* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download cell cycle - Chair of Computational Biology
Cell membrane wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Signal transduction wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
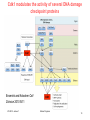
Programmed cell death wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
V7: cell cycle The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication (replication). In cells without a nucleus (prokaryotes), the cell cycle occurs via a process termed binary fission. In cells with a nucleus (eukaryotes), the cell cycle can be divided in 2 brief periods: interphase—during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA—and the mitosis (M) phase, during which the cell splits itself into two distinct cells, often called "daughter cells". Each turn of the cell cycle divides the chromosomes in a cell nucleus. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 1 Phases The cell cycle consists of 4 distinct phases: - G1 phase, - S phase (synthesis), - G2 phase (collectively known as interphase) - and M phase (mitosis). Activation of each phase is dependent on the proper progression and completion of the previous one. Cells that have temporarily or reversibly stopped dividing are said to have entered a state of quiescence called G0 phase. Schematic of the cell cycle. Outer ring: I = Interphase, M = Mitosis; Inner ring: M = Mitosis, G1 = Gap 1, G2 = Gap 2, S = Synthesis. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 2 Activity during 4 phases M phase itself is composed of 2 tightly coupled processes: - mitosis, in which the cell's chromosomes are divided between the two daughter cells, and - cytokinesis, in which the cell's cytoplasm divides in half forming distinct cells. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 3 Regulation of the eukaryotic cell cycle Regulation of the cell cycle involves processes crucial to the survival of a cell, including the detection and repair of genetic damage as well as the prevention of uncontrolled cell division. The molecular events that control the cell cycle are ordered and directional; that is, each process occurs in a sequential fashion. It is impossible to "reverse" the cycle. Leland Hartwell Tim Hunt Paul Nurse Noble Price in Physiology/Medicine 2001 „for their discoveries of key regulators of the cell cycle“ Two key classes of regulatory molecules, cyclins and cyclin-dependent kinases (CDKs), determine a cell's progress through the cell cycle. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 4 protein kinase A Susan S. Taylor UC San Diego Masterson et al. Nat Chem Biol. 6, 825 (2010) WS 2010 – lecture 7 Cellular Programs Taylor et al. Phil Trans R.Soc. B (1993) 5 Cyclin – cdk2 complex crystal structure Cyclin A – cdk 2 complex red: PSTAIRE motif yellow: activation loop Nikola Pavletich Memorial Sloan-Kettering Cancer Center Cyclin A – cdk2 phosphorylated at Thr160 www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 6 Crystal structure p27 (Kip1) is shown bound to the CyclinA-Cdk2 complex, provoking profound changes in the kinase active site and rendering it inactive. p27(Kip1)-CyclinA-Cdk2 Complex p27 also interacts with the secondary substrate recognition site on the cyclin. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 7 Targets of Cdk1 (also known as Cdc28) Sofar, 75 targets of Cdk1 are known. Cdk1 is involved in positive and negative feedback loops that regulate transcriptional programs to control cell cycle progression; Clb, Cln: cyclins WS 2010 – lecture 7 Cellular Programs Enserink and Kolodner Cell Division 2010 5:11 8 Cdk1-phosphorylation sites Cdk1 substrates frequently contain multiple phosphorylation sites that are clustered in regions of intrinsic disorder, and their exact position in the protein is often poorly conserved in evolution, indicating that precise positioning of phosphorylation is not required for regulation of the substrate. Cdk1 interacts with nine different cyclins throughout the cell cycle. Expression of human cyclins through the cell cycle. Enserink and Kolodner Cell Division 2010 5:11 WS 2010 – lecture 7 www.wikipedia.org Cellular Programs 9 Cdk1 modulates the activity of several DNA damage checkpoint proteins Enserink and Kolodner Cell Division 2010 5:11 WS 2010 – lecture 7 Cellular Programs 10 Cd1-controlled targets and processes Abstract The cyclin dependent kinase Cdk1 controls the cell cycle, which is best understood in the model organism S. cerevisiae. Research performed during the past decade has significantly improved our understanding of the molecular machinery of the cell cycle. Approximately 75 targets of Cdk1 have been identified that control critical cell cycle events, such as DNA replication and segregation, transcriptional programs and cell morphogenesis. .... Conclusions In conclusion, the identification of Cdk1 targets during the past decade has greatly improved our understanding of the molecular mechanism of the cell cycle. Nonetheless, much work still needs to be done because many targets remain to be identified, the exact phosphorylation sites of many known Cdk1 targets have not been mapped and the consequences of these phosphorylations at the molecular often remain elusive. Enserink and Kolodner Cell Division 2010 5:11 WS 2010 – lecture 7 Cellular Programs 11 Cell cycle checkpoints Cell cycle checkpoints are control mechanisms that ensure the fidelity of cell division in eukaryotic cells. These checkpoints verify whether the processes at each phase of the cell cycle have been accurately completed before progression into the next phase. An important function of many checkpoints is to assess DNA damage, which is detected by sensor mechanisms. When damage is found, the checkpoint uses a signal mechanism either to stall the cell cycle until repairs are made or, if repairs cannot be made, to target the cell for destruction via apoptosis (effector mechanism). All the checkpoints that assess DNA damage appear to utilize the same sensorsignal-effector mechanism. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 12 G1 checkpoint The first checkpoint is located at the end of the cell cycle's G1 phase, just before entry into S phase, making the key decision of whether the cell should divide, delay division, or enter a resting stage. Many cells stop at this stage and enter a resting state called G0. Liver cells, for example, enter mitosis only around once or twice a year. The G1 checkpoint is where eukaryotes typically arrest the cell cycle if environmental conditions make cell division impossible or if the cell passes into G0 for an extended period. In animal cells, the G1 phase checkpoint is called the restriction point, and in yeast cells it is called the Start point. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 13 G2 checkpoint The second checkpoint is located at the end of G2 phase, triggering the start of the M phase (mitosis). In order for this checkpoint to be passed, the cell has to check a number of factors to ensure the cell is ready for mitosis. If this checkpoint is passed, the cell initiates many molecular processes that signal the beginning of mitosis. The CDKs associated with this checkpoint are activated by phosphorylation of the CDK by the action of a "Maturation promoting factor" (or Mitosis Promoting Factor, MPF). The molecular nature of this checkpoint involves the activating phosphatase Cdc25 which under favourable conditions removes the inhibitory phosphates present within the MPF complex. However, DNA is frequently damaged prior to mitosis, and, to prevent transmission of this damage to daughter cells, the cell cycle is arrested via inactivation of the Cdc25 phosphatase. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 14 Metaphase checkpoint The mitotic spindle checkpoint occurs at the point in metaphase where all the chromosomes have/should have aligned at the mitotic plate and be under bipolar tension. The tension created by this bipolar attachment is what is sensed, which initiates the anaphase entry. This sensing mechanism allows the degradation of cyclin B, which harbours a D-box (destruction box). Degradation of cyclin B ensures that it no longer inhibits the anaphase-promoting complex, which in turn is now free to break down securin. The latter is a protein whose function is to inhibit separase, the protein composite responsible for the separation of sister chromatids. Once this inhibitory protein is degraded via ubiquitination and subsequent proteolysis, separase then causes sister chromatid separation. After the cell has split into its two daughter cells, the cell enters G1. www.wikipedia.org WS 2010 – lecture 7 Cellular Programs 15 The classical model of cell-cycle control Nature Reviews Molecular Cell Biology 9, 910-916 (2008) Cyclin-dependent kinases (cDKs) trigger the transition from G1 to S phase and from G2 to M phase by phosphorylating distinct sets of substrates. The metaphase-to-anaphase transition requires the ubiquitylation and proteasome-mediated degradation of mitotic B-type cyclins and various other proteins, and is triggered by the anaphase-promoting complex/cyclosome (APc/c) e3 ubiquitin ligase WS 2010 – lecture 7 Cellular Programs 16 The classical model of cell-cycle control cDK1 and cDK2 both show promiscuity in their choice of cyclin partners and can bind cyclins A, B, D and E, whereas cDK4 and cDK6 only partner Dtype cyclins. Thick lines represent the preferred pairing for each kinase Nature Reviews Molecular Cell Biology 9, 910-916 (2008) WS 2010 – lecture 7 Cellular Programs 17 The classical model of cell-cycle control According to the classical model of cell-cycle control, D-type cyclins and cDK4 or cDK6 regulate events in early G1 phase (not shown), cyclin e–cDK2 triggers S phase, cyclin A–cDK2 and cyclin A–cDK1 regulate the completion of S phase, and cDK1–cyclin B is responsible for mitosis. But see Paper 7 .... Nature Reviews Molecular Cell Biology 9, 910-916 (2008) WS 2010 – lecture 7 Cellular Programs 18 Tim Hunt about these new experiments ... According to the classical model of cell-cycle control ... ... The first serious blow to this oderly scheme was the discovery that mice that lack CDC2, although infertile, are viable and healthy. ... Deletion of other CDKs and cyclins in mice led to a ruther revision of the „specialized CDK“ hypothesis for the mammalian cell cycle. ... Santamaria et al. Recently published the ultimate step in this line of work. ... Nature Reviews Molecular Cell Biology 9, 910-916 (2008) WS 2010 – lecture 7 Cellular Programs 19 Tim Hunt about these new experiments ... Why were the earlier experiments so misleading? Quite simply, they were not sufficiently rigorous. Antibody injection and antisense experiments are inherently difficult to control and interpret correctly, and they cannot substitute for ablations that are achieved using gene-targeting. Although dominant-negative mutants give clear results, they can be problematic if several kinases share the same activating partners. Nature Reviews Molecular Cell Biology 9, 910-916 (2008) WS 2010 – lecture 7 Cellular Programs 20