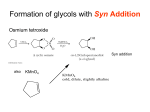
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alkynes
Woodward–Hoffmann rules wikipedia , lookup
Marcus theory wikipedia , lookup
Kinetic resolution wikipedia , lookup
Elias James Corey wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Aromatization wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hydrogenation wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Discodermolide wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hydroformylation wikipedia , lookup
Alkynes Structure sp hybridization Acidity of Terminal Alkynes Stronger base Weaker base Other strong bases that will ionize the terminal alkyne: Not KOH Important Synthetic Method: Dehydrohalogenation 1. Dehydrohalogenation… An alkyl halide can eliminate a hydrogen halide molecule, HX, to produce a pi bond. Recall that HX can be added to a double bond to make an alkyl halide. HX can also be removed by strong base, called dehydrohalogenation. Preparation of alkene Strong base RCH=CHR + HX RCHXCH2R Or rewriting RCHBrCH2R base RCH=CHR Also, if we start with a vinyl halide and a very strong base (vinyl halides are not very reactive). NaH RCH=CHBr RCCH Synthetic planning (Retrosynthesis) Work Backwards….. Trace the reactions sequence from the desired product back to ultimate reactants. Br Br2 H H H NaNH2 NaNH2 Br CH3 Starting reactant CH3 Br H prop-1-yne CH3 CH3 Target molecule. Overall Sequence converts alkene alkyne H But typical of synthetic problems side reaction occurs to some extent and must be taken into account. C H H H More Sythesis: Nucleophilic Substitution Use the acidity of a terminal alkyne to create a nucleophile which then initiates a substitution reaction. Note that we still have an acidic hydrogen and, thus, can react with another alkyl group in this way to make RCCR’ Alkyl halides can be obtained from alcohols Reactions: alkyne with halogen RCCR + Br2 RBrC=CBrR No regioselectivity with Br2. Stereoselective for trans addition. Reactions: Addition of HX The expected reaction sequence occurs, formation of the more stable carbocation. Markovnikov orientation for both additions. Now for the mechanism…. Mechanism The expected reaction sequence occurs, formation of the more stable carbocation. Addition of the second mole, another example of resonance. Reactions: Acid catalyzed Hydration (Markovnikov). Markovnikov addition, followed by tautomerism to yield, usually, a carbonyl compound. Reactions: Anti Markovnikov Hydration of Alkynes, Regioselectivity BH3 overall: R R' Step 1 R H R' B H2O2, NaOH Step 2 RCH2CR' o Similar to formation of an anti-Markovnikov alcohol from an alkene Step 1, Internal Alkyne: addition to the alkyne with little or no regioselectivity issue. Alternatively Asymmetric, terminal, alkyne if you want to have strong regioselectivity then use a borane with stronger selectivity for more open site of attack. Less exposed site. More exposed site. sia2BH Aldehyde not ketone. Tautomerism, enol carbonyl Step 2, Reaction of the alkenyl borane with H2O2, NaOH would yield an enol. Enols are unstable and rearrange (tautomerize) to yield either an aldehyde or ketone. catalyzed by base or acid H2O2 H H H H NaOH OH O B enol either an aldehyde or a ketone Overall… internal alkyne ketone (possibly a mixture, next slide) Terminal alkyne aldehyde Examples Used to insure regioselectivity. As before, for a terminal alkyne. But for a non-terminal alkyne frequently will get two different ketones Get mixture of alkenyl boranes due to low regioselectivity. Reduction, Alkyne Alkene 1. Catalytic Hydrogenation If you use catalysts which are also effective for alkene hydrogenation you will get alkane. You can use a reduced activity catalyst (Lindlar), Pd and Pb, which stops at the alkene. You obtain a cis alkene. Syn addition Reduction - 2 2. Treatment of alkenyl borane with a carboxylic acid to yield cis alkene. BH3 CH3CO2H hex-3-yne H B Instead of H2O2 / NaOH Alkenyl borane 3. Reduction by sodium or lithium in liquid ammonia to yield the trans alkene. Plan a Synthetic Sequence Retrosynthesis Synthesize butan-1-ol from ethyne. Work backward from A big the alkyne target can molecule. be formed via nucleophilic Is read as “comes from”. substitution. This is the chance to make thedone. C-C Major problem: make big from small. Be alert for Catalytic when the “disconnect” can be 1. BH3 bond we need. Lindlar OH 2. H2O2, reduction NaOH YES! butan-1-ol Target molecule Convert ethyne to anion Do a “disconnect” here. and react with EtBr. Catalytic Br reduction Addition Lindlar of HBr. bromoethane Now, fill in the “forward reaction” details Can we get alkyne from smaller Not How yet! about Soan joining how can molecules we get it? to get an alkene? Ask yourself! Do we know how tomolecules? join any two Not yet!! So howtogether can we to getyield an alkene? molecules an alcohol? ethyne