* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download genetic et.al - UniMAP Portal
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
DNA profiling wikipedia , lookup
Genomic library wikipedia , lookup
SNP genotyping wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Gel electrophoresis wikipedia , lookup
Point mutation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Non-coding DNA wikipedia , lookup
Molecular cloning wikipedia , lookup
Metalloprotein wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
Community fingerprinting wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Real-time polymerase chain reaction wikipedia , lookup
DNA supercoil wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biosynthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Deoxyribozyme wikipedia , lookup
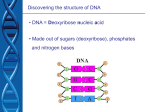
Lect Dec 2013 Genetic Review -->Additional enzyme app: essential oil extraction 1. Nucleotides 2. Central Dogma : 3. PCR, 4. ELECTROPHORESIS 5. SOUTHERN BLOT Gene extrc A nucleotide is base + sugar + phosphate covalently bonded together The BASES The purines NH O N H uracil O pyrimidines DNA= deoxy ribo nucleic acid RNA = Ribo nucleic acid Sugar Exp : sugar + base (adenosine) 3,5 phosphodiester bonds formed between The double helix ● ● ● Each base is hydrogen bonded to a base in the opposite strand to form a planar base pair. Each adenine residue must pair with a thymine residue and vice versa, and each guanine residue must pair with a cytosine residue and vice versa. These hydrogenbonding interactions, a phenomenon known as complementary base pairing, result in the specific association of the two chains of the double helix. Build the dna Comparison between DNA & RNA The base composition of DNA • ● Chargaff’s rules. DNA has equal numbers of adenine and thymine residues (A = T) and equal numbers of guanine and cytosine (G = C). 5/23/2017 Central dogma of molecular biology replication transcription DNA RNA translation PROTEIN DNA directs its own replication to produce new DNA molecule; DNA is transcribes into RNA; RNA is translated into protein. gene1 DNA REPLICATION The two strands separate, then each serves as a template for the synthesis of a complementary strand Each of the two new DNA molecules contains one old strand and one new strand.) Hence it is called semiconservative TRANSCRIPTION ● ● ● messenger RNA (mRNA) carry genetic information from chromosomes to ribosomes ribosomal RNA (rRNA) combine with ribosomal polypeptides to form ribosomes-the organelles that synthesize polypeptides transfer RNA (tRNA) deliver amino acids to the ribosomes TRANSLATION ● Translation is the process whereby ribosomes use the genetic information of nucleotide sequences to synthesize protein. quiz ● ● ● ● ● Code Quiz Click for the answer. 1. CCU codes for: ? 2. CGA codes for: ? 3. UCA codes for: ? 1. pro 2. arg 3. ser Multiplying DNA in vitro: The Polymerase Chain Reaction (PCR) ● ● The polymerase chain reaction (PCR) is a technique to produce a large number of identical molecules of DNA in vitro. Using PCR, we start with a single molecule of DNA and generate billions of exact replicate within hours. PCR PCR steps • Denaturation. • Priming. Extension Exposure to heat (about 94°C) Separates the double strands DNA A mixture containing an excess of DNA primers, DNA polymerase, and the four deoxyribonucleotide triphosphates (A, T, G, and C) is added to the target DNA This mixture is then cooled to about 65°C, enabling double-stranded DNA to reform. Raising the temperature to about 72°C increases the rate at which DNA polymerase replicates each strand to produce more DNA These steps are repeated over and over, so the number of DNA molecules increases exponentially Separating DNA Molecules: Gel Electrophoresis Southern Blot Electrophoresis ● ● ● ● Gel electrophoresis is a technique for separating molecules by size, shape, and electrical charge. It involves drawing DNA molecules (negative charge), through a gel by an electric current toward the positive electrode Smaller DNA fragments move faster and farther than larger ones. Size of a fragments is determined by comparing the distance it travels to the distances traveled by standard DNA fragment of known size. gelelectroanim Southernblot ● ● ● The Southern blot technique is the extension of gel electrophoresis to stabilize specific DNA sequences and then localize them using DNA dyes or probes. Once the DNA fragments have been separated by size, the liquid in the electrophoresis gel is blotted out, the DNA is denatured with NaOH, and its single strands are transferred and bonded to anitrocellulose membrane. If the gel separates DNA and the DNA is detected with a DNA probe, it is called a Southern blot. Southern blot technique CITRIC ACID CYCLE Electron transport Chain (ETC) PHOTOSYNTHESIS Types of Cell Respirations: Obligate anaerobes, organisms that grow only in the absence of oxygen, avoid the gas by living in highly reduced environments such as soil. They use fermentative processes to satisfy their energy requirements. Aerotolerant anaerobes, also depend on fermentation for their energy needs, possess detoxifying enzymes and antioxidant molecules that protect against oxygen's toxic products. Facul- tative anaerobes not only possess the mechanisms needed for detoxifying oxygen metabolites, they can also generate energy by using oxygen as an electron acceptor when the gas is present. Obligate aerobes highly dependent on oxygen for energy production. They protect themselves from the potentially dangerous consequences of exposure to oxygen with elaborate mechanisms composed of enzymes and antioxidant molecules. Respiration Main steps: 1. Oxidation of organic fuels (fatty acids, glucose, and some amino acids) yields acetyl-CoA. 2. Oxidation of acetyl groups in the citric acid cycle includes four steps in which electrons are abstracted. 3. Electrons carried by NADH and FADH2 are funneled into a respiratory chain, ultimately reducing O2 to H2O. This electron flow drives the production of ATP. citric acid kreb Explanation: The citric acid cycle is a metabolic pathway where 2 carbon fragments derived from organic fuel molecules are oxidized to form CO2 and the coenzymes NAD+ and FAD are reduced to form NADH and FADH2, which act as electron carriers. The electron transport Chain (ETC) is a mechanism where electrons are transferred from reduced coenzymes to an acceptor (usually O2). mido e transport.swf Oxidative phosphorylation, synthesis of ATP from ADP ( the energy released by electron transport is captured in the form of a proton gradient that drives the synthesis of ATP, the energy currency of living organisms. Main Reactions of the Citric Acid Cycle. Oxaloacetate (4 C ) + acetyl-CoA (2C) ---->citrate (6 C) Also formed : 2 molecules of CO2 3 molecules of NADH, 1 molecule of FADH2, In each turn of the citric acid cycle: 2 carbon atoms enter as the acetyl group of acetyl-CoA and 2 molecules of CO2 are released. OVERALL REACTION glycolysis After its transport into the mitochondrial matrix, pyruvate is converted to acetyl- CoA in a series of reactions catalyzed by the enzymes in the pyruvate dehydrogenase complex. The net reaction, an oxidative decarboxylation, is as follows: The net reaction for the citric acid cycle is as follows: Electron Transport Chain. Complexes I and II transfer electrons from NADH and succinate, respectively, to UQ. Complex III transfers electrons from UQH2 to cytochrome c. Complex IV transfers electrons from cytochrome c to O2. Complex I, called NADH dehydrogenase complex, catalyzes the transfer of electrons from NADH to UQ. The major sources of NADH include several reactions of the citric acid complex II ( The succinate dehydrogenase complex ) mediates the transfer of electrons from succinate to UQ. Complex III (cytochrome c complex). transfers electrons from reduced coenzyme Q (UQH2) to cytochrome c. Complex IV (Cytochrome oxidase) catalyzes the 4-electron reduction of O2 to form H2O. Summary of the flow of electrons and protons through the four complexes of the respiratory chain. Electrons reach Q through Complexes I and II. QH2 serves as a mobile carrier of electrons and protons. It passes electrons to Complex III, which passes them to another mobile connecting link, cytochrome c. Complex IV then transfers electrons from reduced cytochrome c to O2. etc The Chemi osmotic Theory Electrons from NADH and other oxidizable substrates pass through the inner membrane. Electron flow is accompanied by proton transfer across the membrane, producing pH and charge gradient. The inner mitochondrial membrane is impermeable to protons; protons can re-enter the matrix only through proton-specific channels (Fo). The proton-motive force that drives protons back into the matrix provides the energy for ATP synthesis Photosynthesis : the trapping of light energy and its conversion to chemical energy, which then reduces carbon dioxide and incorporates it into organic molecules Photorespiration: a light-dependent process occurring in plant cells actively engaged in photosynthesis that consumes oxygen and liberates carbon dioxide The essential feature of photosynthesis is the absorption of light energy by pigment molecules. The chlorophylls are green pigment molecules that absorp light energy to drive photochemical events. PHOTOSYNTHESIS • Photosynthesis Reaction • 12H20 + 6CO2 ----- light -----> 6O2+ C6H12O6 + 6H20 Photosynthesis Photorespiration • It is a light-dependent process • consuming oxygen and releasing CO2. EXERCISES : SOME EXAMPLE OF QUESTIONS Example Question : citric acid 1. Define citric acid cycle. Write the different types of biochemical reactions involved in this cycle. 2. Acetyl-CoA is an important compound in citric acid cycle. Explain how this compound is formed and explain its role in this cycle. Answer: 1. The citric acid cycle is a central pathway for recovering energy from the three major metabolic fuels: carbohydrates, fatty acids, and amino acid. These fuels are broken down to yield acetyl-CoA, which enters the citric acid cycle by condensing with the C4 compound oxaloacetate. The citric acid cycle is a series of reactions in which 2 CO2 are released for every acetyl-CoA that enters the cycle, so that oxaloacetate is always reformed. QUESTION EXAMPLES : PHOTOSYNTHESIS: 1. Differentiate between the light reactions and dark (light independent) reactions in photosynthesis 2. Differentiate between photorespiration photosynthesis and Answer : 1. In the light reactions, organisms capture light energy to synthesize ATP and generate reducing equivalents in the form of NADH. In the dark reactions, carbon dioxide is converted to carbohydrates using the ATP and NADPH generated in the light reactions. 2. Photosynthesis & Photorespiration Question Example . ETC 1. The electron transport chain (ETC) components are organized into four complexes. Name the complexes and explain general activities occuring within each of the four complexes. Answer • Complex I, called NADH dehydrogenase complex, catalyzes the transfer of electrons from NADH to UQ. The major sources of NADH include several reactions of the citric acid • Complex II ( The succinate dehydrogenase complex ) mediates the transfer of electrons from succinate to UQ. • Complex III (cytochrome c complex). transfers electrons from reduced coenzyme Q (UQH2) to cytochrome c. • Complex IV (Cytochrome oxidase) catalyzes the 4-electron reduction of O2 to form H2O.