* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Age-related changes in the hippocampal subdivisions of the rat
Activity-dependent plasticity wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Optogenetics wikipedia , lookup
Dendritic spine wikipedia , lookup
Synaptogenesis wikipedia , lookup
Subventricular zone wikipedia , lookup
Development of the nervous system wikipedia , lookup
Hippocampus wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Aging brain wikipedia , lookup
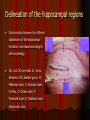
Age-related changes in the hippocampal subdivisions of the rat Mohammad Hosseini-sharifabad, PhD Department of Anatomy Yazd University of Medical sciences E-mail:[email protected] Background Normal aging is commonly linked to a decline in learning and memory. • Understanding the mechanisms responsible for age-related cognitive changes remains a critical challenge in neurobiology. Many studies examining cellular substrates of this agerelated cognitive decline have focused on the hippocampal formation because its structural integrity is crucial for normal learning and memory and because it is especially vulnerable to the process of aging. Background The hippocampal formation shows early signs of agerelated changes in the brains of normal humans. Age differences in total volume of the hippocampus have been observed in healthy humans with the use of both brain–imaging methods and stereological techniques. Studies using modern stereological methods of quantification have established that, in rats, mice, monkeys and humans, the total number of granule cells in the dentate gyrus, and pyramidal neurons in CA3 and CA1 fields, preserved over the life span. Background These findings support the hypothesis that an agerelated decline in hippocampal-dependent learning and memory may result from changes in other morphometric parameters, rather than a loss of hippocampal neurons. age-related changes in dendrites are of particular interest since dendrites are the targets of the majority of synapses and since dendrites remain subject to structural changes even into adulthood. Recent experimental studies and models of dendritic processing suggest that both the extent and pattern of the dendritic arbor could influence how synaptic inputs are integrated. Background In human studies, the influence of age on the hippocampus is confounded with other variables, such as inadequate nutrition, psychological and physical stress. Therefore, this study aimed to determine the effects of normal advanced aging on the hippocampal subdivisions using animal model of rat. Background The present study investigated the effects of aging on the volumes of the layers in hippocampal subregions, where the respective cell bodies or its processes were located. we also performed a quantitative morphological analysis of dendritic architecture of Golgi-impregnated hippocampal neurons from young and aged rats. Animals and Housing Male wistar rats were housed in a temperature- controlled (22± 2 ºC) animal room and on a 12 hr light/ dark cycle (light on at 07.00–19.00 hours) and provided food and water until sacrifice at 6(young) and 24 (old) months of age. Methods Rats were deeply anesthetized with urethan and transcardially perfused with a phosphate-buffered solution of 4% formaldehyde and 1% glutaraldehyde. Each brain was numbered and cerebellum and olfactory bulb were removed. The brains divided into hemispheres. One hemisphere was selected at random for estimating the volumes of layers, and the other for morphometric analysis of neuronal dendrites. Posterior portion of each hemisphere, which contained hippocampus, was taken. Coronal sections of 100µm thickness were cut serially with a calibrated vibratome into a bath of 3% potassium dichromate in distilled water. Delineation of the hippocampal regions Discrimination between the different subdivisions of the hippocampal formation was made according to cell morphology. CA1 and CA3 are fields of Cornu Ammonis; DG, dentate gyrus; M; Molecular layer; G, Granular layer; H, Hilus, O, Oriens layer; P, Pyramidal layer; R, Radiatum layer. Hematoxilin stain. Volume estimation The Cavalieri principle used to estimate the reference volume of the constituent layers of the hippocampal formation. A grid with a tessellation of points, randomly positioned on each section, and the points hitting each layer of hippocampal layer were counted. Volume estimation The number of points, P, multiplied with the area associated with each point, a (P), to obtain an unbiased estimate of sectional area of each profile. The sum of sectional areas of the layers was used to estimate reference volume, V (ref), from the following relationship, where t represents the distance between sections. V (ref) = t. P. a (p) = t. A Staining Incubate in 3% potassium dichromate in distilled water overnight Rinse in distilled water Mount on slides and glue coverslip over the sections at four corners Incubate in 1.5% silver nitrate in distilled water overnight in the dark The following day, dismantle the slide assemblies Rinse the tissue sections Rinse in distilled water Dehydrate in 95%, followed by absolute ethanol. Clear the sections in xylene Mount onto gelatinized slides Coverslip Cell selection The criteria employed for selecting the neurons to be measured were the following: (1) Dark and consistent impregnation throughout the extent of dendrites (2) Cell bodies located in the middle part of the section thickness to minimize branch segments cut off at the plan of the section (3) Relative isolation from neighbouring impregnated cells in order not to irresolvable overlap the dendrites of adjacent cells. Number of dendritic segments A Camera lucida drawing of the granule cell in which the classification of the dendritic segments is shown. The numbers represent the degree of dendrites: 1: terminal segments ; 2: intermediate segments originating two terminals ; 3, 4: intermediate segments divided in a terminal and an intermediate segment. The centrifugal ordering of dendritic trees was used to estimate the number of dendritic segments per cell. Dendritic branching density The branching density of dendritic trees was evaluated by applying the method of concentric rings. The number of dendritic intersections crossing each concentric ring centered in the cell body was counted. The concentric rings were calculated at interval of 20 µm for granule cells and 25 µm for CA3 and CA1 pyramidal cells. Whenever the dendrites extended beyond 375 µm (circle 15), they were included in circle 15. Results Statistical analysis revealed there is only a significant influence of age in the stratum radiatum and lacunosum-molecular of the CA1 pyramidal field, in where the volume was lower in aged than young rats (P<0.05). Results also showed that there is no significant difference in total volume of hippocampus between aged and young rats. Results T-test revealed a significant effect of normal aging on the total number of dendritic segments per cell in the CA1 pyramidal cells ( P=0.01) but not in the dentate granule cells and CA3 pyramidal cells ( table 1). The number of dendritic intersections showed the effect of aging was significant for circles 11, 12, 13, 14 and 15 in CA1 pyramidal cells (P<0.05). In these circles aged had a lower number of intersections than young rats. No significant age-related difference was detected in the dendritic branching density of granule cells and CA3 pyramidal cells. The total number of dendritic segments in granule cells and CA3 and CA1 pyramidal cells of rat hippocampus in young and aged rats. Animals Hippocampal Region Granule cells CA3 CA1 Young 20.9 ±2.0 41.0±3.6 41.0±3.6 Aged 18.5 ±2.0 39.1 ±3.6 36.5±1.8 P=0.210 P=0.314 P=0.01 Graphic representation of the dendritic branching density of hippocampal granule cells in the aged and young rats. Vertical bars represent SD. Aged (24 months) Young (6 months) Number of intersections Granule cells 8 7 6 5 4 3 2 1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Graphic representation of dendritic branching density of CA1 pyramidal cells in the aged and young rats. Vertical bars represent SD. Circles 11, 12, 13, 14 and . 15, P<0.05 Number of Intersections CA1 pyramidal cells 8 7 6 5 * 4 * 3 * * 2 * 1 0 1 B 2 3 4 5 6 7 8 Circles 9 10 11 12 13 14 15 Graphic representation of dendritic branching density of CA3 pyramidal cells in the aged and young rats. Vertical bars represent SD. Number of intersections CA3 pyramidal cells 9 8 7 6 5 4 3 2 1 0 1 C 2 3 4 5 6 7 8 Circles 9 10 11 12 13 14 15 Discussion Our present findings revealed the alterations in the terminal segments of the apical arbors of the CA1 hippocampal cells due to normal aging. Reports on age-related alterations in morphology of the rodent CA fields have been inconsistent, however. Some indicate a reduction in synaptic contacts and dendrites while others suggest preservation of connectivity. It is likely that methodological differences, such as rodent strain, age of subjects, and precise hippocampal region examined, contribute to the disparity of findings. Discussion Although there are several descriptions of age-related dendritic changes, the mechanisms controlling dendritic morphology in the adult and aging brain have not been elucidated. Since individual trophic factors promote dendritic growth of specific populations of neurons and can act within restricted dendritic domains, it is reasonable to propose that differential trophic support continues across the lifespan and that age-related declines in trophic support lead to dendritic regression in some neural regions. Discussion Throughout development and adulthood the availability of growth factors is likely to differ among cortical regions and layers due to differences in capillary density and blood flow. As levels of many blood-borne trophic factors decline with age, the focal differences in blood flow that exist throughout the lifespan, coupled with age-related vascular changes, may result in local deficiencies in one or more critical trophic factors that lead to dendritic regression among some neurons. Discussion The CA1 region is the hippocampal subdivision in which the cytoarchitectural organization and size have changed most during mammalian and its structural integrity has been reported to be particularly susceptible to ischemia. It is also reported that Microtubule –associated 2 protein (MAP2) has been decreased in the dendrites and axons of hippocampal CA1 neurons in aged mice. These findings demonstrate that dendrites and axons in the hippocampal CA1 neurons are particularly susceptible to aging processes. Conclusion This and similar descriptive studies demonstrate that throughout adulthood and senescence dendritic extent is regulated locally and that changes affect specific populations of neurons and restricted regions within the dendritic arbors. Recognition of which neurons are subject to dendritic regression and of how dendritic geometry is altered will facilitate experimental studies to assess the significance of dendritic change for neural function, as well as the mechanisms by which dendritic extent is regulated throughout the lifespan. Thank you for your attention