* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture02
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell nucleus wikipedia , lookup
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
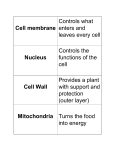
Welcome to Biology 160! General Biology w/ Lab Dr. Colleen Sheridan Mon/Wed 6-9:20p Rm AS 1617 Section 08 Logistics Attendance/Wait List Class Website: Please download and print Lab #2:Cell Diversity TODAY: Media Reading Conference and Review Ch1,2,3 from last week 20 min break Lecture Ch 4 & 5 Presentations Media Reading During your conference: 1) Each person read ONE summary 2) All discuss what evidence was given for and against a reliable resource The Scope of Life – Biology is the scientific study of life. Life is structured on a size scale ranging from the molecular to the global. Biology’s scope stretches across the enormous diversity of life on Earth. Biosphere Ecosystem African savanna Community All organisms in savanna Organism Zebra Population Herd of zebras Organ system Circulatory system Organ Heart Cell Tissue Heart muscle cell Heart muscle tissue Molecule DNA Atom Oxygen atoms Ecosystems Each organism interacts continuously with its environment and each is affected by the other. Inflow of light energy Loss of heat energy Chemical energy (food) Ecosystem dynamics: Producers - Cycling of nutrients - Flow of energy Cycling of nutrients Consumers (plants and other photosynthetic organisms) (such as animals) Decomposers Ecosystem The prokaryotic cell is simple, small, and contains no organelles. The eukaryotic cell is larger and more complex and contains organelles. DNA! – All cells use DNA as the chemical material of genes. • Genes are the units of inheritance that transmit information from parents to offspring. – The language of DNA contains just four letters: • A, G, C, T The Three Domains of Life – The three domains of life are: • Bacteria • Archaea • Eukarya Prokaryotes Eukaryotes At least four kingdoms of Eukarya -Plantae -Fungi -Animalia -Protists (a group of multiple kingdoms) The Darwinian View of Life The evolutionary view of life came into focus in 1859 when Charles Darwin published The Origin of Species. Observing Natural Selection – There are many examples of natural selection in action. • The development of antibiotic-resistant bacteria is one. Tuberculosis - MDR-TB - XDR-TB Staphylcoccus aureus (staph) - CA-MRSA Observations Question Hypothesis Prediction Test does not support hypothesis; revise hypothesis or pose new one Test Test supports hypothesis Chapter 2 Essential Chemistry for Biology Matter: Elements and Compounds – Matter is anything that occupies space and has mass. – Matter is found on the Earth in three physical states: •Solid •Liquid •Gas – Twenty-five elements are essential to life. • Four of these make up about 96% of the weight of the human body. • Trace elements occur in smaller amounts. Atoms – Atoms are composed of subatomic particles. • A proton is positively charged. • An electron is negatively charged. • A neutron is electrically neutral. Example: a helium atom Isotopes – Isotopes are alternate mass forms of an element. • They have the same number of protons and electrons. • But they have a different number of neutrons. – In radioactive isotopes, • The nucleus decays, giving off particles and energy. – Radioactive isotopes have many uses in research and medicine. • Example: PET scans – Uncontrolled exposure to radioactive isotopes can harm living organisms by damaging DNA. • Example: the 1999 Chernobyl nuclear accident Chemical Bonding and Molecules – Chemical reactions enable atoms to give up or acquire electrons in order to complete their outer shells. • These interactions usually result in atoms staying close together. • The atoms are held together by chemical bonds. Ionic Bonds – When an atom loses or gains electrons, it becomes electrically charged. • Charged atoms are called ions. • Ionic bonds are formed between oppositely charged ions. Covalent Bonds – A covalent bond forms when two atoms share one or more pairs of outer-shell electrons. Hydrogen Bonds – Water is a compound in which the electrons in its covalent bonds are shared unequally. • This causes it to be a polar molecule, one with opposite charges on opposite ends. + - – The polarity of water results in weak electrical attractions between neighboring water molecules. • These interactions are called hydrogen bonds. Chemical Reactions – Cells constantly rearrange molecules by breaking and forming chemical bonds. • These processes are called chemical reactions. – Chemical reactions cannot create or destroy matter, • They only rearrange it. Water’s Life-Supporting Properties – The polarity of water molecules and the hydrogen bonding that results explain most of water’s life-supporting properties: • • • • Water’s cohesive nature Water’s ability to moderate temperature Floating ice Versatility of water as a solvent To describe the acidity of a solution, we use the pH scale Chapter 3 The Molecules of Life Carbon Chemistry – Carbon is a versatile atom. • It has four electrons in an outer shell that holds eight. • Carbon can share its electrons with other atoms to form up to four covalent bonds. – The simplest organic compounds are hydrocarbons. • These are organic molecules containing only carbon and hydrogen atoms. • The simplest hydrocarbon is methane. – The unique properties of an organic compound depend not only on its carbon skeleton but also on the atoms attached to the skeleton. • These atoms are called functional groups. – Most macromolecules are polymers. • Polymers are made by stringing together many smaller molecules called monomers. • Cells link monomers by dehydration reactions. – Organisms also have to break down macromolecules. • Cells do this by a process called hydrolysis. Biological Molecules – There are four categories of large molecules in cells: • Carbohydrates • Lipids • Proteins • Nucleic acids Monosaccharides small sugar molecules – Monosaccharides are simple sugars. • Glucose is found in sports drinks. • Fructose is found in fruit. Monomer - means “one” unit Dimer - means “two” units Polymer - means “many” units Isomers – The monosaccharides glucose and fructose are isomers. • They have the same formula, but their atoms are arranged differently. – In aqueous solutions, monosaccharides form rings. Monosaccharides are the main fuel that cells use to do work. Disaccharides – A disaccharide is a double sugar. • It is constructed from two monosaccharides. – Disaccharides are joined through a dehydration reaction. Polysaccharides – Complex carbohydrates are called polysaccharides. • They are long chains of sugar units. • They are polymers of monosaccharides. Fats – Dietary fat consists largely of the molecule triglyceride. • Triglyceride is a combination of glycerol and three fatty acids. – Unsaturated fatty acids • Have less than the maximum number of hydrogens bonded to the carbons. (double and triple bonds) – Saturated fatty acids • Have the maximum number of hydrogens bonded to the carbons. (all single bonds) Saturated Fatty Acids: Too much of a good thing can be bad – Most animal fats have a high proportion of saturated fatty acids, which can be unhealthy. • Example: butter – Most plant oils tend to be low in saturated fatty acids. • Example: corn oil WHY are too many saturated fats unhealthy? – Synthetic anabolic steroids are controversial. • They are variants of testosterone. Proteins – A protein is a polymer constructed from amino acid monomers. – Proteins perform most of the tasks the body needs to function. Structural Proteins Receptor Proteins Storage Proteins Enzymes Contractile Proteins Hormonal Proteins Transport Proteins Sensory Proteins Defensive Proteins Gene Regulatory Proteins Structure of an Amino Acid Proteins as Polymers – Cells link amino acids together by dehydration reactions. • The resulting bond between them is called a peptide bond. Protein Shape – Proteins have four levels of structure. Nucleic Acids – Nucleic acids are information storage molecules. • They provide the directions for building proteins. Transcription Translation – Nucleic acids are polymers of nucleotides. – Each DNA nucleotide has one of the following bases: – Nucleotide monomers are linked into long chains. • These chains are called polynucleotides, or DNA strands. • A sugar-phosphate backbone joins them together. – Two strands of DNA join together to form a double helix. – RNA, ribonucleic acid, is different from DNA. • Its sugar has an extra OH group. • It has the base uracil (U) instead of thymine (T). Evolution Connection: DNA and Proteins as Evolutionary Tape Measures You can use DNA and protein sequences to test how closely related two species are in evolution. Copyright © 2007 Pearson Education, Inc. publishing as Pearson Benjamin Cummings Figure 4.6a The Microscopic World of Cells – Organisms are either: • Single-celled, such as most bacteria and protists • Multicelled, such as plants, animals, and most fungi Microscopes as a Window on the World of Cells – The light microscope is used by many scientists. • Light passes through the specimen. • Lenses enlarge, or magnify, the image. Euglena in a light microscope QuickTime™ and a YUV420 codec decompressor are needed to see this picture. Characteristics of Microscopes – Magnification • Is an increase in the specimen’s apparent size. – Resolving power • Is the ability of an optical instrument to show two objects as being separate. The Discovery of Cells and the Cell Theory – Cells were first discovered in 1665 by Robert Hooke. – The accumulation of scientific evidence led to the cell theory. • All living things are composed of cells. • All cells are formed from previously existing cells. – The electron microscope (EM) uses a beam of electrons. • It has a higher resolving power than the light microscope. – The electron microscope can magnify up to 100,000X. • Such power reveals the diverse parts within a cell. – The scanning electron microscope (SEM) is used to study the detailed architecture of the surface of a cell. – The transmission electron microscope (TEM) is useful for exploring the internal structure of a cell. The Two Major Categories of Cells – The countless cells on earth fall into two categories: • Prokaryotic cells • Eukaryotic cells – Prokaryotic and eukaryotic cells differ in several respects. The Two Major Categories of Cells – The countless cells on earth fall into two categories: • Prokaryotic cells • Eukaryotic cells – Prokaryotic and eukaryotic cells differ in several respects. Figure 4.4 – Prokaryotic cells • Are smaller than eukaryotic cells. • Lack internal structures surrounded by membranes. • Lack a nucleus. A Panoramic View of Eukaryotic Cells – An idealized animal cell – An idealized plant cell Cytoplasmic Streaming QuickTime™ and a YUV420 codec decompressor are needed to see this picture. Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Membrane Structure – The plasma membrane separates the living cell from its nonliving surroundings. The Plasma Membrane: A Fluid Mosaic of Lipids and Proteins – The membranes of cells are composed mostly of: • Lipids • Proteins Copyright © 2007 Pearson Education, Inc. publishing as Pearson Benjamin Cummings – The lipids belong to a special category called phospholipids. – Phospholipids form a two-layered membrane, the phospholipid bilayer. – Most membranes have specific proteins embedded in the phospholipid bilayer. – Membrane phospholipids and proteins can drift about in the plane of the membrane. – This behavior leads to the description of a membrane as a fluid mosaic: • Molecules can move freely within the membrane. • A diversity of proteins exists within the membrane. Cell Surfaces – Most cells secrete materials for coats of one kind or another • That are external to the plasma membrane. – These extracellular coats help protect and support cells • And facilitate interactions between cellular neighbors in tissues. – Plant cells have cell walls, • Which help protect the cells, maintain their shape, and keep the cells from absorbing too much water. – Animal cells have an extracellular matrix, • Which helps hold cells together in tissues and protects and supports them. Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella The Nucleus and Ribosomes: Genetic Control of the Cell – The nucleus is the manager of the cell. • Genes in the nucleus store information necessary to produce proteins. Structure and Function of the Nucleus – The nucleus is bordered by a double membrane called the nuclear envelope. • It contains chromatin and a nucleolus. Chromatin: long strands of DNA and associated proteins Nucleolus: makes the component parts of ribosomes Ribosomes: Protein Synthesis – Ribosomes are responsible for protein synthesis. How DNA Controls the Cell – DNA controls the cell by transferring its coded information into RNA. • The information in the RNA is used to make proteins. Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella The Endomembrane System: Manufacturing and Distributing Cellular Products – Many of the membranous organelles in the cell belong to the endomembrane system. The Endoplasmic Reticulum – The endoplasmic reticulum (ER) • Produces an enormous variety of molecules. • Is composed of smooth and rough ER. Rough ER – The “roughness” of the rough ER is due to ribosomes that stud the outside of the ER membrane. – The functions of the rough ER include: • Producing two types of membrane proteins – Membrane proteins – Secretory proteins • Producing new membrane Rough ER – The “roughness” of the rough ER is due to ribosomes that stud the outside of the ER membrane. – The functions of the rough ER include: • Producing two types of membrane proteins – Membrane proteins – Secretory proteins • Producing new membrane – After the rough ER synthesizes a molecule, it packages the molecule into transport vesicles. Smooth ER – The smooth ER lacks the surface ribosomes of ER and produces lipids, including steroids. The Golgi Apparatus – The Golgi apparatus • Works in partnership with the ER. • Refines, stores, and distributes the chemical products of cells. Lysosomes – A lysosome is a membrane-enclosed sac. • It contains digestive enzymes. • The enzymes break down macromolecules. – Lysosomes have several types of digestive functions. • They fuse with food vacuoles to digest the food. – They break down damaged organelles. Vacuoles – Vacuoles are membranous sacs. • Two types are the contractile vacuoles of protists and the central vacuoles of plants. – A review of the endomembrane system Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Energy Conversion: Chloroplasts & Mitochondria – Cells require a constant energy supply to do all the work of life. Chloroplasts – Chloroplasts are the sites of photosynthesis, the conversion of light energy to chemical energy. Mitochondria – Mitochondria are the sites of cellular respiration, which involves the production of ATP from food molecules. – Mitochondria and chloroplasts share another feature unique among eukaryotic organelles. • They contain their own DNA. – The existence of separate “mini-genomes” is believed to be evidence that • Mitochondria and chloroplasts evolved from free-living prokaryotes in the distant past. Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella The Cytoskeleton: Cell Shape and Movement – The cytoskeleton is an infrastructure of the cell consisting of a network of fibers. Maintaining Cell Shape – One function of the cytoskeleton • Is to provide mechanical support to the cell and maintain its shape. – The cytoskeleton can change the shape of a cell. • This allows cells like amoebae to move. Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Structure Meets Function in a Eukaryotic Cell • Plasma Membrane and Cell Surface • Nucleus and Ribosomes • Endomembrane System – – – – Endoplasmic Reticulum (ER) Golgi Body Lysosomes Vacuoles • Energy Conversion: Chloroplasts and Mitochondria • Cytoskeleton • Cilia and Flagella Cilia and Flagella – Cilia and flagella are motile appendages. – Flagella propel the cell in a whiplike motion. – Cilia move in a coordinated back-and-forth motion. Paramecium Cilia QuickTime™ and a YUV420 codec decompressor are needed to see this picture. – Some cilia or flagella extend from nonmoving cells. • The human windpipe is lined with cilia. Using scientific data some computer scientists put together a movie of the inside of a living cell: http://www.studiodaily.com/main/technique/tprojects/6850.html Some Basic Energy Concepts – Energy makes the world go around. • What is energy? Conservation of Energy – Energy is defined as the capacity to perform work. – Kinetic energy is the energy of motion. – Potential energy is stored energy. – Energy can be changed from one form to another. • However, it cannot be created or destroyed. • This is the conservation of energy principle. Heat • Is a type of kinetic energy. • Is a product of all energy conversions. Entropy – Scientists use the term entropy as a measure of disorder, or randomness. • All energy conversions increase the entropy of the universe. Chemical Energy • Is a form of potential energy. • Is found in food, gasoline, and other fuels. – Living cells and automobile engines use the same basic process to make chemical energy do work. Combustion Cellular Respiration – Cellular respiration • Is the energy-releasing chemical breakdown of fuel molecules. • Provides energy for the cell to do work. Food Calories – A calorie is the amount of energy that raises the temperature of one gram of water by one degree Celsius. – The kilocalorie is • 1,000 calories. • The unit used to measure the energy in food. Potential (Chemical) Energy In Foods Kinetic Energy Used by Activities ATP and Cellular Work – The chemical energy of organic molecules is released in cellular respiration to make ATP in the mitochondria. ATP is the currency of the cell. The Structure of ATP – ATP (adenosine triphosphate) • Consists of adenosine plus a tail of three phosphate groups. • Is broken down to ADP, accompanied by the release of energy. Phosphate Transfer – ATP can energize other molecules by transferring phosphate groups. • This energy can be used to drive cellular work. The ATP Cycle – Cellular work spends ATP. – ATP is recycled from ADP and phosphate through cellular respiration. Enzymes – Metabolism is the sum total of all chemical reactions that occur in organisms. – Few metabolic reactions occur without the assistance of enzymes. Biology and Society: Stonewashing Without the Stones – The sturdy cotton fabric denim has been worn because of its toughness and appeal. – Stonewashing jeans with pumice stone can damage the fabric. – Recently the enzyme cellulase has been used to achieve better results. Copyright © 2007 Pearson Education, Inc. publishing as Pearson Benjamin Cummings Activation Energy – Activation energy • Is the energy that activates the reactants in a chemical reaction. • Triggers a chemical reaction to proceed. – Enzymes • Lower the activation energy for chemical reactions. Figure 5.8 Induced Fit – Each enzyme is very selective. • It catalyzes specific reactions. – Each enzyme recognizes a specific substrate. • The active site fits to the substrate, and the enzyme changes shape slightly. • This interaction is called induced fit. – Enzymes can function over and over again. How Enzymes Work Enzyme Inhibitors – Enzyme inhibitors • Can inhibit a metabolic reaction. • Bind to the active site, as substrate imposters. – Other inhibitors • Bind at a remote site, changing the enzyme’s shape. • In some cases, this is called feedback regulation. Membrane Function – Working cells control the transport of materials to and from the environment with membranes. Transport of materials Passive Transport: Diffusion Across Membranes – Molecules contain heat energy. • They vibrate and wander randomly. – Diffusion is one result of the movement of molecules. • Molecules tend to spread into the available space. • Diffusion is passive transport; no energy is needed. – Another type of passive transport is facilitated diffusion, the transport of some substances by specific transport proteins that act as selective corridors. Osmosis and Water Balance in Cells – Osmosis is the passive transport of water across a selectively permeable membrane. [solute] = [solute] [solute] Plasmolysis Water Balance in Cells – Osmoregulation is the control of water balance in animals. Contractile vacuoles in Protists Kidneys and gills in freshwater fish – Water balance in plant cells is different. • They have rigid cell walls. • They are at the mercy of the environment. Membrane Function – Working cells control the transport of materials to and from the environment with membranes. – They do this with the aid of membrane proteins. Active Transport: The Pumping of Molecules Across Membranes – Active transport requires energy to move molecules across a membrane. Exocytosis and Endocytosis: Traffic of Large Molecules – Exocytosis • Secretes substances outside of the cell. – Endocytosis • Takes material into the cell. – In phagocytosis (“cellular eating”), a cell engulfs a particle and packages it within a food vacuole. – In pinocytosis (“cellular drinking”), a cell “gulps” droplets of fluid by forming tiny vesicles. – Phagocytosis at work… A macrophage (big eater) Plasma membrane = green QuickTime™ and a decompressor are needed to see this picture. Infectious agent = red – Receptor-mediated endocytosis • Is triggered by the binding of external molecules to membrane proteins. The Role of Membranes in Cell Signaling – Cellular communication • Begins with the reception of an extracellular signal. – The signal transduction pathway • Consists of proteins and other molecules that relay the signal. Figure 5.20