* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Plant Physiology I: PLS622 2006 Introduction: Cell division
Cell nucleus wikipedia , lookup
Tissue engineering wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cell membrane wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Signal transduction wikipedia , lookup
Cell encapsulation wikipedia , lookup
Programmed cell death wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Endomembrane system wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
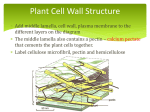
Plant Physiology I: PLS622 2006 Introduction: Cell division, expansion, cell-cell communication: The goals for this lecture include an understanding of: 1) The basics of the cell cycle. 2) The rudiments of control of the cell cycle. 3) The primary components of the cell wall. 4) The basic interactions among these components. 5) The acid-growth theory 6) Apoplastic and Symplastic information retrieval and propagation. How do plants increase in size? 1) more cells. 2) larger cells. How does plant organ-shape arise? Organization of cells creates strict geometric shapes. The basics of the cell cycle. Cell division: Arguably one of the most fundamental events of life, cell division depends on a progenitor increasing its intracellular content, apportioning it, duplicating its genetic material, dividing it in exact replica, and partitioning one part of the previous cell from the other, in the process forming two cells with identical genetic information. This procedure has continued, nonstop, since the beginning of life, leading to the current model of the cell-division cycle, or cell cycle (Fig. 1). Nuclear division Prophase Prometaphase Metaphase Anaphase Telophase G “gap” S “DNA synthesis” M “mitotic division” Nuclear division + Cytokinesis M G2 S G1 Chromosomes at the end of G1 Chromosomes at the end of S Figure 1: The standard cell cycle. 1 The mitotic phase of the cell cycle (“M” in the diagram above) is comprised of Prophase, Premetaphase, Metaphase, Anaphase and Telophase. The objective of mitosis is to divide the chromatin into two identical chromosome assemblages and enclose each portion in a nucleus. To do this, the duplicated chromosomes shorten and thicken (becoming chromatids) while the nucleolus and nucleus eventually break down signifying the end of prophase. Next, microtubules (formed from tubulin polymers) emanate from opposite poles of the plant cell to form the spindle (commencement of Metaphase). One important difference between plant and animal cells is that plant cells do not have centrosomes at the poles to which the microtubules attach as occurs in animal cells (Fig. 2). The chromatids (duplicated, condensed chromosomes) attach to the spindles via the kinetochore (centromer). The chromatids align (or congress) at the equator of the cell (metaphase plate) demarcating the end of metaphase. In anaphase, the kinetochores separate and migrate towards opposite poles, dividing the chromatid into component daughter chromosomes. By the end of anaphase two identical sets of chromosomes are at opposite poles of the cell. At this point, the chromosomes de-condense and become once again enclosed in a nucleus (Telophase). The cell now forms two daughter cells by the formation of the cell plate (phragmoplast) (Fig. 3) which can be considered as the termination of telophase. Animal Mitosis 1 x 2n 2 x 2n Two centrioles forming a centrosome situated at the poles of the cell. Microtubules are closely associated with the centrosomes at either pole. Mid Prophase Metaphase Late Anaphase Contractile ring eventually pinches cell into two cells. Figure 2: Animal Mitosis. 2 Plant Mitosis 1 x 2n 2 x 2n Mid Prophase Congression of the chromatids at the equator of the cell. Metaphase Late Anaphase Phragmoplast (cell plate) Figure 3: Plant Mitosis. A specialized form of chromatin apportioning, meiosis, leads to the production of gametes. In the case of a diploid organism, a single cell replicates its DNA, and then undergoes two successive divisions which reduces the chromosomal content so that each of the four resulting cells is haploid (Fig. 4). The rudiments of control of the cell cycle. Considerable research is currently focussed on elucidating how the events required for successful cell division are regulated. The G-phases of the cell cycle have been determined to be periods of rest for the cell, providing check points determining whether the next phase of the cycle is entered and, if so, when. There is at least one major check in G1 and G2, both of which cue on the size of the cell and, in yeast, the environmental conditions conducive to cellular proliferation. Central to this control are the cyclin-dependent protein kinases (cdks) that can phosphorylate proteins (thereby changing their activation) responsible for forcing cells into the next phase of the cell cycle. The cdk’s are present at all stages of the cell cycle but, as their name implies, are dependent on the presence of a second family of labile proteins called cyclins for their phosphorylating ability. There are two types of cyclins, mitotic cyclins that control entry into the mitotic phase of the cell cycle and G1 cyclins that control exit from G1 and entry into the Sphase. These proteins undergo rapid accumulation when conditions are favorable for entry into 3 the mitotic- and S-phases of the cell cycle, respectively, and are rapidly degraded once the cell has committed to entry into the respective phase. The root meristemless mutants of arabidopsis (rml1 and rml2) provided a glimpse into just how complex the control of cell division in plants can get. These mutants result in primary roots of less than 2 mm length due to a lack of cell division in the root apical meristem following germination. However, the shoot is capable of normal cell division as is callus. It was found that the rml1 defect was in a gene in the glutathione biosynthetic pathway. Further investigation into the lack of cell division in rml1 plants defined an absolute requirement for adequate levels of glutathione for the G1-to-S phase transition. Apparently, glutathione is not necessary for cell division in the shoot apical meristem or it is synthesized by another, non-mutant gene. Plant Meiosis 1 x 2n DNA replication Homolog paring First cell division Cell plate Second cell division 4 x 1n gametes Figure 4: Meiosis. How do you stop dividing?: In vertebrate cells, withdrawal from the cell cycle is mediated by cdk’s. The degradation of mitotic cyclin and the absence of G1 cyclin traps the newly formed daughter cells in the G1 resting phase. In plants cells there is evidence to suggest that the same mechanism may be in place. Cyclin-dependent kinase inhibitors (CDIs) have been identified that result in maize endosperm arrest. 4 Formative vs proliferative cell division. - Formative cell division serves to create cells that will eventually take on distinctly different fates. - Proliferative division serves to increase the number of the same type of cell. The primary components of the cell wall. Cell expansion: Water uptake drives cell expansion in plants which is an irreversible increase in cell volume. By taking up water into the central vacuole, plants have developed an economical method of increasing their size by orders of magnitude while maintaining approximately the same cytoplasmic volume, albeit, now dispersed over the periphery of a larger cell. Water uptake into the vacuole to increase turgor will not be effective in driving cell expansion unless the rigid cell wall is somehow induced to weaken, allowing the pressure within to force the cell wall to extend. This is not analogous to air forcing a balloon to expand since the wall of the balloon becomes increasingly thin until it ultimately fails. The plant cell wall deposits and incorporates new wall material into the expanding cell wall so that no "thinning" occurs. The ability of the cell wall to expand and incorporate new cell wall material while under stress is the result of its complex makeup and precise partial disassembly by some select wall modifying enzymes. The plant cell wall is thought to be comprised of two major classes of components, the cellulose-hemicellulose network and the pectic network. The former is cemented within the matrix of the latter. Cellulose microfibril Lipid bilayer Cellulose microfibril Tubulin microtubule A single component of a rosette. Terminal rosette A more detailed view One cellulose synthase molecule extruding a glucan chain. I have colored the cytoplasmic portion of the protein red and the membrane spanning portion green. The glucan chain is the solid black line on the apoplastic side of the plasmamembrane. SIDE VIEW. Plasma membrane Six cellulose synthase molecules make up one hexameric subunit of a rosette each one extruding a glucan chain for a total, so far, of 6 glucan chains. These 6 chains typically associate as a beta sheet TOP VIEW. ONE ROSETTE IN THE CIRCLE Six hexameric subunits associate in a rosette to extrude a glucan polymer (cellulose) comprised of 36 individual glucan chains from 36 individual cellulose synthase enzymes and arranged as 6 beta-sheets. The 6 beta sheets self assemble into one cellulose microfibril. TOP VIEW. time Figure 5 + 6: Cellulose synthesis at the plasmamembrane. There are five main components to plant cell walls which in dicots have the following approximate percent compositions; 1) cellulose (~30% wall dry weight); 2) hemicelluloses (~30% wall dry weight); 3) pectins (~35% wall dry weight); 4) proteins (~1-5% wall dry weight) and; 5) water (~75% wall wet weight). Ions such as calcium make up a sixth component but in truly negligible amounts relative to the first 5. 5 The basic interactions among these components. Cellulose is the backbone of the cell wall, imparting, depending on the predominant orientation of the fibrils, the wall’s ability to undergo expansion or not. In those instances where cellulose microfibril orientation predisposes the wall to expansion, fibril orientation also dictates the directionality of this expansion. Cellulose in the cell wall is a rigid semi-crystalline polymer that is laid down on the inner cell wall surface (outer surface of the plasmamembrane) by a complex assemblage of proteins arranged in a rosette often referred to as the terminal rosette (Fig. 5). The rosette is embedded in the plasmamembrane, spanning it and presumably anchored to and travelling along tubulin microtubules aligned on the inner surface of the cell membrane (Fig. 6). How the cellulose microfibrils are arranged determines if and how the cell expands. T h is fig u r e is o f a p la n t c e ll w a ll in w h ic h th e c e llu lo s e m ic r o fib r ils h a v e b e e n la id d o w n is o t r o p ic a lly . T h e m ic r o fib r ils w ill e ith e r r e s is t c e ll e x p a n s io n in a n y d ir e c tio n o r s lip p a s t e a c h o th e r a llo w in g e q u a l c e ll e n la r g e m e n t in a ll d ir e c tio n s a t o n c e in a p r o c e s s c a lle d “ p o ly m e r c r e e p ” . Isotropic or no growth In th is fig u r e , d u r in g p la n t c e ll e n la r g e m e n t, c o n tr o l o f th e d ir e c tio n o f c e ll g r o w th is im p o s e d b y a r r a n g in g th e m ic r o fib r ils p e r p e n d ic u la r to th e d ir e c tio n o f g r o w th . T h e m ic r o fib r ils r e s is t e x p a n s io n la te r a lly m u c h m o r e th a n th e y d o lo n g itu d in a lly ( in th e d ir e c tio n o f th e a r r o w s ) . T h is c o n tr o l o f p la n t c e ll e x p a n s io n is c r u c ia l to p la n t d e v e lo p m e n t s in c e it is th e d ir e c tio n a n d a m o u n t o f c e ll e x p a n s io n th a t d e te r m in e s fin a l o r g a n s h a p e a n d s iz e in p la n ts . Anisotropic (directional) growth Cellulose Hemicellulose Pectin Cell wall proteins Figure 7: Diagram of the plant cell wall from two different cells, one that is not expanding or expanding isotropically and a second expanding directionally or anisotropically. If the microfibrils are secreted in a non-parallel manner (isotropically), then the microfibrils may prevent cell expansion since there is no direction the cell may extend without breaking a microfibril (Fig. 7). To date, there have been no reports of cellulases produced by plants that are capable of hydrolyzing crystalline microfibrils. The cellulases that have been discovered are all thought to act on hemicellulose substrates such as xyloglucan. The single exception is a putatively membrane-bound cellulase involved in cellulose synthesis, presumably by hydrolysing a single 1-4 glucan chain from the cellulose synthesizing apparatus comprising one component of a rosette (Fig. 5 and 6). Alternatively, in response to internal signals, microtubules can arrange themselves in parallel with the result that the enzyme rosettes extruding cellulose microfibrils to the inner wall surface exterior to the plasmamembrane lay down the microfibrils in parallel (anisotropically, Fig. 7). Hence, the configuration of the cellulose in the stress bearing region of the cell wall resembles a spring or slinky. It is difficult to expand the spring laterally but much easier to extend the spring longitudinally. Recently, a mutation in the membrane-bound cellulase 6 involved in cellulose synthesis has been isolated. Dubbed korrigan (kor), the mutant plants are dwarfed and exhibit aberrant cell expansion. The hemicellulosic component of plant cell walls is thought to be intimately associated with the cellulose microfibrils, either laying along the microfibrils for much of their length associated by hydrogen bonds, or actually embedded in the interior of the microfibrils. The hemicellulosic constituents are delivered to the cell wall from Golgi derived vesicles in which they are synthesised. Upon arrival at the plasma membrane the vesicles fuse with the membrane and release the contents into the wall. These wall components are then somehow incorporated into the stress bearing wall, probably being linked together enzymatically to form longer, more highly branched polymers than were synthesized in the Golgi. This process appears to be operational in the deposition of xyloglucan into the cell wall of plants using xyloglucan endo-transglycosylase (XET) to polymerize short, vesicle-deposited xyloglucan chains into larger polymers of xyloglucan. Additionally, the simultaneous extrusion of semi-crystalline cellulose at the inner wall surface and a marked affinity of xyloglucan for cellulose ensures their tight association, even the possibility of xyloglucan embedding itself into the elongating microfibril. To permit cell wall expansion, these hemicelluloses must be either disassociated from the cellulose microfibrils and themselves by a recently discovered enzyme christened “expansin” or disentangled from the matrix usually though partial polymer hydrolysis by endo-glycosylases (EGases). Pectin forms a “gel” in which the other cell wall components are embedded. Although it is most abundant in the middle lamella between cells, cementing them together, pectin is found throughout the plant cell wall. Pectin is initially deposited at the inner cell wall surface in discrete lengths by Golgi derived vesicles where it is formed. This pectin is highly methyl esterified when deposited making it resistant to hydrolysis and relatively unreactive with cell wall calcium and other ions. Upon deposition, the pectin is typically de-esterified by pectin methyl esterase (PME) imparting to it a negative charge and allowing it to form rigid configurations and bind tenaciously to calcium. It is also now susceptible to modification by pectin hydrolysing enzymes (polygalacturonases, PGs). The degree to which pectin is hydrated, its degree of esterification, and the bonds it is participating in are all thought to control wall porosity which in turn determines the sieve size of the cell wall, dictating what size particle, protein or polysaccharide, can pass through the matrix. Protein in the cell wall can be structural, such as extensins thought to impart inflexibility to the cell wall, or enzymatic, such as expansins, XETs, EGases, PMEs, PGs, etc. Although they comprise only a small percentage of the total cell wall by dry weight, they are absolutely crucial to its ability to expand. Water is often ignored as a major component of the living, dynamic plant cell wall but, besides imparting solvent and lubrication, it also determines the concentration of the components of the wall and their hydration determines wall porosity. Its influx into the vacuole of cells dictates the turgor they are capable of exerting and no plant cell, however disposed to cell wall loosening, can expand without turgor pressure. Plant cell walls isolated from regions undergoing natural expansion are susceptible to elongation due to a decrease in pH, so-called acid growth (see assigned reading; Hager 2003. J. Plant Res. 116: 483-505 parts thereof). Acid growth is thought to to be induced by the plant hormone auxin by the stimulation of plasma membrane H+-ATPases that pump protons from the cytoplasm out of the cell thereby acidifying the apoplast. This stimulates the cell wall modifying enzymes allowing wall components to "creep" past each other and growth to occur. Additionally, inward rectifying potassium channels are activated to allow potassium ions into the cell to balance the charge differential across the plasma membrane resulting from H+ efflux. This eventually results in a decrease in vacuolar osmotic potential, the influx of water, and maintenance of turgor in the expanding cell. Protease treatment has been shown to eliminate acid growth from plant cell walls normally responding to decreased pH. This led to Daniel Cosgrove isolating two proteins from cucumber hypocotyls that potentiate acid growth. These were named expansins and are 7 purportedly solely responsible for acid growth of plant cell walls without cleaving any cell wall constituent. The family of expansin genes in plants is large with different members putatively involved in a diversity of processes that require cell expansion. In Arabidopsis, alterations in the expression of one expansin family member, AtEXP10, results in aberrant leaf morphology and pedicel abscission. The expansin-mediated growth of plant cell walls is enhanced by pretreatment of cell walls by hemicellulases and presumably this is also the case in vivo, but the hemicellulases tested to date contribute little to cell wall expansion relative to expansin. Yet, in plant parts that normally do not expand, the cells are resistant to acid-mediated elongation. There is therefore, a point in the life of a cell where it becomes rigidly fixed in size and unresponsive to molecules and environments that promoted cell elongation in the tissue at some earlier developmental stage. This is termed rigidifying the cell wall and generally fixes cell size irreversibly. The process of regidification is thought to involve the reduction of cell wall loosening processes, a more comprehensive cross-linking of cell wall components resisting cell wall expansion, and an alteration in the components of the cell wall, stiffening it against extension. Cell-cell communication: Apoplastic and Symplastic information retrieval and propagation. Communication among cells takes place both apoplastically and symplastically in the plant (apoplast and symplast defined on the required terms page). Apoplastic signaling is dependent on the assembly and accessibility of a variety of receptors on the plasmalemma (plasma membrane). The receptor must span the plasma membrane completely and be capable of initiating a biochemical change on the interior surface as a consequence of stimulation on the exterior surface, usually by interaction with, and/or attachment to other proteins. The former event is depicted by the ethylene receptors. These receptors have an Nterminus that contains three membrane-spanning domains that embed the receptor in the membrane and also serve to bind ethylene in the presence of a copper cofactor. These proteins span the plasma membrane as homodimers. The presence of ethylene at the surface of the cell is communicated to the interior of the cell by a change in the phosphorylation status of the receptors cytoplasmic surface. This, in turn, triggers further signal transduction, ultimately changing gene expression in the nucleus. The second form of receptor is best depicted by a receptor that, when stimulated, activates phospholipase-C. This is a very common form of receptor in animal cells. The activated phospholipase-C hydrolyses inositol containing phospho-lipids, liberating a number of bio-active "secondary messengers" on the cytoplasmic face of the membrane which stimulate a signal cascade that results in a change in gene expression. Cell-cell communication in plants includes the regulation of plant growth and development by phytohormones, the receptor-ligand signaling evident in pollen-stigma interactions leading to self-incompatibility responses, and intercellular trafficking of substances through plasmodesmata (PD). PD are unique to the plant, a consequence of how cell division occurs in these organisms. The fact that: 1) there is incomplete separation of the cytoplasm during cytokinesis and; 2) a cell plate forms de novo across the cell but leaves some regions of cytoplasmic continuity between the two daughter cells, gives rise to plasmodesmata between the cell clones. Hence, every set of daughter cells are symplastically continuous through what has become known as primary, unbranched PD. Cells that are not derived from the same mother cell can also form PD connections and do so frequently. The mechanism by which these PD are formed varies from that of primary PD in that secondary branched PD can form de novo in preexisting cell walls. They usually consist of multiple cytoplasmic strands that converge between the two cells in a central cavity before branching into multiple channels again to enter the second cell. Symplastic domains: The cells of the embryos of those plants studied to date have been shown to be all symplastically continuous. However, soon after the completion of germination, this symplastic continuity is disrupted so that some assemblages of cells in the seedling lose their PD connections with other assemblages. This establishes groups of cells that, although in direct 8 symplastic contact with each other, are isolated symplastically from additional groups of cells setting up domains of cells that, although in close proximity to each other may be in very different physiological states. The symplastic continuity of cells and their isolation from neighboring cells has vast implications for plant cell differentiation which is dependent on cell position. For example, the guard cells comprising the stomata are completely symplastically isolated by the time the stomata are completely differentiated. The PD connections between the guard cells and their surrounding cells are lost though protein degradation of PD components. In addition, evidence from cell ablation studies has shown that more mature cells in the plant root tip dictate to recently divided cells more distal in the same file, their developmental fate. Moreover, there exists a mechanism to dictate the direction of propagation of such a signal so that laterally adjacent cells are unaffected by signals produced in the more mature cell, only those cells within the same file and less mature than the cell producing the signal are influenced. One mechanism allowing this type of developmental control would be to have the cells within a file, all clones of their initial at the root apex, symplastically continuous with each other through primary PD and symplastically isolated from adjacent files of cells. This is only a hypothesis but is a possible explanation for this developmental phenomenon. In support of this hypothesis are two observations made with diffusable dyes. The first is that the more mature cells of the arabidopsis epidermis are symplastically isolated from the underlying cells of the cortex. Additionally, root hairs are also symplastically isolated from the epidermal cells surrounding them. A mutant in cell wall development (knolle) artificially maintains symplastic connections between symplastic domains not normally associated. As a probable result, the knolle mutant has many abnormalities in development. Plasmodesmatal connections can also undergo developmental changes during the differentiation of plant cells. In young buds and flowers of Setcreasea purpurea the PD size exclusion limits (SEL) of stamen hairs are less than 1000 Daltons while in senescing flowers, the SEL is greater than 4.4 kD. SEL also increases due to environmental stimuli. Anoxia increases SEL in wheat roots and osmotic stress increases SEL in pea roots while changes in light quality alters symplastic trafficking rates in maize. One of the most studied consequences of SEL is the mechanism of symplastic phloem loading used by plants that transport the raffinose-family oligosaccharides. Additionally, turgor differences between adjacent cells can close existing plasmodesmata so that the cells are symplastically isolated despite the existence of plasmodesmata. Differences in turgor pressure gating plasmodesmata is a sound strategy to prevent catastrophic dehydration should one cell in a symplastic domain become injured. Plasmodesmata are also closed by callose plugs in the case of injury to the cell. The developmental changes that regulate PD function and SELs has profound implications for plant-virus interactions. Plant viruses have been shown to be capable of altering the SEL of PD considerably. However, this ability to alter SEL is modified by host developmental stage and physiological state. Hence, arabidopsis plants grown under long-days mature early and are resistant to the cauliflower mosaic virus (CaMV) while those grown under short-days mature slowly and are not as resistant to CaMV infection. By anology, if pathogenic molecules/organisms can move systemically though PD, then it is anticipated that endogenous protective molecules, so called pathogen related (PR) molecules, may also turn out to be targeted to PD for further transport into neighboring cells. 9 Closed Plasmodesmata Extracellular sphincter Open Plasmodesmata Neck Central cavity Cell wall Plasmamembrane Cytoplasmic sleeve Desmotubule Redrawn from The Plant Cell 9: 1047. Figure 8: Diagramatic representation of one model of plasmodesmata structure. Plasmodesmata are complex organelles, certainly not a simple "hole-in-the-wall". The cytoplasmic sleeve of plasmodesmata appears to be subdivided into many smaller microchannels (Fig. 8). The "spokes" detected in the lumen of the plasmodesmata are probably comprised of actin, a protein that has been localized to the PD. Alterations in the size exclusion limit of PD has led to intensive study of various viral and plant proteins thought to function in the modification of the SEL of the PD. These proteins are capable of transporting select, very large proteins and nucleic acid polymers through the plasmodesmata. These so called movement proteins are currently under intense scrutiny to determine how, mechanistically, they permit such trafficking. With their discovery has come the realization that the PD probably play a hither to vastly undervalued role in cell-to-cell communication in plants. Models of transport through PD: Cell biologists use inert, usually fluorescent, dextrans of precisely defined size to determine the SEL of plasmodesmata. Using these dextrans in concert with plant or viral movement proteins has revealed some interesting results regarding the probably method of transport through PD. Evidence exists that there is more than one type of protein facilitated movement through PD. Relatively short distances between mesophyll cells leads to short PD which, upon being dilated by movement proteins in the presence of large dextran molecules, permit the transport of both since the whole route is gated open simultaneously. However, longer PD linking trichome cells with other cells may either; 1) be capable of unfolding proteins with associated transport signals and transporting them through the undilated PD to refold on the other side or; 2) gate the PD open very quickly and propagate the open state of the PD along the PD so that the PD is not open from neck to neck simultaneously in response to a movement protein signal. Either scenario results in the trafficking of the movement protein only and the exclusion of the dextrans from transport into neighboring cells unlike movement through PD in mesophyll cells. Literature: Chang C., and Shockey JA. 1999. The ethylene-response pathway: signal perception to gene regulation. Current Opinions in Plant Biology. 2: 352-358. 10 Cheng, J.-C., Seeley, K.A., and Sung, Z.R. (1995) RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip, Plant Physiol. 107, 365-376. Cho H-T, Cosgrove DJ. 2000. Altered expression of expansin modulates leaf growth and pedicel abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 97: 9783-9788. Hanzawa Y, Takahashi T, and Komeda Y. 1997. ACL5: an Arabidopsis gene required for internodal elongation after flowering. The Plant J. 12: 863-874. Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, and Komeda Y. 2000. ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. The EMBO J. 19: 4248-4256. Nicol F, His I, Jauneau A, Vernhettes S, Canut H, and Höfte H. 1998. A plasma membrane-bound putative endo-1,4-beta-D-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. The EMBO J. 17: 5563-5576. Vernoux, T., Wilson, R.C., Seeley, K.A., Reichheld, J.-P., Muroy, S., Brown, S., Maughan, S.C., Cobbett, C.S., Van Montagu, M., Inzé, D., May, M.J., and Sung, Z.R. (2000) The ROOT MERISTEMLESS1/ CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development, Plant Cell 12, 97-109. 11