556