* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Powerpoint
Mitogen-activated protein kinase wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Reactive oxygen species wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Biochemical cascade wikipedia , lookup
Lipid signaling wikipedia , lookup
Zinc finger nuclease wikipedia , lookup
Paracrine signalling wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Proteases in angiogenesis wikipedia , lookup
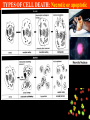
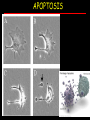
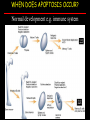
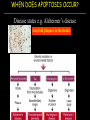
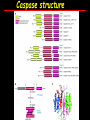
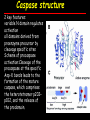
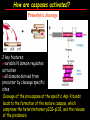
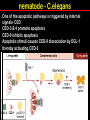
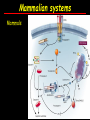
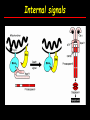
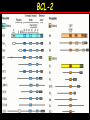
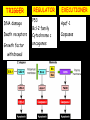
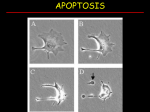
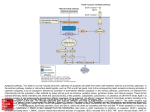
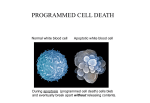
Apoptosis – mechanisms and role in cancer therapy TYPES OF CELL DEATH: Necrotic or apoptotic APOPTOSIS External signals WHEN DOES APOPTOSIS OCCUR? Normal development e.g. immune system WHEN DOES APOPTOSIS OCCUR? Disease states e.g. Alzheimer’s disease Amyloid plaques in the brain Caspases – key executioners of apoptosis (cysteinyl aspartate specific proteases) Highly conserved proteases inactive zymogens Caspases divided into Group I Inflammatory caspases Caspases 1,4,5,11,12,13,14 Group II Initiator caspases Caspases 2,8,9,10 Group III Effector caspases: caspases 3,6,7 Caspase structure Properties of proteases Irreversible Autocatalytic: triggered by cofactor binding or inhibitor removal Proteases can regulate their own activation protease inhibitors specificity Caspase structure 3 domains 1) highly variable NH2 domain 2) large subunit (p20; ~20kD) 3) small subunit ( p10; ~10kD) Highly specific absolute requirement for cleavage after aspartic acid recognition of at least 4 amino acids NH2 terminals to the cleavage site Caspase structure 2 key features: variable N domain regulates activation all domains derived from proenzyme precursor by cleavage specific sites Scheme of procaspase activation:Cleavage of the procaspase at the specific Asp-X bonds leads to the formation of the mature caspase, which comprises the heterotetramer p202– p102, and the release of the prodomain. Structure of caspase-3 heterotetramer Each heterodimer is formed by hydrophobic interactions resulting in the formation of mostly parallel ß-sheets, composed of 6 antiparallel ß-strands. Two heterodimers fit together with formation of a 12-stranded ß-sheet that is sandwiched by a helices. N and C termini of the small and large protease subunits are indicated Basic apoptotic machinery DNA fragmentation, chromatin condensation, membrane blebbing, cell shrinkage & disassembly into apoptotic bodies engulfment Initiator caspases inactivate proteins that protect cells from apoptosis Effector caspases are responsible for cellular changes associated with apoptosis. How do caspases disassemble a cell? It slices, it dices! Selective cleavage of specific proteins eg bcl-2, or CAD/ICAD e.g. nuclear lamins eg. Gelsolin What triggers apoptosis? • Growth factor withdrawal • Specific ‘death ligands • Loss of contact with surroundings • Irreparable internal damage • Conflicting signals for cell division How are caspases activated? Proteolytic cleavage 2 key features: variable N domain regulates activation all domains derived from precursor by cleavage specific sites Cleavage of the procaspase at the specific Asp-X bonds leads to the formation of the mature caspase, which comprises the heterotetramer p202–p102, and the release of the prodomain. How are caspases activated? Induced proximity aggregation of multiple procaspase-8 molecules into close proximity somehow results in cross-activation How are caspases activated? Holoenzyme formation Activation of caspase-9 is mediated by means of conformational change, not proteolysis nematode - C.elegans One of the apoptotic pathways is triggered by internal signals- CED CED-3 & 4 promote apoptosis CED-9 inhibits apoptosis Apoptotic stimuli causes CED-9 dissociation by EGL-1 thereby activating CED-3. Caspase signaling in Mammalian systems IN Lavrik et al The Journal of Clinical Investigation 115(10):2665-72. (October 2005) Mammalian systems Mammals External signals driven by death receptors (DR) e.g. CD95 (or Fas/Apo) Each CD95L trimer binds to 3 CD95 leading to DD clustering. FADD ( Fas associated death domain/ Mort 1) binds via its own DD Caspase –8 oligomerisation drives activation through self cleavage Caspase –8 then activates downstream effector caspases like caspase –9 (CED-9 homolog) Internal signals BCL-2 TRIGGER DNA damage Death receptors Growth factor withdrawal REGULATOR P53 Bcl-2 family Cytochrome c oncogenes EXECUTIONER Apaf-1 Caspases Green and Kroemer The Journal of Clinical Investigation 115(10):2610-17 (October 2005) References Chapter 12: Cellular & Mol Biology by Knowles and Selby AND/OR Science (1998) Vol 281: No 5381; pgs 1298-1326 AND/OR J. Clin Invest (10 Oct 2005) 115(10):2665-72 AND/OR Cancer Biology by RJB King pgs 160-167 AND/OR NATURE | VOL 407 | 12 OCTOBER 2000 pp770-776 The biochemistry of apoptosis by M.O. Hengartner Optional NATURE REVIEWS MOLECULAR CELL BIOLOGY Vol 5 | NOV 2004 | 897 Molecular mechanisms of caspase regulation during apoptosis Stefan J. Riedl and Yigong Shi (Only read it if you want to know more about caspase structure)