* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Notes on Alkynes
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
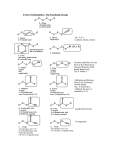
Chemistry 212 Clark College Notes on Alkynes Compounds with triple bonds afford a variety of features above those of an alkene – a linear geometry, an acidic C–H proton, C – C bond forming reactions and traditional addition reactions. We will explore the structure, acidity and reactions of alkynes, after a brief overview of the nomenclature of alkynes. Nomenclature Molecules with one triple bond have the suffix –yne for the name of the molecule, with a number preceding the parent name indicating the position of the start of the triple bond. Molecules with more than one triple bond are named as diynes, triynes, etc…, with numbers for each triple bond. 6-bromo-5,5-dimethyl-2-heptyne 1,4-hexadiyne Br If multiple functional groups are present, the triple bond is often the lower priority. Non-carbon functional groups, such as alcohols, ketones and aldehydes, take precedence over a triple bond, as do double bonds. O R-3-methylpent-4-yn-2-one Z-3-methyloct-6-yn-3-ene Structure and Acidity of the Alkyne sp-hybridized carbons give rise to two pi bonds Increased s-character in the C–H bond increases the acidity of that bond, as the anion formed has extra electron density in a short sp-hybridized orbital, close to the nucleus. As a result, the pKa of an alkyne H is approximately 25. This proton can then be removed by a strong nitrogen base. R H Na NH2 H NH3 Keq = 10+13 The acidity of this proton can be exploited as it generates a carbon nucleophile. We will explore nucleophilic substitution reactions with the alkynyl ion. Reactions of Alkynes Preparation of Alkynes In general, an alkyne can be prepared from an geminal or vicinal dihalide through a double-elimination process. X X A geminal dihalide: The two halogens are attached to the same carbon. X A vicinal dihalide: X The two halogens are attached to neighboring carbons. Alkynes can be prepared in a two-step process from alkenes: Br H 2 NaNH2 Br2/CH2Cl2 -orHBr/H2O2 H or fused KOH Br Note that in order to form an alkyne, the starting alkene must have at least one H on either side of the double bond. If a terminal alkene is used to make a terminal alkyne, the acidity of the alkyne must be considered as a competing reaction. To compensate for this, additional base is added to completely Alkyne Notes Chapter 7 Page 1 of 3 Chemistry 212 Clark College form and deprotonate the triple bond, and water is added to reprotonate the alkyne and quench the reaction. Br 1) 3 NaNH2 H 2) H O 2 H Br H An internal alkyne can be isomerized to a terminal alkyne through the “zipper reaction,” simply by heating an internal alkyne with excess NaNH2 and quenching the reaction with water. 1) xs NaNH2 2) H2O H The "zipper" reaction isomerizes an internal alkyne into a terminal alkyne. The bonds zip up and down an unbranched chain until water is added, which traps the alkyne at the end. Nucleophilic Substitution Reactions After deprotonating the alkyne CH, the anion can be used as a nucleophile/Lewis base in a substitution reaction. The electrophile/Lewis acid for this reaction is a 1° alkyl halide–the partial positive charge on the carbon, caused by the dipole of the C-X bond, attracts the alkyne anion. As the new carbon-carbon bond is formed, the carbon-bromine bond breaks, leaving a bromide ion in solution. 1) NaNH2 H 2) The substrate for this SN2 reaction must be a 1° bromide. Br Addition Reactions With two pi bonds, alkynes often mimic the behavior of alkenes, but twice as much! The presence of the pi bond allows alkynes to undergo electrophilic addition reactions, using either one or both sets of pi electrons. These reactions still follow the rules of addition with respect to carbocation stability – the more substituted carbocation is formed as the intermediate of the addition reaction. Halide additions Halides add to alkynes to result with the Markovnikov product. Sequential additions can be somewhat controlled with reaction stoichiometry – 1 mole of HX or X2 adds once, 2 or more (excess, or xs) moles will add twice. If the alkyne is terminal, only one product, the Markovnikov product, will result. However, an internal alkyne will result in a mixture of products. excess H-Br H a geminal dihalide Br Br excess H-Br Br Br Br excess Br2 Br Br Br Br Br Br 1 equiv Br2 LiBr, CH3COOH Alkyne Notes Br Chapter 7 This reaction can be controlled by stoichiometry and the addtion of LiX and acetic acid. Page 2 of 3 Chemistry 212 Clark College Addition of Water Additions of water occur only once to the alkyne, diols do not form. Upon the addition of the first –OH, an enol forms. This enol exists in equilibrium with a keto (carbonyl) form. This equilibrium, called a tautomerization, heavily favors the carbonyl form, such that it is the only form isolated. At this point, there is no alkene, so a second addition does not occur. Once again, products change with reagent, and whether the alkyne is internal or terminal. OH O This transformation of internal alkynes, with the exact same products can be done with H2SO4/HgSO4 or with 1) BH3, 2) OH-, H2O2, H2O O OH With terminal alkynes, the lack of steric bulk requires specific reagents, however it will also allow us to specify a product. OH 1) HB(sia)2 H 2) NaOH, H2O2, H 2O H HgSO4 H2SO4 O H The anti-Markovnikov product yields an aldehyde. A modified H boron reagent must be used to ensure a clean transformation. Oxymercuration results in a 2-ketone H O OH Reduction/Addition of Hydrogen Addition of hydrogen to a triple bond can generate the alkane, the E alkene or the Z alkene, depending on the reagent. H2, Pt Pd or Ni H2, Lindlar catalyst Lindlar catalyst = Pd/CaCO3, Pb2+ 1) BH3 2) CH3COOH Na0, NH3 -78°C The preceeding reactions show how to apply alkene reactions to alkynes, and what modifications must be made to deal with the triple bond. However, one of the most useful features of the triple bond rests in the enhanced acidity of the sp-hybridized C-H bond. Upon deprotonating, we now have a great carbon nucleophile that we can use in a SN2 reaction to make a new carbon-carbon bond. Alkynes are very useful in organic synthesis for this reason; they provide a method to make carbon-carbon bonds, and introduce a new functional group that can be transformed into others. Alkyne Notes Chapter 7 Page 3 of 3