* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 6 - EukDNAexpression2007 - Cal State LA
Transposable element wikipedia , lookup
Genealogical DNA test wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Genetic engineering wikipedia , lookup
DNA damage theory of aging wikipedia , lookup
Mitochondrial DNA wikipedia , lookup
Cancer epigenetics wikipedia , lookup
DNA polymerase wikipedia , lookup
Human genome wikipedia , lookup
Adeno-associated virus wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Minimal genome wikipedia , lookup
Designer baby wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Nucleic acid double helix wikipedia , lookup
Molecular cloning wikipedia , lookup
Epigenomics wikipedia , lookup
DNA vaccination wikipedia , lookup
DNA supercoil wikipedia , lookup
Point mutation wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Microevolution wikipedia , lookup
Genome evolution wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Genomic library wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Non-coding DNA wikipedia , lookup
Genome editing wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
History of genetic engineering wikipedia , lookup
Primary transcript wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
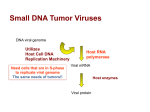
Expression and Replication of the Viral Genome in Eukaryotic Cells: The DNA Viruses (How do DNA viruses express their genomes in Eukaryotic cells?) Eukaryotic DNA Viruses The DNA viruses have a larger range in size than do the RNA viruses. Since their genome is of the same molecular type (DNA) as their host’s genome, they can use the host cellular machinery for replication, transcription and translation. If they are dependent upon host cell enzymes for replication they need to get to the host cell nucleus, and problems that they may encounter in replication are: Eukaryotic DNA Viruses The host cells must be in the S phase of the cell cycle when the enzymes for DNA replication are made and would be available for the virus to use. To overcome this problem: Small DNA viruses only infect cells that have naturally entered the S phase of the cell cycle. Other viruses have a way to force the host cells into the S phase of the cell cycle. Larger viruses simply encode their own enzymes for this process. That way they are not dependent on the host cell enzymes. If these viruses also bring in the enzymes for transcription, they may replicate entirely within the cytoplasm since they don’t need replication or transcription enzymes/proteins present in the host cell nucleus. Eukaryotic DNA Viruses If the genome is linear, DNA synthesis can’t occur at the 5’ ends of the template (lagging strand synthesis). This results in 3’ overhangs. To overcome this problem: The virus may use protein primers The virus may form concatamers The virus may resort to the use of reverse transcription. Expression Early genes encode nonstructural regulatory proteins for DNA replication Late genes are expressed after DNA replication and they encode the structural components of the virus. Eukaryotic DNA Viruses Outcomes when a DNA virus infects a cell Productive, acutely cytopathic infection – leads to the manufacture and release of new virions and, in the process, the infected cell dies. Lysis Apoptosis Persistent infection - the virus remains present for long periods of time without killing or seriously impairing cell function. A balance is struck between the virus and the host. This can also occur with RNA viruses, but it occurs more often with DNA viruses. There are two types of persistent infection: Eukaryotic DNA Viruses Chronic infection –can always recover infectious virus from the host Due to specific virus-host interactions Due to antibody or interferon production that limits, but does not completely inhibit virus production Due to production of defective-interfering particles Due to a combination of all three of the above Latent infection – the viral genome persists, but infectious virus can only be recovered during reactivation periods (more on this later on) Eukaryotic DNA Viruses Transformation – viral infection changes the cellcell communication and growth regulation in the host cell which then takes on the characteristics of a tumor cell (more about this later on). The viral genome or part of it is retained, but infectious virus is usually not produced. In general, for DNA viruses that are capable of causing transformation, in a permissive host there is a productive infection and the host cell dies rather than being transformed. In a nonpermissive host transformation may occur. Eukaryotic DNA Viruses Double stranded DNA viruses Papovaviruses – includes 2 subfamilies – polyomavirinae (polyoma and SV40), and papillomavirinae They are small icosahedral viruses They have a circular DS DNA genome that encodes 5-7 proteins. We will look at SV40 as an example: The DNA is complexed with histones and encodes 6 proteins, 2 nonstructional proteins (early genes = large T Ag and small T Ag) and 4 structural proteins (VP1,VP2, VP3, and agnoprotein) SV40 Transcription Uses host RNA polymerase II Early genes encode the T Ags. that are expressed from differentially spliced mRNAs Late genes encode VP1, VP2, VP3, and the agnoprotein which are all expressed from 2 differentially spliced mRNAs. VP2 and VP3 are expressed from the same mRNA using different start codons. The agnoprotein is expressed from the same mRNA as VP1 using a different start codon. SV40 Virus SV40 virus Early gene expression Late gene expression SV40 Virus The early promoter contains a TATA box and is activated by host cell transcription factors and low concentrations of one of its products, the large T antigen The late promoter does not contain a TATA box. The shift from early to late gene expression occurs after DNA synthesis has begun. The late promoter region has multiple binding sites for a cellular repressor, Ibp. Once viral replication begins, the concentration of the late promoter becomes sufficiently high such that not all Ibp binding sites are occupied and the promoter becomes accessible to cellular transcription factors. The large T Ag binds to the promoter to help activate late gene transcription and to suppress early gene transcription. SV40 Virus – late gene expression SV40 Replication - The Large T Ag plays a role and has: ATPase activity Binding to DNA origin of replication Can serve as a DNA unwinding helicase. It opens the DNA at the origin of replication to let in a host cell primase complex to initiate bidirectional DNA replication. This results in a pair of relaxed DS molecules that get supercoiled by host topoisomerase II Gene activator activity for genes in the host cell that are involved in controlling the host cell cycle. Cellular DNA synthesis is induced so the the enzymes that the virus requires to replicate its genome are available. Large T Ags presence in tissue culture cells can result in immortalization of the cells ( cells are permanently going through the cell cycle, therefore they can be passaged again and again) Replication of the circular genome involves a theta form SV40 T Ag activities SV40 genome replication Eukaryotic DS DNA Viruses Adenovirus Has a naked, icosahedral capsid Has a linear, double-stranded DNA genome with Inverted terminal redundancies A 55 kd terminal protein attached covalently to the 5’ ends Transcription Both strands serve as templates for transcription by the host cell DNA dependent RNA polymerase II. Therefore, the strands are called the right and the left strand to indicate the direction of transcription. Immediate early gene – expression of E1A, the immediate early gene, is needed for the expression of the remaining early genes Early genes – use 5 different promoters with each product being spliced in at least three different ways. The early gene products are involved in the regulation of viral and cellular synthetic activities (S phase activation) Late genes are expressed after the onset of DNA synthesis and from a single promoter with a complex number of splicing reactions of the product. Adenovirus Adenovirus Adenovirus Replication – how can the virus replicate the ends of its linear genome? There is a terminal protein (TP) at the 5’ end of the genome that is involved as is a viral DNA polymerase, a viral single stranded binding protein, and two host cell transcription factors that the virus borrows to assist in its replication. All viral DNA synthesis is continuous. The host cell factors bind to and alter the conformation of the 3’ end of the DNA to promote binding of the viral DNA polymerase - TP precursor(80 kd protein) complex. The 80 kd TP precursor serves as a primer for DNA synthesis. Elongation from an OH a serine side chain causes displacement of the corresponding genomic DNA strand. Both strands may initiate synthesis at the same time or only one strand may initiate synthesis. Both strands use the 80 kd terminal protein precursor as a primer. It is cleaved to the 55 kd terminal protein during viral maturation. Adenovirus Adenovirus genome replication Eukaryotic DS DNA Viruses Herpesviruses Have an envelope and an icosahedral capsid Between the capsid and the envelope is a matrix called the tegument The genome is a linear, double stranded DNA with nicks or gaps The herpes viruses are neurotropic The and herpesviruses are lymphotropic The herpesviruses have been linked to human cancers (EBV and Kaposi sarcoma-associated virus) All form latent infections after the initial primary infection Include HSV 1 and 2, varicella-zoster, EBV, Kaposi sarcoma-associated virus, and CMV Herpesviruses Transcription Some use differential splicing and/or alternate promoters HSV-1 uses neither, but has a complex way of regulating the timing of gene expression Immediate early genes () – products are involved in regulating the expression of all three sets of genes (, , and ) Early genes () – products are involved in nucleic acid metabolism and DNA synthesis (they duplicate cellular genes) Late genes () - products are the structural proteins The control of all three sets of genes is by feedback loops HSV-1 A gene product ( trans induction factor or VP16) which is a virion tegument protein, enters the host nucleus with the genome and activates gene transcription. HSV-1 Replication Many gene products are involved in DNA replication – only 7 encode required functions Host cells do not need to be in the S phase of the cell cycle since the virus provides its own replication machinery A model involving circularization of the linear genome due to single nucleotides extensions that are complementary at each 3’ end Initial circle amplification Rolling circle replication to create concatamers Cleavage of concatamers during incorporation into virions HSV-1 genome replication 5’ end ’ Herpesvirus – proposed model for DNA replication Eukaryotic DS DNA Viruses Poxviruses Are the largest of all known viruses Have a core plus 2 lateral bodies and a lipoprotein coat plus an envelope Has a double stranded linear DNA genome with closed ends and termini with inverted repeats Carry all their own enzymes for replication and transcription, both of which occur in the cytoplasm Poxvirus genome Poxviruses Transcription Begins as soon as the viral core is released into the cytoplasm. All enzymes required to produce what looks like eukaryotic mRNA are part of the virus itself. Half of the genome is expressed from the early gene promoters. Late gene transcription occurs during and after DNA replication and is controlled by regulatory proteins and the configuration of the newly made DNA. After DNA replication begins, early mRNAs are not transcribed as efficiently, therefore less early mRNA is made. Those that are made are also degraded more rapidly. Poxviruses Replication Concatamers that are head to head and tail to tail rather than head to tail are formed A nick is made near 1 terminal repeat. This creates a free 3’ OH that serves as a primer for synthesis. The sequence now available to serve as template contains a set of bases that are self-complementary The newly added bases on the 3’ end are also selfcomplementary so they can fold back on themselves to begin a process of displacement synthesis The displaced DNA serves as a template to form a 2 genome long concatamer. The growing point doubles back on itself to use the newly replicated DNA as a template to create 4 genome length concatamers. Eventually staggered cuts and ligation create 4 genomes. Poxviruses Poxviruses Replication can occur from both ends at the same time Eukaryotic DNA Viruses Single stranded DNA viruses Parvoviridae Naked and small with a linear, SS DNA genome Have no means of forcing the host into the S phase of the cell cycle Those that infect mammalian or avian cells have (-) strand DNA. Others package either (+) or (-) strand DNA. There are three genera Parvoviridae Densoviruses – reproduce in insect cells Parvoviruses – reproduce in suitable mammalian hosts Dependoviruses – are replication defective. An example is the adeno associated viruses (AAV). They require that the host be infected with another virus to provide helper functions necessary for replication and they can package either the (+) or the (-) DNA strand. Autonomous viruses have all the information necessary to reproduce in a suitable host cell and they package (-) sense DNA strands as their genome. The genomes of the parvoviruses contain selfcomplementary sequences at the ends that form hairpin loops. The sequences at the two ends are the same fro the dependoviruses, but different from each other for the autonomous viruses. Parvovirus Parvovirus Expression of the parvovirus genome involves the use of three different promoters (two for the early, nonstructural and regulatory genes, and one for the late, structural genes), use of differential splicing, and alternative AUGs Adeno-associated Virus Replication Replication of parvoviruses is very complicated and involves self-priming because of the self-complementary sequences at each end. It involves SS displacement synthesis leading to concatamer formation. The replication defective AAV have self-complementary, inverted repeats at their 5’ and 3’ ends. The hairpin loop in the 3’ terminus serves as a primer for elongation down the entire length of the genome A nick in the original strand permits the hairpin to straighten out and thus serve as template for elongation of the newly created 3’ end Note that each of the resulting DNA molecules is a hybrid of new and old DNA Note that the sequence of some of the bases within the terminal repeat has been reversed. Adeno-associated Virus Replication Autonomous parvovirus genome replication Eukaryotic DNA Viruses Hepadenaviruses Are very small with a capsid about the size of parvovirus Have an envelope with glycoprotein spikes Genome is partially SS, circular, nicked DNA The longer strand is the (-) strand. It has a protein attached to the 5’ end and it serves as a template for transcription of all viral mRNAs. The shorter strand is of varying length. It has a 19 basepair RNA attached to its 5’end. Hepadenaviruses The two strands basepair in an overlapping fashion with breaks in both strands. Note that there is a pair of direct repeated sequences, DR1 and DR2. Hepadenaviruses We will look at the human hepatitis B virus Immediately upon entering a host cell, the genome is trasnsported to the nucleus and the shorter strand elongates to the length of the longer strand, both strands become unblocked at their 5’ ends, and the genome is converted to a covalently closed circular DNA. Transcription uses the host RNA polymerase II and occurs from 4 viral promoters. Using these promoters, 1 genomic and 3 subgenomic mRNAs are made. HBV The 4 mRNAs encode seven proteins. The mRNAs all start at different 5’ sites, but have the same 3’ terminus The largest is longer than the entire genome – the promoter is upstream of the 3’ cleavage/poly A site, but the poly A site is not used until the second time it is encountered . Two of the mRNAs (for the genomic and S promoters) show heterogenous 5’ ends. Expression of P from the genomic mRNA may occur from ribosome slippage during translation. HBV HBV Replication Uses an RNA intermediate. Uses the 2 direct repeat sequences, DR1 and DR2 Begins with synthesis of a pregenomic RNA equivalent to the longest mRNA. HBV Reverse transcriptase synthesizes a (-) DNA strand using the pregenomic RNA as a template. Synthesis begins at the 3’ DR1 Synthesis is primed by the viral DNA polymerase acting as a protein primer attached to the 5’ end. All the pregenomic RNA except the DR1 region is degraded by the RNAse H activity of the RT. Synthesis of the (+) strand requires a jumping reaction in which the DR1 at the 5’ end of the (+) strand basepairs to the DR2 at the 5’ end of the (-) strand. HBV HBV Circularization of the negative strand allows the viral polymerase to synthesize the (+) strand using the RNA as a primer. Assembly occurs during these steps. DNA synthesis ceases when the particle leaves the cell. Therefore different lengths of the (+) strand are made. HBV - summary