* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download programmed cell death
Cell nucleus wikipedia , lookup
Extracellular matrix wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Cellular differentiation wikipedia , lookup
Paracrine signalling wikipedia , lookup
Signal transduction wikipedia , lookup
List of types of proteins wikipedia , lookup
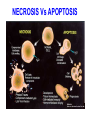
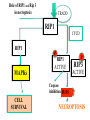
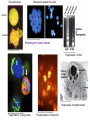
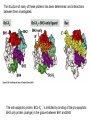
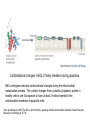
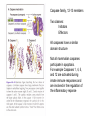
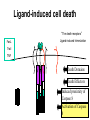
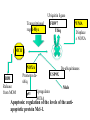
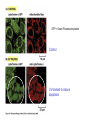
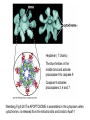
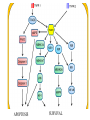
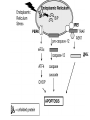
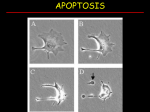
PROGRAMMED CELL DEATH Normal white blood cell Apoptotic white blood cell During apoptosis (programmed cell death) cells bleb and eventually break apart without releasing contents. Why should a cell commit suicide? ! Apoptosis is needed for proper development Examples: – The resorption of the tadpole tail – The formation of the fingers and toes of the fetus – The sloughing off of the inner lining of the uterus – The formation of the proper connections between neurons in the brain ! Apoptosis is needed to destroy cells Examples: – Cells infected with viruses – Cells of the immune system – Cells with DNA damage – Cancer cells Apoptosis is also important in the development of the nervous system NECROSIS Vs APOPTOSIS Wilde, 1999 Role of RIP1 and Rip 3 in necroptosis TRADD RIP1 RIP1 MAPKs CYCD P RIP1 ACTIVE P RIP3 ACTIVE Caspase inhibition ROS Cell CELL survival SURVIVAL NECROPTOSIS Two lymphocytes Healthy Staurosporin-treated HeLa cells Apoptosis Apoptosis Apoptotic Shrinking of nuclear volume Fragmentation of DNA Pyknotic nuclear fragments Apoptotic cell Phagocytosis of apoptotic bodies Fragmentation of Golgi bodies Phosphorylation of Histone 2B Apoptosis: Pathways Extrinsic Pathway Death Ligands Death Receptors Intrinsic Pathway DNA damage & p53 Mitochondria/ Cytochrome C Initiator Caspase 8 Effector Caspase 3 Initiator Caspase 9 PCD Evolutionary conservation of apoptotic pathways Apoptosis can be intrinsic (signaled from within or extrinsic to the cell i.e. from cell receptors The intrinsic apoptotic pathway The extrinsic pathway THE PLAYERS INVOLVED. BCL-2 family of proteins important in apoptotic pathways = anti-apoptotic The structure of many of these proteins has been determined and interactions between them investigated. The anti-apoptotic protein, BCL-XL , is inhibited by binding of the pro-apoptotic BH3 only protein (orange) in the groove between BH1 and BH3 Conformational changes in BCL-2 family members during apoptosis. BAX undergoes extensive conformational changes during the mitochondrial translocation process. The protein changes from a soluble cytoplasmic protein in healthy cells to one that appears to have at least 3 helices inserted in the mitochondrial membrane in apoptotic cells. Youle and Strasser (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology, 9, 47-59 Death receptors Caspase family, 12-13 members Two classes: Initiators Effectors All caspases have a similar domain structure Not all mammalian caspases participate in apoptosis. For example Caspases 1, 4, 5, and 12 are activated during innate immune responses and are involved in the regulation of the inflammatory reponse Ligand-induced cell death The death receptors FasL Ligand-induced trimerization Trail TNF Death Domains Death Effectors Induced proximity of Caspase 8 Activation of Caspase 8 3 mechanisms of caspase activation a. Proteolytic cleavage e.g. procaspase 3 b. Induced proximity, e.g. procaspase 8 c. Oligomerization, e.g. cyt c, Apaf-1 & caspase 9 Back The mitochondrial pathway DNA damage Growth factor receptors Fas Casp8 p53 PI3K Bid Bid Bax Bid Bax Akt casp3 BAD Bcl2 casp9 Apaf1ATP Cyt.C IAPs casp3 Smac/ DIABLO H2O2 AIF Pollack etal., 2001 Transcriptional c-Myc regul. Ubiquitin ligase FBW7 Ubiq MCl1 Release from MOM Displace s NOXA Mcl-1 NOXA BIM PUMA De-ubiquitinases Promotes deubiq. p53 Upregulates NOXA USP9X Mule Apoptosis: regulation of the levels of the antiapoptotic protein Mcl-1. GFP = Green Fluorescent protein Control UV-treated to induce apoptosis Heptamer ( 7 chains) The blue helices in the middle bind and activate procaspase 9 to caspase 9 Caspase 9 activates procaspases 3, 6 and 7 Weinberg Fig 9.28 The APOPTOSOME is assembled in the cytoplasm when cytochrome c is released from the mitochondria and binds to Apaf-1 APOPTOSIS SURVIVAL P53 & Apoptosis p53 first arrests cell growth between G1 → S This allows for DNA repair during delay If the damage is too extensive then p53 induces gene activation leading to apoptosis (programmed cell death) BACK JNK