* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PharmacoLecture 7 - pharmacology1lecnotes
Survey
Document related concepts
Cell encapsulation wikipedia , lookup
Cell nucleus wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Programmed cell death wikipedia , lookup
Paracrine signalling wikipedia , lookup
Transcript
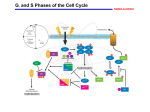
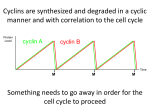
Cellular mechanism: Cell proliferation and apoptosis Objective/ learning out come: Introduction. The cell cycle. Positive regulators of the cell cycle. Negative regulators of the cell cycle. Angiogenesis. Apoptosis and cell removal. Therapeutic implications Introduction: Cell proliferation is involved in many physiological and pathological processes including growth, healing, repair, hypertrophy, hyperplasia and development of tumours. Proliferating cells go through cell cycle, during which the cell replicates all its components and then bisects itself into two identical daughter cells. Receptor tyrosine kinase and mitogen -activated kinase cascade are very important and these leads to transcription of the genes that control the cycle. The cell cycle: The cell cycle is an ordered series of events consisting of several sequential phase: G1,S,G2 and M M is the phase of mitosis. S phase is the phase of DNA synthesis. G1 is the gap between the mitosis that gave rise to the cell and S phase; during G1the cell is preparing for DNA synthesis. G2 is the gap between S phase and the mitosis that will give rise to two daughter cells; during G2 the cell is preparing for the mitotic division into two daughter cells. S phase (DNA replication) and M phase (Mitosis) are two very critical event in cell division. Therefore entry into each of these phases is carefully regulated by two check points known as (restriction points) in the cycle. DNA damage results in the cycle being stopped at one or other of these, therefore the integrity of the check points is critical for the maintenance of genetic stability. Positive regulators of the cell cycle The cell cycle is initiated when a growth factor acts on a quiescent cell provoking it to divide. They achieve these through the stimulation of the various cell cycle regulators. Cyclins and cyclin - dependence kinase (cdks) are two major proteins that determine the progress of cell cycle. The cdks phosphorylate various proteins (e.g. enzymes) activating some and inhibiting others to coordinate their activities. Each cdk is inactive until it binds to a cyclin, the binding enabling the cdk to phosphorylate the protein(s) necessary for a particular step in the cycle. It is the cyclin that determines which protein(s) are phosphorylated. .There are eight main groups of cyclins, for control of cell cycle cyclin A,B,D and E are very important. Each cyclin is associated with and activates particular cdk (s). Cyclin A activates cdks 1 and 2; Cyclin B, cdk 1; cyclin D,cdks 4 and 6; cyclin E cdk 2. The activity of these cyclin/cdk complexes is modulated by various negative regulatory forces. cells in G0- cyclin D is present in low concentration. Helps in phosphorylation of Rb proteins. phase G1- prepare for S-phase by synthesizing RNAs (mRNAs) and proteins needed for DNA replication. At this phase the concentration of cyclin D increases and the cyclin D/cdk complex phosphorylates and activates the necessary proteins. S-phase- Cyclin E/cdk and cyclin A/cdk regulate progress through S-phase, phosphorylating and thus activating proteins/enzymes involved in DNA synthesis. G2 phase – the chromosomes in cell is doubled, cellular components are duplicated synthesis of mRNAs and proteins occur. Cyclin A/ cdk and cyclin B/cdk complexes are active during G2 phase and are necessary for entry into M phase, i.e. for passing check point 2. The presence of cyclin B/cdk complexes in the nucleus is required for mitosis to commence. Unlike cyclins C,D and E, which are short lived, cyclins A and B remain stable throughout interphase but undergo proteolysis by a ubiquitin - dependent pathway during mitosis. Mitosis Mitosis is a continuous process but can be considered to consists of four stages: prophase, metaphase, anaphase and telophase. Negative regulators of the cell cycle One of the main negative regulator of cell cycle is the Rb protein, which holds the cycle in check while it is hypophosphorylated. Another negative regulatory mechanism is the action of the inhibitors of cdks. These bind to and inhibit the action of the complexes, their main action is on the check point 1. There are two families of inhibitors: the CIP family – (cdk inhibitory proteins); also termed KIP or kinase inhibitory proteins): p21, p27 and p57 the Ink family ( inhibitors of kinase) : p16, p19,p15. The action of p21 serves as an example of the role of a cyclin/cdk inhibitor. Protein p21 is under the control of p53 gene- a particularly important negative regulator that operates at check point 1. Inhibition of the cycle at check point 1 p53 gene guardian of genome, it codes for a protein transcription factor-p53 protein. normal healthy person concentration of p53 is low, but when there is DNA damage, the protein accumulates and activates the transcription of several genes one of which codes for p21. Protein p21 inactivates cyclin/cdk complexes, thus preventing Rb phosphorylation, which means arresting the cycle at check point 1 This allows for DNA repair. If the repair is successful, the cycle proceeds past check point 1 into S phase. If the repair is unsuccessful, the p53 gene triggers apoptosis-cell suicide. Inhibition of the cycle at check point 2 There is evidence that DNA damage can result in the cycle being stopped at check point 2 but the mechanism involved are less clear than those at check point 1. Inhibition of the accumulation of cyclin B/cdk complex in the nucleus seems to be a factor. Angiogenesis Angiogenesis, which normally accompanies cell proliferation, is the formation of new capillaries from existing small blood vessels. Angiogenic stimuli, in the context of cell proliferation, includes the action of various growth factors and cytokines, in particular VEGF. The sequence of events is as follows: The basement membrane is degrade locally by proteases Endothelial cells migrate out forming a sprout Endothelial cells following the leading cells proliferate under the influence of VEGF Matrix is laid down around the new capillary. Apoptosis and Cell Removal Apoptosis is cell suicide by a built-in self destruct mechanism; it consists of a generally programmed sequence of biochemical events. It is, therefore, unlike necrosis, which is a disorganized disintegration of damaged cells resulting in products that trigger the inflammatory response. There are two main pathways to activation of the effectors caspases : the death receptor pathway and the mitochondrial pathway. The death receptor pathway involves stimulation of members of the tumor necrosis factor receptor (TNFR) family; and the main initiator caspase is caspase 8 The mitochondrial pathway is activated by internal factors such as DNA damage, which results in transcription of gene p53. The p53 protein activates a subpathway that results in release from mitochondrion of cytochrome c. This, in turn, complexes with protein Apaf-1 and together they activate initiator caspase 9. In undamaged cells, survival factors (cytokines, hormones, cell-to-cell contact factors) continuously activate antiapoptotic mechanisms. Withdrawal of survival factor stimulation causes cell death through the mitochondrial pathway. The effector caspases (e.g. caspase 3) start a pathway that results in cleavage of cell constituents: DNA, cytoskeleton components, enzymes, etc. This reduces the cell to a cluster of membrane-bound entities that are eventually phagocytosed by macrophages. Pathophysiological Implications of Apoptosis Cell proliferation and apoptosis are involved in many physiological and pathological processes: the growth of tissues and organ the replenishment of lost or time-expired cells such as leucocytes, gut epithelium, uterine endometrium, etc. healing and repair after injury or inflammation the hyperplasia (increase in cell number) associated with chronic inflammatory, hypersensitivity and autoimmune disease. the growth, invasion and metastasis of tumor Therapeutic Implication Targets For New Drug Development Angiogenesis has a critical role in numerous bodily processes, some physiological (e.g. growth, repair) some pathological (e.g. tumor growth, chronic inflammatory conditions) Angiogenesis Inhibitors Angiogenesis Inhibitors are being sought for use in pathological angiogenesis and there are currently 30 compounds in clinical trial. The approaches being used include: Interference with endothelial cell growth, for example by the use of monoclonal antibodies that prevent the interaction of VEGF and FGF with their receptors Interference with the necessary adherence of endothelial cells in the endothelial sprout to the matrix; an antiintergrin monoclonal antibody has shown promise Interference with the necessary degradation of the matrix round the developing endothelial sprout; inhibitors of metalloproteinases are under test. Angiogenesis Stimulators Angiogenesis Stimulators are being investigated for use in various ischaemic condition, for example coronary disease, limb ischaemia, and gastrointestinal ulcers associated with insufficient local perfusion. The main compound under investigation is VEGF. In pilot studies, naked DNA encoding the gene for VEGF has been injected directly into the relevant tissue along with a viral promoter. Apoptotic Mechanisms Defective apoptosis is a factor in several diseases, and compounds that modify it are under investigation Examples of defective apoptosis include cancer cell proliferation, resistance to cancer chemotherapy and ineffective eradication of virus – infected cells. Examples of over-exuberant apoptosis of T cells in human immunodeficiency virus (HIV) infection, allograft rejection, loss of neurons in neurodegenerative diseases, and loss of chondrocytes in osteoarthritis Several anti – apoptosis compounds are in clinical trials for neurodegenerative and inflammatory diseases and numerous pro – apoptosis compounds are in clinical trials for cancer. Various approaches to apoptosis – based therapies are being explored.