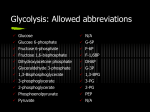
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 16 Gluconeogenesis
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Microbial metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Phosphorylation wikipedia , lookup
Blood sugar level wikipedia , lookup
Citric acid cycle wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
CHAPTER 16 Gluconeogenesis 16.1 Glucose Can Be Synthesized from Noncarbohydrate Precursors 16.2 Gluconeogenesis and Glycolysis Are Reciprocally Regulated 16.3 Metabolism in Context: Precursors Formed by Muscle Are Used by Other Organs 16.4 Glycolysis and Gluconeogenesis Are Evolutionarily Intertwined Fasting is a part of many cultures and religions, including those of the Teton Sioux. Fasting is believed to cleanse the body and soul and to foster spiritual awakening. Gluconeogenesis is an important metabolic pathway during times of fasting because it supplies glucose to the brain and red blood cells, tissues that depend on this vital fuel. [Edward S. Curtis Collection, ”Fasting Indians,”Library of Congress.] W e now turn to the synthesis of glucose from noncarbohydrate precursors, a process called gluconeogenesis. Maintaining levels of glucose is important because the brain depends on glucose as its primary fuel and red blood cells use glucose as their only fuel. The daily glucose requirement of the brain in a typical adult human being is about 120 g, which accounts for most of the 160 g of glucose needed daily by the whole body. The amount of glucose present in body fluids is about 20 g, and that readily available from glycogen, the storage form of glucose, is approximately 190 g. Thus, the direct glucose reserves are sufficient to meet glucose needs for about a day. Gluconeogenesis is especially important during a longer period of fasting or starvation. 251 252 16 Gluconeogenesis The major site of gluconeogenesis is the liver, with a small amount also taking place in the kidney. Little gluconeogenesis takes place in the brain, skeletal muscle, or heart muscle. Rather, gluconeogenesis in the liver and kidney helps to maintain the glucose level in the blood where it can be extracted by the brain and muscle to meet their metabolic demands. In this chapter, we begin by examining the reactions that constitute the gluconeogenic pathway. We then investigate the reciprocal regulation of gluconeogenesis and glycolysis, and end the chapter with a look at how gluconeogenesis and glycolysis are coordinated between tissues. 16.1 Glucose Can Be Synthesized from Noncarbohydrate Precursors Although glucose is usually available in the environment of most organisms, this molecule is so important biochemically that a pathway exists in virtually all forms of life to synthesize it from simple precursors. The gluconeogenic pathway converts pyruvate into glucose. Noncarbohydrate precursors of glucose are first converted into pyruvate or enter the pathway at later intermediates (Figure 16.1). The major noncarbohydrate precursors are lactate, amino acids, and glycerol. Lactate is formed by active skeletal muscle through lactic acid fermentation when the rate of glycolysis exceeds the rate at which muscle can process pyruvate aerobically (p. 235). Lactate is readily converted into pyruvate in the liver by the action of lactate dehydrogenase. Amino acids are derived from proteins in the diet and, during starvation, from the breakdown of proteins in skeletal muscle (p. 483). The hydrolysis of triacylglycerols (p. 404) in fat cells yields glycerol and fatty acids. Glycerol is a precursor of glucose, but animals cannot convert fatty acids into glucose, for reasons that will be given later (p. 413). Unlike lactate and amino acids, glycerol can be metabolized by glycolysis and can be converted into glucose by gluconeogenesis. Glycerol may enter either the gluconeogenic or the glycolytic pathway at dihydroxyacetone phosphate. CH2OH HO C H CH2OH ATP ADP + H+ Glycerol kinase CH2OH HO C H CH2OPO32– NAD+ Glycerol phosphate dehydrogenase Glycerol phosphate Glycerol NADH + H+ CH2OH O C CH2OPO32– Dihydroxyacetone phosphate Gluconeogenesis Is Not a Reversal of Glycolysis In glycolysis, glucose is converted into pyruvate; in gluconeogenesis, pyruvate is converted into glucose. However, gluconeogenesis is not a reversal of glycolysis. Several reactions must differ because the equilibrium of glycolysis lies far on the side of pyruvate formation. The actual ⌬G for the formation of pyruvate from glucose is about ⫺84 kJ mol⫺1 (⫺20 kcal mol⫺1) under typical cellular conditions. Most of the decrease in free energy in glycolysis takes place in the three essentially irreversible steps catalyzed by hexokinase, phosphofructokinase, and pyruvate kinase. Hexokinase Glucose + ATP 9999: glucose 6-phosphate + ADP ¢G = - 33 kJ mol - 1 ( - 8.0 kcal mol - 1) Phosphofructokinase Fructose 6-phosphate + ATP 999999: fructose 1,6-bisphosphate + ADP ¢G = - 22 kJ mol - 1 ( - 5.3 kcal mol - 1) Pyruvate kinase Phosphoenolpyruvate + ADP 99999: pyruvate + ATP ¢G = - 17 kJ mol - 1 ( - 4.0 kcal mol - 1) 253 16.1 Gluconeogenesis CH2OH O Glucose OH Glucose 6-phosphatase OH HO Pi OH CH2OPO32– H2O O Glucose 6-phosphate OH HO Phosphoglucose isomerase OH 2–O 3POH2C O Fructose 6-phosphate OH CH2OH HO OH Pi Fructose 1, 6-bisphosphatase HO 2– O 3POH2C H2O CH2OPO32– O HO Fructose 1,6-bisphosphate Glycerol OH OH Aldolase Dihydroxyacetone phosphate Triose phosphate isomerase Glyceraldehyde 3-phosphate CH2OH O C CH2OPO32– Glyceraldehyde 3-phosphate dehydrogenase H NADH 2– C H C H 2-Phosphoglycerate H OH OPO32– C CH2OH H2O Phosphoenolpyruvate O – GTP O C C H – H O H2 C C O C ADP + Pi ATP, HCO3– OPO32– C GDP, CO2 Oxaloacetate Pyruvate C CH2OPO32– O – O C Phosphoglycerate mutase Lactate Some amino acids OH CH2OPO32– O – O C 3-Phosphoglycerate Pyruvate carboxylase O O3PO ATP Some amino acids OH CH2OPO32– ADP Phosphoenolpyruvate carboxykinase C Pi, NAD Phosphoglycerate kinase Enolase C + 1,3-Bisphosphoglycerate 2X O H O – O O C O O C O C CH3 – Figure 16.1 The pathway of gluconeogenesis. The distinctive reactions and enzymes of this pathway are shown in red. The other reactions are common to glycolysis. The enzymes for gluconeogenesis are located in the cytoplasm, except for pyruvate carboxylase (in the mitochondria) and glucose 6-phosphatase (membrane bound in the endoplasmic reticulum). The entry points for lactate, glycerol, and amino acidsp are shown. 254 16 Gluconeogenesis In gluconeogenesis, the following new steps bypass these virtually irreversible reactions of glycolysis: 1. Phosphoenolpyruvate is formed from pyruvate by way of oxaloacetate through the action of pyruvate carboxylase and phosphoenolpyruvate carboxykinase. Pyruvate carboxylase Pyruvate + CO2 + ATP + H2O 9999999: oxaloacetate + ADP + Pi + 2 H + Phosphoenolpyruvate carboxykinase Oxaloacetate + GTP 99999999999: phosphoenolpyruvate + GDP + CO2 2. Fructose 6-phosphate is formed from fructose 1,6-bisphosphate by the hydrolysis of the phosphate. Fructose 1,6-bisphosphatase catalyzes this exergonic hydrolysis. Fructose 1,6-bisphosphate + H2O ¡ fructose 6-phosphate + Pi 3. Glucose is formed by the hydrolysis of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphatase. Glucose 6-phosphate + H2O ¡ glucose + Pi We will examine each of these steps in turn. The Conversion of Pyruvate into Phosphoenolpyruvate Begins with the Formation of Oxaloacetate The first step in gluconeogenesis takes place inside the mitochondria. In this step, pyruvate is carboxylated by pyruvate carboxylase to form oxaloacetate at the expense of a molecule of ATP. COO– O O C Pyruvate carboxylase – O + CO2 + ATP + H2O C C O H C H + ADP + Pi + 2H+ COO– CH3 Pyruvate Oxaloacetate Then, oxaloacetate is decarboxylated and phosphorylated by phosphoenolpyruvate carboxylase to yield phosphoenolpyruvate, at the expense of the high phosphoryl-transfer potential of GTP. COO– O Phosphoenolpyruvate carboxylase C H C H + GTP O – O + GDP + CO2 C COO– C H Oxaloacetate OPO32– C H Phosphoenolpyruvate The sum of these reactions is Pyruvate + ATP + GTP + H2O ¡ phosphoenolpyruvate + ADP + GDP + Pi + 2 H + (B) (A) Activated CO2 Lysine Biotin Lysine Figure 16.2 The structure of carboxybiotin. (A) Biotin is shown with CO2 attached. (B) The biotin-binding domain of pyruvate carboxylase shows that biotin is on a flexible tether, allowing it to move between the ATP-bicarbonate site and the pyruvate site. [(B) Drawn from 1BDO.pdb.] Pyruvate carboxylase requires the vitamin cofactor biotin, a covalently attached prosthetic group that serves as a carrier of activated CO2 in that it readily donates the CO2 to other molecules. The carboxylate group of biotin is linked to the side chain of a specific lysine residue by an amide bond (Figure 16.2). Note that biotin is attached to pyruvate carboxylase by a long, flexible chain. The carboxylation of pyruvate takes place in three stages: HCO3- + ATP Δ HOCO2-PO32- + ADP Biotin A vitamin used in CO2 transfer and carboxylation reactions. Biotin deficiency is characterized by muscle pain, lethargy, anorexia, and depression. This vitamin is synthesized by microflora in the intestinal tract and can be obtained in the diet from liver, soybeans, nuts, and many other sources. Biotin–enzyme + HOCO2-PO32- Δ CO2 - biotin–enzyme + Pi CO2 - biotin–enzyme + pyruvate Δ biotin–enzyme + oxaloacetate In aqueous solutions, CO2 exists primarily as HCO3⫺, which is activated to carboxyphosphate by the addition of a phosphate group from ATP. This activated CO2 is subsequently bonded to the biotin ring attached to pyruvate carboxylase to form the carboxybiotin–enzyme intermediate (see Figure 16.2). However, this step can take place only in the presence of acetyl CoA. Biotin is not carboxylated unless acetyl CoA is bound to the enzyme. The allosteric activation of pyruvate carboxylase by acetyl CoA is an important physiological control mechanism that will be discussed on page 289. The CO2 attached to biotin is activated. The ⌬G°⬘ for its cleavage [Charles Brutlag/FeaturesPics.] CO2 - biotin–enzyme + H + ¡ CO2 + biotin- enzyme is ⫺20 kJ mol⫺1 (⫺4.7 kcal mol⫺1). This negative ⌬G°⬘ indicates that carboxybiotin is able to transfer CO2 to acceptors without the input of additional free energy. The long, flexible link between biotin and the enzyme enables the carboxybiotin to rotate from one active site of the enzyme (the ATP-bicarbonate site) to the other (the pyruvate site). The activated carboxyl group is then transferred from carboxybiotin to pyruvate to form oxaloacetate. 255 Oxaloacetate Is Shuttled into the Cytoplasm and Converted into Phosphoenolpyruvate Cytoplasm Matrix Pyruvate CO2 + ATP ADP + Pi Oxaloacetate NADH + H+ NAD+ Malate Malate NAD+ Pyruvate carboxylase is a mitochondrial enzyme, whereas the other enzymes of gluconeogenesis are present primarily in the cytoplasm. Oxaloacetate, the product of the pyruvate carboxylase reaction, must thus be transported to the cytoplasm to complete the pathway. Oxaloacetate is transported from a mitochondrion in the form of malate: oxaloacetate is reduced to malate inside the mitochondrion by an NADH-linked malate dehydrogenase. After malate has been transported across the mitochondrial membrane, it is reoxidized to oxaloacetate by an NAD⫹-linked malate dehydrogenase in the cytoplasm (Figure 16.3). The formation of oxaloacetate from malate also provides NADH for use in subsequent steps in gluconeogenesis. Finally, oxaloacetate is simultaneously decarboxylated and phosphorylated by phosphoenolpyruvate carboxykinase to generate phosphoenolpyruvate. The phosphoryl donor is GTP. The CO2 that was added to pyruvate by pyruvate carboxylase comes off in this step. Why are a carboxylation and a decarboxylation required to form phosphoenolpyruvate from pyruvate? Recall that, in glycolysis, the presence of a phosphoryl group traps the unstable enol isomer of pyruvate as phosphoenolpyruvate (p. 233). However, the addition of a phosphoryl group to pyruvate is a highly unfavorable reaction: the ⌬G°⬘ of the reverse of the glycolytic reaction catalyzed by pyruvate kinase is ⫹31 kJ mol⫺1 (⫹7.5 kcal mol⫺1). In gluconeogenesis, the use of the carboxylation and decarboxylation steps results in a much more favorable ⌬G°⬘. The formation of phosphoenolpyruvate from pyruvate in the gluconeogenic pathway has a ⌬G°⬘ of ⫹0.8 kJ mol⫺1 (⫹0.2 kcal mol⫺1). Instead, a molecule of ATP is used to power the addition of a molecule of CO2 to pyruvate in the carboxylation step. That molecule of CO2 is then removed from oxaloacetate to power the formation of phosphoenolpyruvate in the decarboxylation step. Decarboxylations often drive reactions that are otherwise highly endergonic. NADH + H+ Oxaloacetate Figure 16.3 Compartmental cooperation. Oxaloacetate used in the cytoplasm for gluconeogenesis is formed in the mitochondrial matrix by the carboxylation of pyruvate. Oxaloacetate leaves the mitochondrion by a specific transport system (not shown) in the form of malate, which is reoxidized to oxaloacetate in the cytoplasm. The Conversion of Fructose 1,6-bisphosphate into Fructose 6-phosphate and Orthophosphate Is an Irreversible Step On formation, phosphoenolpyruvate is metabolized by the enzymes of glycolysis but in the reverse direction. These reactions are near equilibrium under intracellular conditions; so, when conditions favor gluconeogenesis, the reverse reactions will take place until the next irreversible step is reached. This step is the hydrolysis of fructose 1,6-bisphosphate to fructose 6-phosphate and Pi. 2–O 3POH2C O CH2OPO32– + H2O HO Fructose 1,6-bisphosphatase 2–O 3POH2C O HO OH OH Fructose 1,6-bisphosphate CH2OH + Pi OH OH Fructose 6-phosphate The enzyme responsible for this step is fructose 1,6-bisphosphatase. Like its glycolytic counterpart, it is an allosteric enzyme that participates in regulation—in this case, of gluconeogenesis. We will return to its regulatory properties later in the chapter. The Generation of Free Glucose Is an Important Control Point The fructose 6-phosphate generated by fructose 1,6-bisphosphatase is readily converted into glucose 6-phosphate. In most tissues, gluconeogenesis ends here. Free glucose is not generated; rather glucose 6-phosphate is commonly converted 256 257 Ca2+-binding protein 16.1 Gluconeogenesis Cytoplasmic side Glucose 6phosphatase H2O + glucose 6-phosphate Pi + glucose ER lumen Figure 16.4 The generation of glucose from glucose 6-phosphate. Several endoplasmic reticulum (ER) proteins play a role in the generation of glucose from glucose 6-phosphate. One transporter brings glucose 6-phosphate into the lumen of the ER, whereas separate transporters carry Pi and glucose back into the cytoplasm. Glucose 6-phosphatase is stabilized by a Ca2⫹-binding protein. [After A. Buchell and I. D. Waddel. Biochem. Biophys. Acta 1092:129–137, 1991.] into glycogen, the storage form of glucose. The final step in the generation of free glucose takes place primarily in the liver, a tissue whose metabolic duty is to maintain adequate levels of glucose in the blood for use by other tissues. Free glucose is not formed in the cytoplasm. Rather, glucose 6-phosphate is transported into the lumen of the endoplasmic reticulum, where it is hydrolyzed to glucose by glucose 6-phosphatase, which is bound to the ER membrane (Figure 16.4). Glucose and Pi are then shuttled back to the cytoplasm by a pair of transporters. Six High-Transfer-Potential Phosphoryl Groups Are Spent in Synthesizing Glucose from Pyruvate As we have seen in the preceding sets of reactions, the formation of glucose from pyruvate is energetically unfavorable unless it is coupled to reactions that are favorable. Compare the stoichiometry of gluconeogenesis with that of the reverse of glycolysis. The stoichiometry of gluconeogenesis is Pyruvate + 4 ATP + 2 GTP + 2 NADH + 6 H2O ¡ glucose + 4 ADP + 2 GDP + 6 Pi + 2 NAD + + 2 H + ¢G°¿ = - 38 kJ mol - 1 ( - 9 kcal mol - 1) In contrast, the stoichiometry for the reversal of glycolysis is Pyruvate + 2 ATP + 2 NADH + 2 H2O ¡ glucose + 2 ADP + 2 Pi + 2 NAD + + 2 H + ¢G°¿ = + 84 kJ mol - 1 ( + 20 kcal mol - 1) Note that six nucleoside triphosphate molecules are hydrolyzed to synthesize glucose from pyruvate in gluconeogenesis, whereas only two molecules of ATP are generated in glycolysis in the conversion of glucose into pyruvate. Thus, the extra cost of gluconeogenesis is four high-phosphoryl-transfer-potential molecules for each molecule of glucose synthesized from pyruvate. The four additional highphosphoryl-transfer-potential molecules are needed to turn an energetically unfavorable process (the reversal of glycolysis) into a favorable one (gluconeogenesis). Here, we have a clear example of the coupling of reactions: NTP hydrolysis is used to power an energetically unfavorable reaction. QUICK QUIZ 1 What barrier prevents glycolysis from simply running in reverse to synthesize glucose? How is this barrier overcome in gluconeogenesis? 258 16 Gluconeogenesis 16.2 Gluconeogenesis and Glycolysis Are Reciprocally Regulated Gluconeogenesis and glycolysis are coordinated so that, within a cell, one pathway is relatively inactive while the other is highly active. If both sets of reactions were highly active at the same time, the net result would be the hydrolysis of four nucleoside triphosphates (two ATP molecules plus two GTP molecules) per reaction cycle. Both glycolysis and gluconeogenesis are highly exergonic under cellular conditions, and so there is no thermodynamic barrier to such simultaneous activity. However, the activities of the distinctive enzymes of each pathway are controlled so that both pathways are not highly active at the same time. The rate of glycolysis is also determined by the concentration of glucose, and the rate of gluconeogenesis by the concentrations of lactate and other precursors of glucose. The basic premise of the reciprocal regulation is that, when glucose is abundant, glycolysis will predominate. When glucose is scarce, gluconeogenesis will take over. Energy Charge Determines Whether Glycolysis or Gluconeogenesis Will Be Most Active The first important regulation site in the gluconeogenesis pathway is the interconversion of fructose 6-phosphate and fructose 1,6-bisphosphate (Figure 16.5). Consider first a situation in which energy is needed. In this case, the concentration of AMP is high. Under this condition, AMP stimulates phosphofructokinase Glucose GLYCOLYSIS GLUCONEOGENESIS Fructose 6-phosphate F-2,6-BP + AMP + ATP − Citrate − Phosphofructokinase Fructose 1, 6-bisphosphatase − F-2,6-BP − AMP + Citrate H+ − Fructose 1,6-bisphosphate Several steps Phosphoenolpyruvate F-1,6-BP + ATP − Phosphoenolpyruvate carboxykinase Pyruvate kinase − ADP Oxaloacetate Alanine − Pyruvate carboxylase Pyruvate + Acetyl CoA − ADP Figure 16.5 The reciprocal regulation of gluconeogenesis and glycolysis in the liver. The level of fructose 2,6-bisphosphate (F-2,6-BP) is high in the fed state and low in starvation. Another important control is the inhibition of pyruvate kinase by phosphorylation during starvation. but inhibits fructose 1,6-bisphosphatase. Thus, glycolysis is turned on and gluconeogenesis is inhibited. Conversely, high levels of ATP and citrate indicate that the energy charge is high and that biosynthetic intermediates are abundant. ATP and citrate inhibit phosphofructokinase, whereas citrate activates fructose 1,6-bisphosphatase. Under these conditions, glycolysis is nearly switched off and gluconeogenesis is promoted. Why does citrate take part in this regulatory scheme? As we will see in Chapter 18, citrate reports on the status of the citric acid cycle, the primary pathway for oxidizing fuels in the presence of oxygen. High levels of citrate indicate an energy-rich situation and the presence of precursors for biosynthesis. Glycolysis and gluconeogenesis are also reciprocally regulated at the interconversion of phosphoenolpyruvate and pyruvate in the liver. The glycolytic enzyme pyruvate kinase is inhibited by allosteric effectors ATP and alanine, which signal that the energy charge is high and that building blocks are abundant. Conversely, pyruvate carboxylase, which catalyzes the first step in gluconeogenesis from pyruvate, is inhibited by ADP. Likewise, ADP inhibits phosphoenolpyruvate carboxykinase. Pyruvate carboxylase is activated by acetyl CoA, which, like citrate, indicates that the citric acid cycle is producing energy and biosynthetic intermediates (Chapter 18). Hence, gluconeogenesis is favored when the cell is rich in biosynthetic precursors and ATP. 259 16.2 Regulation of Glycolysis and Gluconeogenesis QUICK QUIZ 2 What are the regulatory means that prevent high levels of activity in glycolysis and gluconeogenesis simultaneously? What would be the result if both pathways functioned rapidly at the same time? The Balance Between Glycolysis and Gluconeogenesis in the Liver Is Sensitive to Blood-Glucose Concentration In the liver, rates of glycolysis and gluconeogenesis are adjusted to maintain blood-glucose levels. Recall that fructose 2,6-bisphosphate is a potent activator of phosphofructokinase (PFK), the primary regulatory step in glycolysis (p. 241). Fructose 2,6-bisphosphate is also an inhibitor of fructose 1,6-bisphosphatase. When blood glucose is low, fructose 2,6-bisphosphate loses a phosphoryl group to form fructose 6-phosphate, which no longer binds to PFK. How is the concentration of fructose 2,6-bisphosphate controlled to rise and fall with bloodglucose levels? Two enzymes regulate the concentration of this molecule: one phosphorylates fructose 6-phosphate and the other dephosphorylates fructose 2,6-bisphosphate. Fructose 2,6-bisphosphate is formed from fructose 6-phosphate in a reaction catalyzed by phosphofructokinase 2 (PFK2), a different enzyme from phosphofructokinase. In the reverse direction, fructose 6-phosphate is formed through hydrolysis of fructose 2,6-bisphosphate by a specific phosphatase, fructose bisphosphatase 2 (FBPase2). The striking finding is that both PFK2 and FBPase2 are present in a single 55-kd polypeptide chain (Figure 16.6). This bifunctional enzyme contains an N-terminal regulatory domain, followed by a kinase domain and a phosphatase domain. Kinase domain Regulatory domain Phosphatase domain Figure 16.6 The domain structure of the bifunctional regulatory enzyme phosphofructokinase 2/fructose 2,6-bisphosphatase. The kinase domain (purple) is fused to the phosphatase domain (red). The bar represents the amino acid sequence of the enzyme. [Drawn from 1BIF.pdb.] Glucagon stimulates PKA when blood glucose is scarce. FBPase 2 is activcated. Glycolysis is inhibited, and gluconeogenesis is stimulated. GLUCOSE ABUNDANT (glycolysis active) Fructose 2,6-bisphosphate (stimulates PFK) GLUCOSE SCARCE (glycolysis inactive) Protein kinase A ADP ATP PFK2 Pi ADP FBPase2 PFK2 Fructose 6-phosphate (no PFK stimulation) FBPase2 Pi PFK more active ATP Pi Fructose 6-phosphate + H2O Phosphoprotein phosphatase H2O Fructose 2,6-bisphosphate High levels of fructose 6-phosphate stimulate phosphoprotein phosphatase. PFK2 is activated. Glycolysis is stimulated, and gluconeogenesis is inhibited. Figure 16.7 Control of the synthesis and degradation of fructose 2,6-bisphosphate. A low blood-glucose level as signaled by glucagon leads to the phosphorylation of the bifunctional enzyme and, hence, to a lower level of fructose 2,6-bisphosphate, slowing glycolysis. High levels of fructose 6-phosphate accelerate the formation of fructose 2,6-bisphosphate by facilitating the dephosphorylation of the bifunctional enzyme. What controls whether PFK2 or FBPase2 dominates the bifunctional enzyme’s activities in the liver? The activities of PFK2 and FBPase2 are reciprocally controlled by the phosphorylation of a single serine residue. When glucose is scarce, as it is during a night’s fast, a rise in the blood level of the hormone glucagon triggers a cyclic AMP signal cascade (p. 177), leading to the phosphorylation of this bifunctional enzyme by protein kinase A (Figure 16.7). This covalent modification activates FBPase2 and inhibits PFK2, lowering the level of F-2,6-BP. Gluconeogenesis predominates. Glucose formed by the liver under these conditions is essential for the viability of the brain. Glucagon stimulation of protein kinase A also inactivates pyruvate kinase in the liver (p. 244). Conversely, when blood-glucose is abundant, as it is after a meal, glucagon concentration in the blood falls and insulin levels in the blood rise. Now, gluconeogenesis is not needed and the phosphoryl group is removed from the bifunctional enzyme. This covalent modification activates PFK2 and inhibits FBPase2. The resulting rise in the level of F-2,6-BP accelerates glycolysis. The coordinated control of glycolysis and gluconeogenesis is facilitated by the location of the kinase and phosphatase domains on the same polypeptide chain as the regulatory domain. Clinical Insight Insulin Fails to Inhibit Gluconeogenesis in Type 2 Diabetes Insulin, the hormone that signifies the presence of fuels in the blood or the fed state, normally inhibits gluconeogenesis. When glucose is present in the blood after a meal, as signaled by the appearance of insulin, there is no need to synthesize glucose. Recall from Chapter 12 that insulin normally starts a signaling cascade that increases the expression of genes that encode the proteins of glycolysis. Insulin also turns off the gene that encodes phosphenolpyruvate carboxykinase (PEPCK), thereby inhibiting gluconeogenesis. However, in type 2 diabetes (or non-insulin-dependent diabetes), insulin, although present, fails to inhibit the expression of the gene that encodes PEPCK and other genes of gluconeogenesis. This metabolic circumstance is called insulin resistance and is the defining feature of type 2 diabetes. The higher-than-normal concentration of PEPCK results in an 260 increased output of glucose by the liver even when glucose from the diet is present. Blood glucose rises to abnormally high levels (hyperglycemia). High levels of blood glucose result in excessive thirst, frequent urination, blurred vision, fatigue, and frequent or slow-healing infections. The cause of type 2 diabetes, the most common metabolic disease in the world, is unknown, although obesity may be a contributing factor. Untreated, type 2 diabetes can progress to type 1, or insulin-dependent, diabetes. The treatment of type 2 diabetes includes weight loss, a healthy diet, exercise, and drug treatment to enhance sensitivity to insulin (Figure 16.8). ■ Substrate Cycles Amplify Metabolic Signals A pair of reactions such as the phosphorylation of fructose 6Figure 16.8 Diet can help to prevent the development of type 2 phosphate to fructose 1,6-bisphosphate in the glycolytic pathdiabetes. A healthy diet, one rich in fruits and vegetables, is an way and its hydrolysis back to fructose 6-phosphate in the important step in preventing or treating type 2 diabetes. gluconeogenic pathway is called a substrate cycle. As already [Photodisc/Getty Images.] mentioned, both reactions are not simultaneously fully active in most cells, because of reciprocal allosteric controls. However, the results of isoATP ADP tope-labeling studies have shown that some fructose 6-phosphate is phosphorylated to fructose 1,6-bisphosphate even in gluconeogenesis. There is also a limited 100 degree of cycling in other pairs of opposed irreversible reactions. This cycling was A B regarded as an imperfection in metabolic control, and so substrate cycles have 90 sometimes been called futile cycles. Indeed, there are pathological conditions, such as malignant hyperthermia, in which control is lost and both pathways proceed H2O Pi rapidly. In malignant hyperthermia, there is rapid, uncontrolled hydrolysis of ATP, Net flux of B = 10 which generates heat and can raise body temperature to 44 °C (111 °F). Muscles may become rigid and destroyed as well. ATP ADP Despite such extraordinary circumstances, substrate cycles now seem likely to be biologically important. One possibility is that substrate cycles amplify meta120 bolic signals. Suppose that the rate of conversion of A into B is 100 and of B into A B A is 90, giving an initial net flux of 10. Assume that an allosteric effector 72 increases the A S B rate by 20% to 120 and reciprocally decreases the B S A rate by 20% to 72. The new net flux is 48, and so a 20% change in the rates of H2O Pi the opposing reactions has led to a 380% increase in the net flux. In the examNet flux of B = 48 ple shown in Figure 16.9, this amplification is made possible by the rapid hydrolysis of ATP. The flux of each step of the glycolytic pathway has been sugFigure 16.9 A substrate cycle. This ATPgested to increase as much as 1000-fold at the initiation of intense exercise, driven cycle operates at two different rates. when a lot of ATP is needed. Because the allosteric activation of enzymes alone A small change in the rates of the two seems unlikely to explain this increased flux, the existence of substrate cycles opposing reactions results in a large may partly account for the rapid rise in the rate of glycolysis. change in the net flux of product B. 16.3 Metabolism in Context: Precursors Formed by Muscle Are Used by Other Organs Lactate produced by active skeletal muscle and red blood cells is a source of energy for other organs. Red blood cells lack mitochondria and can never oxidize glucose completely. Recall that, in contracting skeletal muscle during vigorous exercise, the rate at which glycolysis produces pyruvate exceeds the rate at which the citric acid cycle oxidizes it. In these cells, lactate dehydrogenase reduces excess pyruvate to lactate to restore redox balance (p. 235). However, lactate is a dead end in metabolism. It must be converted back into pyruvate before it can be metabolized. Both pyruvate and lactate diffuse out of these cells through carriers into the blood. In contracting skeletal muscle, the formation and 261 262 16 Gluconeogenesis IN LIVER GLUCONEOGENESIS Glucose GLYCOLYSIS B Glucose L 6 ~P Figure 16.10 The Cori cycle. Lactate formed by active muscle is converted into glucose by the liver. This cycle shifts part of the metabolic burden of active muscle to the liver. The symbol ⬃P represents nucleoside triphosphates. IN MUSCLE Pyruvate O 2 ~P Pyruvate O Lactate D Lactate release of lactate lets the muscle generate ATP in the absence of oxygen and shifts the burden of metabolizing lactate from muscle to other organs. The pyruvate and lactate in the bloodstream have two fates. In one fate, the plasma membranes of some cells, particularly cells in cardiac muscle, contain carriers that make the cells highly permeable to lactate and pyruvate. These molecules diffuse from the blood into such permeable cells. Inside these well-oxygenated cells, lactate can be reverted back to pyruvate and metabolized through the citric acid cycle and oxidative phosphorylation to generate ATP. The use of lactate in place of glucose by these cells makes more circulating glucose available to the active muscle cells. In the other fate, excess lactate enters the liver and is converted first into pyruvate and then into glucose by the gluconeogenic pathway. Thus, the liver restores the level of glucose necessary for active muscle cells, which derive ATP from the glycolytic conversion of glucose into lactate. These reactions constitute the Cori cycle (Figure 16.10). Studies have shown that alanine, like lactate, is a major precursor of glucose in the liver. The alanine is generated in muscle when the carbon skeletons of some amino acids are used as fuels. The nitrogens from these amino acids are transferred to pyruvate to form alanine (p. 458); the reverse reaction takes place in the liver. This process also helps to maintain nitrogen balance. The interplay between glycolysis and gluconeogenesis is summarized in Figure 16.10, which shows how these pathways help meet the energy needs of different cell types. 16.4 Glycolysis and Gluconeogenesis Are Evolutionarily Intertwined The metabolism of glucose has ancient origins. Organisms living in the early biosphere depended on the anaerobic generation of energy until significant amounts of oxygen began to accumulate 2 billion years ago. We can speculate on the evolutionary relationship between glycolysis and gluconeogenesis if we think of glycolysis as consisting of two segments: the metabolism of hexoses (stages 1 and 2, see Figure 15.1) and the metabolism of trioses (stage 3). The enzymes of stages 1 and 2 are different in some species and are missing entirely in some archaea, whereas the enzymes of stage 3 are quite conserved. In fact, four enzymes of the lower segment are present in all species. This lower part of the pathway is common to glycolysis and gluconeogenesis. This common part of the two pathways may be the oldest part, constituting the core to which the other steps were added. The upper part would have varied according to the sugars that were available to evolving organisms in particular niches. Interestingly, this core part of carbohydrate metabolism can generate triose precursors for ribose sugars, a component of RNA and a critical requirement for the RNA world. Thus, we are left with the unanswered question, was the original core pathway used for energy conversion or biosynthesis? 263 SUMMARY Key Terms 16.1 Glucose Can Be Synthesized from Noncarbohydrate Precursors Gluconeogenesis is the synthesis of glucose from noncarbohydrate sources, such as lactate, amino acids, and glycerol. Several of the reactions that convert pyruvate into glucose are common to glycolysis. Gluconeogenesis, however, requires four new reactions to bypass the essential irreversibility of three reactions in glycolysis. In two of the new reactions, pyruvate is carboxylated in mitochondria to oxaloacetate, which, in turn, is decarboxylated and phosphorylated in the cytoplasm to phosphoenolpyruvate. Two molecules having high phosphoryl-transfer potential are consumed in these reactions, which are catalyzed by pyruvate carboxylase and phosphoenolpyruvate carboxykinase. Pyruvate carboxylase contains a biotin prosthetic group. The other distinctive reactions of gluconeogenesis are the hydrolyses of fructose 1,6-bisphosphate and glucose 6-phosphate, which are catalyzed by specific phosphatases. The major raw materials for gluconeogenesis by the liver are lactate and alanine produced from pyruvate by active skeletal muscle. The formation of lactate during intense muscular activity buys time and shifts part of the metabolic burden from muscle to the liver. 16.2 Gluconeogenesis and Glycolysis Are Reciprocally Regulated Gluconeogenesis and glycolysis are reciprocally regulated so that one pathway is relatively inactive while the other is highly active. Phosphofructokinase and fructose 1,6-bisphosphatase are key control points. Fructose 2,6-bisphosphate, an intracellular signal molecule present at higher levels when glucose is abundant, activates glycolysis and inhibits gluconeogenesis by regulating these enzymes. Pyruvate kinase and pyruvate carboxylase are regulated by other effectors so that both are not maximally active at the same time. Allosteric regulation and reversible phosphorylation, which are rapid, are complemented by transcriptional control, which takes place in hours or days. 16.3 Metabolism in Context: Precursors Formed by Muscle Are Used by Other Organs Lactate that is generated by glycolysis in contracting muscle is released into the bloodstream. This lactate is removed from the blood by the liver and is converted into glucose by gluconeogenesis. This metabolic cooperation between muscle and liver is called the Cori cycle. Alanine is used to transport nitrogen as well as carbon skeletons from muscle to the liver. 16.4 Glycolysis and Gluconeogenesis Are Evolutionarily Intertwined Parts of glycolysis and gluconeogenesis are ancient pathways. In particular, the metabolism of trioses that is common to both pathways may be the oldest part. This common set of reactions formed the basis of carbohydrate metabolism from which glycolysis and gluconeogenesis evolved. Key Terms gluconeogenesis (p. 251) pyruvate carboxylase (p. 254) biotin (p. 255) glucose 6-phosphatase (p. 257) bifunctional enzyme (p. 259) substrate cycle (p. 261) Cori cycle (p. 262) 264 16 Gluconeogenesis Answers to QUICK QUIZZES 1. The reverse of glycolysis is highly endergonic under cellular conditions. The expenditure of 6 NTP molecules in gluconeogenesis renders gluconeogenesis exergonic. 2. Reciprocal regulation at the key allosteric enzymes in the two pathways. For instance, PFK is stimulated by fructose 2,6-bisphosphate and AMP. The effect of these signals is opposite that of fructose 1,6-bisphosphatase. If both pathways were operating simultaneously, a futile cycle would result. ATP would be hydrolyzed, yielding only heat. Problems 1. Road blocks. What reactions of glycolysis are not readily reversible under intracellular conditions? How are these reactions bypassed in gluconeogenesis? 9. Metabolic mutants. What would be the effect on an organism’s ability to use glucose as an energy source if a mutation inactivated glucose 6-phosphatase in the liver? 2. Waste not, want not. Why is it in an organism’s best interest to convert lactic acid from the blood into glucose in the liver? 10. Never let me go. Why does the lack of glucose 6-phosphatase activity in the brain and muscle make good physiological sense? 3. Metabolic mutant. What are the likely consequences of a genetic disorder rendering fructose 1,6-bisphosphatase in the liver less sensitive to regulation by fructose 2,6-bisphosphate? 11. Match ’em 1. The following sequence is a part of the sequence of reactions in gluconeogenesis. 4. Biotin snatcher. Avidin, a 70-kd protein in egg white, has very high affinity for biotin. In fact, it is a highly specific inhibitor of biotin enzymes. Which of the following conversions would be blocked by the addition of avidin to a cell homogenate? (a) Glucose S pyruvate (b) Pyruvate S glucose (c) Oxaloacetate S glucose (d) Malate S oxaloacetate (e) Pyruvate S oxaloacetate (f) Glyceraldehyde 3-phosphate S fructose 1,6-bisphosphate 5. Tracing carbon atoms. If cells synthesizing glucose from lactate are exposed to CO2 labeled with 14C, what will be the distribution of label in the newly synthesized glucose? 6. Working at cross-purposes? Gluconeogenesis takes place during intense exercise, which seems counterintuitive. Why would an organism synthesize glucose and, at the same time, use glucose to generate energy? 7. Powering pathways. Compare the stoichiometries of glycolysis and gluconeogenesis. Recall that the input of one ATP equivalent changes the equilibrium constant of a reaction by a factor of about 108 (p. 207). By what factor do the additional high-phosphoryl-transfer compounds alter the equilibrium constant of gluconeogenesis? 8. Different needs. Liver is primarily a gluconeogenic tissue, whereas muscle is primarily glycolytic. Why does this division of labor make good physiological sense? Pyruvate ¡ Oxaloacetate ¡ Malate ¡ A B C oxaloacetate ¡ Phosphoenolpyruvate D Match the capital letters representing the reaction in the gluconeogenic pathway with parts a, b, c, etc. (a) takes place in the mitochondria. (b) takes place in the cytoplasm. (c) produces CO2. (d) consumes CO2. (e) requires NADH. (f) produces NADH. (g) requires ATP. (h) requires GTP. (i) requires thiamine. (j) requires biotin. (k) is regulated by acetyl CoA. 12. Salvaging resources. In starvation, protein degradation takes place in muscle. Explain how this degradation might affect gluconeogenesis in the liver. 13. Counting high-energy compounds 1. How many NTP molecules are required for the synthesis of one molecule of glucose from two molecules of pyruvate? How many NADH molecules? 14. Counting high-energy compounds 2. How many NTP molecules are required to synthesize glucose from each of the following compounds? (a) Glucose 6-phosphate (b) Fructose 1,6-bisphosphate (c) Two molecules of oxaloacetate (d) Two molecules of dihydroxyacetone phosphate 265 Problems 16. Lending a hand. How might enzymes that remove amino groups from alanine and aspartate contribute to gluconeogenesis? (a) What was the rationale for comparing the activities of these two enzymes? (b) The following data show the activites of both enzymes for a variety of bumblebee species (genera Bombus and Psithyrus). Do these results support the notion that bumblebees use futile cycles to generate heat? Explain. Enzyme activity (units g−1 thorax) Chapter Integration Problems 15. Match ’em 2. Indicate which of the conditions listed in the right-hand column increase the activity of the glycolytic and gluconeogenic pathways. (a) glycolysis 1. increase in ATP (b) gluconeogenesis 2. increase in AMP 3. increase in fructose 2,6-bisphosphate 4. increase in citrate 5. increase in acetyl CoA 6. increase in insulin 7. increase in glucagon 8. fasting 9. fed PFK FBPase 120 100 80 60 40 20 us trin llis P. ci seo co tus inc gri B. oc B. ruf rpl ex us ns pe ga B. ns va B. tie tus B. im pa s ula ini ac aff bim B. B. res ter B. Data Interpretation Problem 17. Cool bees. In principle, a futile cycle that includes phosphofructokinase and fructose 2,6-bisphosphatase could be used to generate heat. The heat could be used to warm tissues. For instance, certain bumblebees have been reported to use such a futile cycle to warm their flight muscles on cool mornings. Scientists undertook a series of experiments to determine if a number of species of bumblebee use this futile cycle. Their approach was to measure the activity of PFK and F-1,6-BPase in flight muscle. tris 0 [After J. F. Staples, E. L. Koen, and T. M. Laverty, J. Exp. Biol. 207:749–754, 2004, p. 751.] (c) In which species might futile cycling take place? Explain your reasoning. (d) Do these results prove that futile cycling does not participate in heat generation? Selected readings for this chapter can be found online at www.whfreeman.com/Tymoczko