* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
List of types of proteins wikipedia , lookup
Messenger RNA wikipedia , lookup
Community fingerprinting wikipedia , lookup
Gene desert wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Non-coding DNA wikipedia , lookup
Biochemistry wikipedia , lookup
Epitranscriptome wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Genomic imprinting wikipedia , lookup
Molecular evolution wikipedia , lookup
Non-coding RNA wikipedia , lookup
Genome evolution wikipedia , lookup
Ridge (biology) wikipedia , lookup
Transcription factor wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Point mutation wikipedia , lookup
Expression vector wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Gene expression profiling wikipedia , lookup
Gene expression wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene regulatory network wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
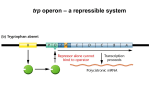
Chapter 17 Regulation of Gene Expression in Bacteria and Bacteriophages Copyright © 2010 Pearson Education Inc. Growth and division genes of bacteria are regulated genes. Their expression is controlled by the needs of the cell as it responds to its environment with the goal of increasing in mass and dividing. Genes that generally are continuously expressed are constitutive genes (housekeeping genes). Examples include protein synthesis and glucose metabolism. All genes are regulated at some level, so that as resources dwindle the cell can respond with a different molecular strategy. Prokaryotic genes are often organized into operons that are cotranscribed. A regulatory protein binds an operator sequence in the DNA adjacent to the gene array and controls production of the polycistronic (polygenic) mRNA. Gene regulation in bacteria and phage is similar in many ways to the emerging information about gene regulation in eukaryotes, including humans. Much remains to be discovered; even in E. coli, one of the most closely studied organisms on earth, 20% of the genomic ORFs have no attributed function. An inducible operon responds to an inducer substance (e.g., lactose). An inducer is a small molecule that joins with a regulatory protein to control transcription of the operon. The regulatory event typically occurs at a specific DNA sequence (controlling site) near the proteincoding sequence. Control of lactose metabolism in E. coli is an example of an inducible operon. E. coli expresses genes for glucose metabolism constitutively, but the genes for metabolizing other sugars are regulated in a “sugar specific” way. Presence of the sugar stimulates synthesis of the proteins needed. Lactose is a disaccharide (glucose + galactose). If lactose is E. coli’s sole carbon source, three genes are expressed: ◦ a. b-galactosidase has two functions: i. Breaking lactose into glucose and galactose. Galactose is converted to glucose, and glucose is metabolized by constitutively produced enzymes. ii. Converting lactose to allolactose (an isomerization). Allolactose is involved in regulation of the lac operon. ◦ b. Lactose permease (M protein) is required for transport of lactose across the cytoplasmic membrane. ◦ c. b-Galactoside transacetylase transfers an acetyl group from acetyl-CoA to bgalactoside for reasons that are not understood. The lac operon shows coordinate induction: ◦ a. In glucose medium, E. coli normally has very low levels of the lac gene products. ◦ b.When lactose is the sole carbon source, levels of the three enzymes increase coordinately (simultaneously) about a thousandfold. i. Allolactose is the inducer molecule. Ii. The mRNA for the enzymes has a short half-life. When lactose is gone, lac transcription stops, and enzyme levels drop rapidly. Lac genes. ◦ ◦ ◦ ◦ a. b-galactosidase is lacZ. b. Permease is lacY. c. Transacetylase is lacA. d. The genes are tightly linked in the order: lacZ-lacY-lacA. The three genes are transcribed on one polycistronic (polygenic) RNA. Premature translation termination prevents this by reducing translation of the downstream genes The operon regulation ◦ lac operator (lacO) just upstream from the lacZ gene. Upstream of lacO is the lac repressor gene (lacI). lacI gene is constitutively expressed at low levels (weak promoter) Without lactose lac repressor proteins bind to the lacO (operator) NEGATIVE REGULATION. No lactose- no induction However, leaky lac operator produces few molecules of the lacZ, lacY and lacA. b-galactosidase in wild-type E. coli growing with lactose as the sole carbon source converts lactose into allolactose. ◦ i. Repressor bound with allolactose changes shape (allosteric shift) and dissociates from the lac operator. Free repressor– allolactose complexes are unable to bind the operator. ◦ ii. Allolactose induces expression of the lac operon by removing the repressor and allowing transcription to occur. The lacOC mutations result in constitutive gene expression. They are cisdominant to lacO+, because repressor cannot bind to the lacOC operator sequence The lacI- mutations change the repressor protein’s conformation and prevent it from binding the operator, resulting in constitutive expression of the operon. ◦ a. In a partial diploid (lacI+ lacO + lacZ- lacY + /lacI- lacO + lacZ + lacY-), the wild-type repressor (lacI+) is dominant over lacImutants. ◦ b. Defective lacI- repressor can’t bind either operator, but normal repressor from lacI + binds both operators and regulates transcription, resulting in functional bgalactosidase and permease a. Binding to the operator region b. Binding with the inducer (allolactose) c. Binding of repressor polypeptide subunits to form an active tetramer Repressor exerts negative control by preventing transcription. Positive control of this operon also occurs when lactose is E. coli’s sole carbon source, with no glucose present. ◦ a. Catabolite activator protein (CAP) binds cyclic AMP (cAMP). ◦ b. CAP–cAMP complex is a positive regulator of the lac operon. It binds the CAP site, a DNA sequence upstream of the operon’s promoter. ◦ c. Binding of CAP–cAMP complex recruits RNA polymerase to the promoter, leading to transcription. When both glucose and lactose are in the medium, E. coli preferentially uses glucose, due to catabolite repression. ◦ a. Glucose metabolism greatly reduces cAMP levels in the cell. ◦ b. The CAP–cAMP level drops and is insufficient to maintain high transcription of the lac genes. ◦ c. Even when allolactose has removed the repressor protein from the operator, lac gene transcription is at very low levels without CAP–cAMP complex bound to the CAP site. ◦ d. Experimental evidence supports this model. Adding cAMP to cells restored transcription of the lac operon, even when glucose was present. If amino acids are available in the medium, E. coli will import them rather than make them, and the genes for amino acid biosynthesis are repressed. When amino acids are absent, the genes are expressed and biosynthesis occurs. Unlike the inducible lac operon, the trp operon is repressible. Generally, anabolic pathways are repressed when the end product is available. a. There are five structural genes, trpA through trpE. b. The promoter and operator are upstream from the trpE gene. c. Between trpE and the promoter-operator is trpL, the leader region. Within trpL is the attenuator region (att). d. The trp operon spans about 7 kb. The operon produces a polygenic transcript with five structural genes for tryptophan biosynthesis. Two mechanisms regulate expression of the trp operon: ◦ a. Repressor–operator interaction. ◦ b.Transcription termination. When tryptophan is present, it will bind to an aporepressor protein (the trpR gene product). ◦ a. The active repressor (aporepressor plus tryptophan) binds the trp operator, and prevents transcription initiation. ◦ b.Repression reduces transcription of the trp operon about 70-fold. When tryptophan is limited, transcription is also controlled by attenuation. ◦ a. Attenuation produces only short (140 bp) transcripts that do not encode structural proteins. ◦ b.Termination occurs at the attenuator site within the trpL region. ◦ c. The proportion of attenuated transcripts to fulllength ones is related to tryptophan levels, with more attenuated transcripts as the tryptophan concentration increases. ◦ d.Attenuation can reduce trp operon transcription 8to 10-fold. Together, repression and attenuation regulate trp gene expression over a 560- to 700-fold range. The molecular model for attenuation: ◦ a. Translation of the trpL gene produces a short polypeptide. Near the stop codon are two tryptophan codons. ◦ b. Within the leader mRNA are four regions that can form secondary structures by complementary base-pairing i. Pairing of sequences 1 and 2 creates a transcription pause signal. Ii. Pairing of sequences 3 and 4 is a transcription termination signal (a rho-independent terminator). Iii. Pairing of 2 and 3 is an antitermination signal, and so transcription will continue. Tight coupling of transcription and translation in prokaryotes makes control by attenuation possible. ◦ i. RNA polymerase pauses when regions 1 and 2 base-pair just after they are synthesized ◦ ii. During the pause, a ribosome loads onto the mRNA and begins translation of the leader peptide. Ribosome position is key to attenuation: ◦ (1) When tryptophan (Trp) is scarce: (a) Trp–tRNAs are unavailable, and the ribosome stalls at the Trp codons in the leader sequence, covering attenuator region 1. (b) When the ribosome is stalled in attenuator region 1, it cannot basepair with region 2. Instead, region 2 pairs with region 3 when it is synthesized. (c) If region 3 is paired with region 2, it is unable to pair with region 4 when it is synthesized. Without the region 3–4 terminator, transcription continues through the structural genes. (2) When Trp is abundant: ◦ (a) The ribosome continues translating the leader peptide, ending in region 2. This prevents region 2 from pairing with region 3, leaving 3 available to pair with region 4. ◦ (b) Pairing of regions 3 and 4 creates a rho-independent terminator known at the attenuator. Transcription ends before the structural genes are reached.