* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download BY 330 Spring 2015Worksheet 4 Name the substrate ligand and
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Lipid signaling wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
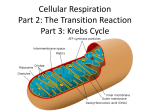
Light-dependent reactions wikipedia , lookup
Gene regulatory network wikipedia , lookup
Paracrine signalling wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemical cascade wikipedia , lookup
Microbial metabolism wikipedia , lookup
Signal transduction wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
BY 330 Spring 2015 Worksheet 4 1. Name the substrate ligand and product for the enzyme pyruvate kinase. Name one regulatory ligand that will increase the Michaelis constant of this enzyme. Substrate ligand – phosphoenol pyruvate Product – pyruvate ATP, alanine are examples of regulatory ligands that will increase the Michaelis constant 2. What are the three pathways that glucose-6-phosphate can take if there is excess glucose in the cell and the energy charge is stable? What additional pathway can it take if the energy charge in the cell is too low? OMIT 3. What is the most powerful modulator of phosofructokinase and how does it originate and work? Fructose-2,6-bisphosphate – turns on PFK (positive modulator). Made from fructose-6-phosphate (PFK2 catalyzes this reaction and glucose-6phosphate turns PFK2 on). 4. How are alanine and citrate important during glycolysis, even though they are not present in the formal pathway? Alanine – negative modulator of pyruvate kinase if there is a lot of alanine (a lot of amino acid) in the cell and not much sugar, cell will burn the alanine and save the sugar/glycolysis for later. Citrate – negative modulator of PFK if there is a lot of citrate, the Krebs cycle is active and the cell does not need to go through glycolysis to produce any more pyruvate 5. Describe and explain energy charge and how it is important to the cell. Energy charge is the normal amounts of ATP, ADP, and AMP the cell would like to maintain to have normal processes occur. Usually, the levels of ATP are high while the levels of ADP and AMP are lower, respectively. (The graph Dr. Watts drew might help to explain this). It is important to maintain a normal energy charge, otherwise the cell will need to make more energy. For example, if the levels of ATP in the cell decrease and the levels of ADP and AMP increase, the cell will need to use glycolysis to make more ATP and get itself back towards a normal energy charge. 6. What is the law of mass action? Which enzymes do not follow this law and how do they work? BY 330 Spring 2015 Worksheet 4 The law of mass action describes enzymes that can work in more than one direction. Whichever direction the equilibrium lies, is the direction that enzyme will work in. For example, if there is too much product present, these enzymes will work in reverse and if there is too much substrate present, the enzyme will work in the forward direction. Hexokinase, pyruvate kinase, and phosphofructokinase do not follow this law. They are rate-limiting enzymes, which means that they will only work in the forward direction. 7. If 10 molecules of isocitrate are converted to succinate in the Krebs cycle, how many molecules of NADH will be produced? AcetylCoA? CO2? GTP? FADH2? Show the cycle. (I won’t show the cycle here, but it comes straight from your notes. Make sure you include sites of energy, NADH, and FADH2 production in your drawing). NADH – 20 AcetylCoA – 0 CO2 – 20 GTP – 10 FADH2 - 0 8. Which three enzymes in the Krebs Cycle lead to the production of NADH? Isocitrate dehydrogenase, alpha-ketogutarate dehydrogenase, and malate dehydrogenase 9. If 12 molecules of glucose are catabolized to malate, how many FADH2 are produced? 24 10. The Km of phosphofructokinase is 0.2. What happens to this value when citrate binds? Glucose-6-phosphate? ATP? Fructose-2,6-bisphosphate? Alanine? Citrate – increases Glucose-6-phosphate – decreases ATP – increases Fructose-2,6-bisphosphate – decreases Alanine – no change as alanine only modulates pyruvate kinase, not PFK 11. What reaction occurs in between glycolysis and the Krebs Cycle? How much NADH and CO2 are produced here for 6 molecules of glucose? What enzyme catalyzes this reaction? Pyruvate dehydrogenase reaction or the oxidative phosphorylation of pyruvate; For 6 glucose – 12 NADH, 12 CO2; pyruvate dehydrogenase catalyzes this reaction 12. Can acetylCoA enter the Krebs Cycle directly? What has to bind to it and what product is produced? What enzyme is used for this reaction? BY 330 Spring 2015 Worksheet 4 No, acetylCoA must be escorted by oxaloacetate. AcetylCoA + oxaloacetate = citrate. Citrate synthase catalyzes this reaction 13. Give two examples of regulation that occurs during the Krebs Cycle. Dr. Watts gave three examples: ATP is a positive modulator for isocitrate dehydrogenase. Succinate is a negative modulator for citrate synthase. Oxaloacetate is a negative modulator for succinate dehydrogenase.