* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell Transport Systems
Cell nucleus wikipedia , lookup
Membrane potential wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell membrane wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
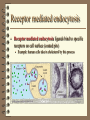
Cell Transport Systems Diffusion • Mixing of materials by their own random motion • Mixing occurs from an area of high to low concentrations Osmosis • • • Basically the diffusion of H2O through a membrane Since H2O molecules are small – they can pass through membranes (even nonpolar part) Follows the same principles of regular diffusion (High to low concentration) Osmotic pressure • When a cell is in fresh water, the concentration of water is greater on the outside than the inside of a cell. This is called a Hypotonic solution. When this happens, water can fill the cell and put pressure on the side of it causing pressure – Osmotic pressure. If osmotic pressure gets too much it can “burst” a cell. This is called Plasmolysis. • Many fresh water organisms have built in “sump pumps” to remove excess H2O. Other Salt water organisms must pump in water • Conversely, in a salt-water environment, cells must pump water into the cell. This is called a hypertonic solution. The cell can lose water and "shrivel" this is called crenation. • Cells in larger organisms are surrounded by solution with roughly equal concentrations of H2O and solvents. This is called an isotonic solution. Osmosis illustrated Isotonic Hypertonic Hypotonic Selective Transports • Used for molecules which cannot easily pass through cell membrane because they are either… • Too large to pass between phospholipids • Are polar and cannot pass through non-polar region eg. ions • Two basic types Facilitated Diffusion • Works by having channel proteins of different sizes (like a sieve) or ion channels which allow larger, or charged molecules to diffuse in/out of cell Active Transports • • • • Lets in/out molecules via membrane proteins which actively pump in/out molecules. This requires energy from the cell. Moves molecules in 1 direction AGAINST THE CONCENTRATION GRADIENT Example is the Sodium-Potassium Pump. Sodium / Potassium Pump (Adv. Bio) Pumps Potassium into & Sodium out-of cell 6 step process 1. 2. 3. 4. 5. 6. 3 Na+ (Sodium) Ions bind to a special receptor protein Energy (from ATP) is added to the protein. Protein changes shape, releasing Na+ from the cell. 2 K+ (Potassium) ions now bind to the protein Phosphate is released, protein returns to its original shape. K+ ions are released into the cell interior Endocytosis • Large particles enter cells via endocytosis • Membrane actively surrounds & engulfs particle or fluid and forms a vacuole. • Phagocytosis – Cell eating – involves large particles. • Pinocytosis – Cell Drinking – involves liquids. Exocytosis • Large particles exit the cell via Exocytosis • Vessicle moves to and merges with the plasma membrane. • Vessicle ruptures outwards, releasing its contents Receptor mediated endocytosis • Receptor mediated endocytosis: ligands bind to specific receptors on cell surface (coated pits) • Example: human cells take in cholesterol by this process