* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download organic chemistry ii
Enantioselective synthesis wikipedia , lookup
Bottromycin wikipedia , lookup
Marcus theory wikipedia , lookup
Elias James Corey wikipedia , lookup
Discodermolide wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Physical organic chemistry wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Stille reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
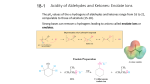
ORGANIC CHEMISTRY II EXAM 3 MATERIAL REVIEW Spring Semester Week 15, 2013 I. Chapter 21: Aldehydes and Ketones –Nucleophilic Addition The carbonyl group is polarized in such a way that the -electron density is concentrated around the oxygen atom thus making the carbon atom electron deficient. This can be visualized using resonance theory: C O C O In terms of reactivity, this means that the carbonyl carbon is susceptible to nucleophilic attack. In most cases nucleophilic addition to aldehydes of ketones follows the general mechanism outlined. Nu C O Nu O C Or Nu C O H Nu O C H if acid catalyzed… There are a large range of nucleophiles which can react with aldehydes of ketones. These can be classified as 1. Carbon nucleophiles 2. Oxygen nucleophiles 3. Nitrogen nucleophiles (classified as “reactions which go beyond the nucleophile addition step”) 1. Carbon Nucleophiles (i) Carbanionic species i.e. cyanide, acetylides (ii) Organometallic species i.e. organolithium compounds, organomagnesium compounds (Grignard reagents) (iii) “Assisted” Carbanionic species (by third-row elements such as phosphorus and sulfur) i.e. phosphorus ylides (classified as “reactions which go beyond the nucleophile addition step”) The following are examples of the products obtained when benzaldehyde undergoes reactions of the types mentioned above: 1(i) nucleophile reaction products OH CN CN OH C C R R Note that the alkyne can be converted into a number of functional groups 1(ii) nucleophile reaction products OH CH 3 CH 2MgBr review reactions of epoxides with Grignard reagents as well 1(iii) nucleophile reaction products CH3 CHP(C 6H5)3 Page 2 2. Oxygen Nucleophiles An aldehyde or a ketone undergoing a reaction with two equivalents of alcohol gives an acetal or ketal. A diol is considered a “two equivalent alcohol” and gives a cyclic acetal or ketal. Most ketals can only be prepared from diols (acyclic ketals are less stable) Acetals/ketals can be hydrolyzed back to the parent aldehyde/ketone under acidic conditions. Given below is an example of a cyclic hemiacetal, acetal, and a ketal OH O OR O O O Acetals are commonly used as protecting groups. 3. Nitrogen Nucleophiles Aldehyde/Ketones + primary amine imine Aldehyde/Ketones + primary amine enamine Note that imines are very basic (Schiff bases) and they can be hydrolyzed back into aldehydes/ketones under acidic conditions. II. Chapter 27: Carbohydrates classification: (i) number of carbons; (ii) aldehyde or ketone; (iii) D or L; (iv) ring size; (v) or Stereochemistry: Fischer structures Conversion from straight chain to ring structures (and from ring to chain); mutarotation (omit Sect. 27.4) Reactions of Monosaccharides: reduction (into alditols) oxidation (into aldaric acids) loss of optical activity (with certain monosaccharides), introduction of a plane of symmetry into the molecule (omit oxidation into aldonic acid) ring extension, Kiliani-Fischer reaction (omit Sect. 27.10A) exhaustive methylation: strategy to determine ring size glycoside formation Page 3 Disaccharides 1,1- and 1,4-linkages (omit Sect. 27.13, 27.14A) N-Glycosides (Sect. 27.14B) nucleosides; nucleotides III. Chapter 22: Carboxylic Acids and their Derivatives –Nucleophilic Acyl Substitution The common features of these classes of compounds is that they can all be hydrolyzed into carboxylic acids Syntheses of Carboxylic Acids: (i) Oxidation of aldehydes and 1o alcohols (remember 1o alcohols will only oxidize to aldehydes if the reaction is carried out in non-aqueous conditions). (ii) Hydrolysis of nitriles (in acid catalyzed conditions) O NaCN ? H3 O ? CN C 6 H 8O C7H10O3 (also an example for conjugate addition) (iii) Carbonylation of Grignard reagents MgBr CO2 H3O ? Types of CA derivatives (in order of reactivity) Acid halides (chlorides) > Acid anhydrides > Esters > Amides (and nitriles) Synthetic Strategy A CA derivative can be prepared starting from the more reactive derivative going to less reactive, i.e. esters and/or amides from acid chlorides or anhydrides; amides from esters, etc. Mechanism of Nucleophilic Acyl Substitution When a nucleophile attacks a CA derivative, a tetrahedral intermediate (unstable) forms, which subsequently dissociates into a new CA derivative (or into a carboxylate) Reactions of Nitriles Nitriles are extremely versatile as precursors; they can be converted into (i) carboxylic acids, (ii) imines, (iii) amines, (iv) aldehydes (v) ketones (upon reaction with Grignard or organolithium reagents) Page 4 Other Reactions Fischer Esterification: an acid catalyzed reaction between a carboxylic acid and an alcohol O R O O H H HO R R OR Note that the carboxylic acid hydroxyl oxygen is protonated and subsequently dehydrated (not the alcohol hydroxyl) An intramolecular esterification of a - (or -) hydroxycarboxylic acid would give a cyclic ester, which is referred to as a “lactone” O OH HO O O -lactone O HO O OH O -lactone Dicarboxylic Acids: Note that -carbonyl carboxylic acids undergo thermal decarboxylation IV. Chapter 23: -Substitution Reactions Aldehydes and ketones which possess -hydrogens can undergo enolization. Most enols are unstable and reactive and instantly equilibrate to the “keto” form. Certain enols, such as -dicarbonyl compounds, among others, are exceptionally stable. Under basic conditions aldehydes and ketones form enolates, which can function as nucleophiles. -Halogenation Enolizable aldehydes and ketones can be halogenated at the -carbon (see iodoform reaction, Sect. 23.7B) -Alkylation Enolizable aldehydes and ketones can be alkylated at the -carbon upon reaction with an alkyl halide. Page 5 In cases where enolate formation can take place at non-equivalent carbons the reaction can take place under (i) thermodynamic or (ii) kinetic conditions (i) a thermodynamic enolate is the more stable (more substituted) enolate which can be formed upon reaction with only a mildly strong base such as an alkoxide (ii) a kinetic enolate is the less stable (less substituted) enolate which can be formed upon reaction with a very strong base such as LDA. Although the more stable enolate is also formed under these conditions, the less stable enolate will undergo alkylation more rapidly. Acetoacetic Ester (or Malonic Ester) Synthesis: Acetoacetic ester (ethyl acetoacetate) is a -keto ester. It will form an enolate under milder conditions. O O H H O OCH2CH3 O O O H The product of AAE synthesis is usually a substituted acetone at the end of the following four steps: (i) enolate formation; (ii) alkylation; (iii) ester hydrolysis; (iv) decarboxylation. The product of an analogous (four-step) reaction with malonic ester is a substituted acetic acid. V. Chapter 24: Enols and Enolates An enolate which is in equilibrium with its keto (non-enolate) form, can undergo a reaction with “itself”, the Aldol Reaction. O C C H H H C O C H OH H C H O C H H + H H H2O Aldol Reactions: Two steps –addition and condensation Addition products: -hydroxy aldehydes (or ketones) Condensation: ,-unsaturated aldehydes (or ketones) Mixed Aldol Reactions: yields are higher if only one of the aldehydes (or ketones) is enolizable (i.e. if the other aldehyde/ketone does not have any -hydrogens) –remember Page 6 the Aldol reaction you carried out in the lab (between acetone and benzaldehyde – anisaldehyde) Intramolecular Aldol Reactions: the staring material contains two carbonyl functionalities, and the product is usually a five- or six-membered ring. HO O O O O C16H24O2 C16H24O2 Ester Enolates Esters which possess -hydrogens can undergo enolization in an analogous fashion to aldehydes and ketones. Similarly ester enolates can function as nucleophiles. Since the ester enolate is in equilibrium with its non-enolate form, it can undergo a reaction with itself. This type of a reaction is referred to as Claisen Condensation. Claisen Condensation: Step 1: formation of the ester enolate (the base used is the same alkoxide as the one in the ester) Step 2: formation of a bond between the -carbon of one ester and the carbonyl carbon of the other (the structure formed is referred to as tetrahedral-intermediate and is relatively unstable) Step 3: dissociation of the tetrahedral intermediate Step 4: formation of a -keto ester and a new enolate Step 5: acid work-up and isolation of the 1,3-dicarbonyl (-dicarbonyl) compound Dieckman Condensation: An intramolecular Claisen Condensation at the end of which a cyclic ester (a lactone) is formed. Reactions of ,-unsaturated aldehydes (or ketones): O two possible electrophilic sites More reactive nucleophiles add to the carbonyl carbon; whereas the less reactive ones (where there is possibility for reversibility) add to the -position. Page 7 Michael Addition Reactions: the nucleophile is an enolate which adds to the -carbon of an ,-unsaturated aldehydes or ketone (the electrophile). The product of a Michael reaction is a 1,5-dicarbonyl compound. O + O O O C16H24O2 C5 H8 O Enolates which take part in Michael additions are usually stable enolates (from -dicarbonyl compounds) Robinson Annulation Reaction: a Michael addition followed by an intramolecular Aldol reaction (see above) Page 8