* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The visual-oculomotor striatum of the cat: functional relationship to
Neuroesthetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Axon guidance wikipedia , lookup
Neuroplasticity wikipedia , lookup
Aging brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Subventricular zone wikipedia , lookup
Optogenetics wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Sexually dimorphic nucleus wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Circumventricular organs wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Synaptic gating wikipedia , lookup
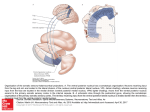
Exp Brain Res (2001) 136:138–142 DOI 10.1007/s002210000606 RESEARCH NOTE John K. Harting · Bruce V. Updyke David P. Van Lieshout The visual-oculomotor striatum of the cat: functional relationship to the superior colliculus Received: 31 August 2000 / Accepted: 4 October 2000 / Published online: 10 November 2000 © Springer-Verlag 2000 Abstract The visual-recipient sector of the cat striatum receives corticostriate input from over 15 higher visual and oculomotor-related areas of the cortex and appears homologous with the physiologically characterized region of mixed visual and oculomotor inputs within the primate caudate nucleus. This area in the cat involves the dorsolateral caudate and a strip of the caudal putamen. In a first series of experiments, the former was injected with a retrograde tracer in several cats. Thalamostriate cells were found in extensive regions, including the intralaminar nuclei, certain motor-related nuclei, and, most notably, across much of the extrageniculate visual thalamus. In another set of experiments, anterograde tracers were also injected into the superior colliculus (SC), and labeled tectothalamic fibers were observed in all thalamic sites projecting to the visual-recipient striatum. These findings highlight for the first time the need for the SC to be considered in models of thalamostriate and visual/ oculomotor-striatal function(s). Moreover, the data bring to light the fact that basal-ganglia outflow reaching the SC via striatonigro-nigrotectal circuitry is well positioned to modulate ascending tecto-thalamic-thalamostriatal signals destined for the visual-recipient striatum. Keywords Striatum · Superior colliculus · Thalamus striate projections in the regulation of saccades is poorly defined. The current findings are novel in revealing a relatively widespread thalamostriate input to the visualrecipient striatum and, most important, the innervation of these extensive projections by the superior colliculus. Materials and methods All surgical procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 80–23, revised 1978). All cats were anesthetized with sodium pentobarbital (38 mg/kg, ip). In several cats, 0.03–0.125 µl of 1.25–1.5% wheat-germ agglutinin conjugated to horseradish peroxidase (WGA-HRP; Sigma) dissolved in saline was injected into the visual-recipient region of the caudate nucleus, identified by Updyke (1993). In several other cats, the same tracer was injected into the superior colliculus. Following all injections, the surgical opening was cleaned, the dura drawn back over the exposed brain, the bone flap replaced, the surgical opening closed, and antibiotics administered. Animals were monitored post-operatively and administered analgesics. Postoperative survivals were 40–50 h, after which the cats were killed with an overdose of sodium pentobarbital and perfused with buffered saline followed with 2 l of 0.5% paraformaldehyde–2.5% glutaraldehyde in 0.1-M phosphate buffer, at pH 7.4, and a post-fix rinse consisting of 1 l of 0.1-M phosphate buffer containing 5% sucrose. The brains were later sectioned at 40 µm, after which a one in five series was reacted for peroxidase activity with the tetramethylbenzidine (TMB) method of Mesulam (1978) and counterstained with neutral red. Introduction The striatum is an integral component of saccadic eyemovement circuits (see Hikosaka 2000). The inputs underlying particular motor, sensory, and cognitive activities of visuo/oculomotor neurons in the caudate are poorly understood. While corticostriate projections are thought to provide saccade-related information to caudate neurons (Hikosaka et al. 1989), the role of thalamoJ.K. Harting (✉) · B.V. Updyke · D.P. Van Lieshout Department of Anatomy, University of Wisconsin, Madison, WI 53706, USA e-mail: [email protected] Fax: +1-608-2627306 Results The left column in Figs. 1 and 2 shows the locations of retrogradely labeled cells following an injection of WGA-HRP into the visual-recipient sector of the caudate nucleus (Updyke 1993). Within the thalamus, extensive regions of the intralaminar complex contained dense concentrations of labeled cells, especially the central anterior, rhomboidalis, central dorsal, and paraventricular nuclei. Scattered cells also lay within the centromedian nucleus as well as in the ventral region of the parafascicular nucleus and the nucleus limitans. 139 The lateral thalamus contained significant numbers of labeled cells. In particular, there was a rostral band of labeled cells within the medial part of the ventroanterior nucleus, which extended into the lateral ventromedial nucleus; a few scattered cells were also present in the Fig. 1 Legend see page 140 ventrolateral nucleus. Just as conspicuous were the numerous labeled cells in the lateral posterior complex. Using the terminology of Updyke (1983), the dorsal, medial, and ventral divisions of the lateral posterior shell and the rostral division of the lateral zone contained labeled 140 ▲ striatal projecting cells. At more caudal levels, retrograde labeling was also present within the lateral posterior shell, the rostral division of the lateral zone, and the interjacent and medial zones. The suprageniculate nucleus also contained heavy labeling rostrally and more sparse label caudally. Scattered labeled cells were also present within the posterior nuclear group, the caudal division of the lateral zone of the lateral posterior nucleus (LP), and the pulvinar nucleus. Data from other experiments (not illustrated) indicate that the characteristic pattern of afferent (and efferent) subcortical connections of the visual-recipient zone is distinct from the adjacent somatosensory/motor region. The right column in Figs. 1 and 2 shows the distribution of ascending anterogradely transported WGA-HRP from the SC (and the locations of tectopetal cell bodies). These projections were also more robust than previous reports indicate (see Huerta and Harting 1984). Ascending SC input reached almost all of the thalamic cell groups that project to the visual striatum. Thus, anterograde labeling was evident within the ventromedial and ventrolateral nuclei, nucleus limitans, and the central dorsal nucleus. Sparser label overlied the centromedian nucleus, and there were dense patches of labeled processes overlying the central lateral nucleus. WGA-HRPpositive axons/terminals were also associated with the intermediate division of the posterior nuclear group, suprageniculate nucleus, and the interjacent and medial zones of the LP complex. Anterograde label lied over the rostral division of the lateral zone of the LP complex and the ventral division of the lateral posterior shell. Labeled axons encroached upon the caudal division of the lateral zone, along its border with the interjacent zone of the LP Fig. 1 Illustrations showing the locations of anterogradely labeled axons/terminals (black lines) and retrogradely labeled cells (black circles) following injections of wheat-germ agglutinin/horseradish peroxidase (WGA-HRP) into the visual-recipient caudate (left column) and superior colliculus (right column). AC Anterior commissure, AM anteromedial nucleus, AV anteroventral nucleus, Cd central dorsal nucleus, CD caudate nucleus, Cl central lateral nucleus, Cm central medial nucleus, CM centromedian nucleus, d dorsal division of the medial geniculate nucleus, DHA dorsal hypothalamic area, EN entopeduncular nucleus, Fr, Fx fornix, GP globus pallidus, LD laterodorsal nucleus, LGNd dorsal lateral geniculate nucleus, LGNv ventral lateral geniculate nucleus, Li nucleus limitans, LPi interjacent zone of lateral posterior nucleus, LPl-c lateral zone of lateral posterior nucleus, caudal division, LPl-r lateral zone of lateral posterior nucleus, rostral division, LPm medial zone of lateral posterior, LPs-d lateral posterior shell, dorsal division, LPs-v lateral posterior shell, ventral division, mc magnocellular division of medial geniculate nucleus, MD mediodorsal nucleus, MTT mammillothalamic tract, Ocn oculomotor nucleus, OT optic tract, P pulvinar nucleus, PAG periaqueductal grey, Pf parafascicular nucleus, POi posterior nuclear group, intermediate division, POm posterior nuclear group, medial division, PU putamen, PV paraventricular nucleus, R red nucleus, Sg suprageniculate nucleus, SNc substantia nigra pars compacta, SNr substantia nigra pars reticulata, TRc thalamic reticular nucleus, v ventral divison of the medial geniculate nucleus, VA ventroanterior nucleus, VL ventrolateral nucleus, VM ventromedial nucleus, VPL ventroposterolateral nucleus, VPM ventroposterior medial nucleus, VTA ventral tegmental area, ZI zona incerta complex. Label present in the pulvinar and parvocellular layers of the dorsal lateral geniculate nucleus can be attributed to the involvement of the pretectum in the injection site. Discussion The distribution of thalamostriate projections observed in the current analysis is more extensive than reported in previous studies of the caudate nucleus (see Mengual et al. 1999 for a review). The intralaminar as well as several motor-related nuclei have been labeled in most of these earlier studies (as well as in ours), so it is the additional widespread distribution of caudate-projecting cells throughout the lateral (primarily visual) thalamus that distinguishes the visual-recipient striatum. The fact that the visual striatum was not specifically injected in most earlier reports (see, however, Takada et al. 1985) explains the absence of thalamostriate cells in the visual thalamus in such analyses. These data support the idea that functionally distinct sectors of the striatum exhibit different patterns of thalamostriate projections (Mengual et al. 1999). Superimposing the distributions of visual striate-related thalamostriate neurons (left column of Figs. 1 and 2) and ascending tectothalamic axons/terminal (right column of Figs. 1 and 2) indicates that all of the former are overlapped by the latter. While double-labeling studies are needed to more accurately demonstrate this overlap, the data certainly suggest that the SC is strategically positioned to influence all thalamic signals reaching the visual striatum. Some of these tectally innervated thalamic nuclei, for example the ventromedial nucleus (VM), are direct targets of the basal-ganglia outflow streams arising in the entopeduncular nucleus (EN) and the substantia nigra pars reticulata (SNr; Hendry et al. 1979). These outflow signals reaching VM can be sent back to the striatum as part of a feedback circuit informing the striatum about basal-ganglia signals reaching the cerebral cortex (Mengual et al. 1999). Ascending tectal signals could play a role in this proposed thalamostriate feedback loop. Moreover, descending cortical as well as ascending brain stem inputs that reach the SC (see Huerta and Harting 1984; Harting et al. 1992) could ultimately influence the message sent from the VM to the visual-recipient striatum. While basal-ganglia-outflow pathways directly innervate the VM, the cell groups of the LP-posterior nucleus complex, which give rise to a far larger portion of the thalamostriate projection to the visual-recipient striatum, lack well-substantiated direct input from EN or SNr (see, however, Takeda et al. 1984). Instead, these nuclei are dominated by ascending tectal (Huerta and Harting 1984) and descending cortical inputs (Updyke 1981, 1983). These connectional differences indicate that information reaching the visual-recipient striatum from the VM and LP complex differ even though ascending tectal projections reach both regions. Moreover, these ascend- 141 Fig. 2 Illustrations showing the locations of anterogradely labeled axons/terminals (black lines) and retrogradely labeled cells (black circles) following injections of WGA-HRP into the visual-recipient caudate (left column) and superior colliculus (right column). Abbreviations as in Fig. 1 ing tectothalamic signals would be modulated by the nigrotectal projection. Thus, the striatonigro-nigrotectal projections would convey the output signal of the basal ganglia to the SC, where such information would modulate ascending tecto-thalamic pathways and, thus, the information reaching the visual-recipient striatum via direct thalamostriate and indirect thalamo-corticostriate paths. Most importantly, the SC signals conveyed to the visualrecipient striatum reflect the ongoing activity in the SC, which involves both the integration of visual, oculomotor, auditory, and somatosensory signals, and their modulation by striato-nigro-tectal pathways. It is noteworthy that the striatonigral projection labeled following WGA-HRP injections into the visualrecipient striatum (Fig. 2, left column) only partially overlaps the locations of retrogradely labeled nigrotectal neurons seen in the experiments involving injections of WGA-HRP into the SC. Functionally distinct striatal sectors project to distinct non-overlapping regions of the SNr (see Maurin et al. 1999), and different parts of the SNr innervate (and modulate) distinct layers and sublayers of the SC (Harting et al. 1988). Since cells in the latter area target different thalamic cell groups innervating both cortical areas and the visual-recipient striatum, it follows that the different thalamofugal projections may be selectively influenced by outflow channels of different functional sectors of the caudate (and putamen; see Updyke 1993). This would facilitate associative “cross talk” within the striatum and the different parallel functional circuits (Alexander et al. 1986). In summary, these findings suggest a broader influence of the superior colliculus on thalamostriate circuitry and on visual/oculomotor-striatal function(s). Basal-ganglia outflow reaching the SC via striatonigro-nigrotectal circuitry not only influences the SC projections that descend to eye-movement centers in the brain stem (see Huerta and Harting 1984), but, just as important, regulate different ascending tectothalamic pathways. The evi- 142 dence presented here for relatively robust and direct tecto-thalamostriate projections suggests to us that the constraints placed upon tectal processing by striatonigral control have important consequences for central perceptuomotor processing at the sriatal and cortical levels. Acknowledgements Supported by Grant NS-37445 to JKH and EY05724 to BVU. We thank Cheryl Vega for technical support. References Alexander GE, DeLong MR, Strick, PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381 Harting JK, Huerta MF, Hashikawa T, Weber JT, Van Lieshout DP (1988) Neuroanatomical studies of the nigrotectal projection in the cat. J Comp Neurol 278:615–631 Harting JK, Updyke BV, Van Lieshout DP (1992) Corticotectal projections in the cat: anterograde transport studies of 25 cortical areas. J Comp Neurol 324:379–414 Hendry S, Jones EG, Graham J (1979) Thalamic relay nuclei for cerebellar and certain related fiber systems in the cat. J Comp Neurol 185:679–714 Hikosaka O, Sakamoto M, Usui S (1989) Functional-properties of monkey caudate neurons. 1. Activities related to saccadic eyemovements. J Neurophysiol 61:780–798 Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978 Huerta MF, Harting JK (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 687–773 Maurin Y, Banrezes B, Menetrey A, Mailly P, Deniau JM (1999) Three-dimensional distribution of nigrostriatal neurons in the rat: relation to the topography of striatonigral projections. Neuroscience 9:1891–1909 Mengual E, Heras S de las, Erro E, Lanciego JL, Gimenez-Amaya JM (1999) Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Anat 16:185–197 Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing afferents and efferents. J Histochem Cytochem 26:106–117 Takada M, Itoh K, Yasui T, Sugimoto T, Mizuno N (1984) Direct projections from the substantia nigra to the posterior thalamic region in the cat. Brain Res 309:143–146 Takada M, Itoh K, Yasui T, Sugimoto T, Mizuno N (1985) Topographic projections from the posterior thalamic regions to the striatum in the cat, with reference to possible tecto-thalamostriatal connections. Exp Brain Res 60:385–396 Updyke BV (1981) Projections from visual areas of the middle suprasylvian sulcus onto the lateral posterior complex and adjacent thalamic nuclei in cat. J Comp Neurol 201:477–506 Updyke BV (1983) A reevaluation of the functional-organization and cytoarchitecture of the feline lateral posterior complex, with observations on adjoining cell groups. J Comp Neurol 219:143–181 Updyke BV (1993) Organization of visual corticostriatal projections in the cat, with observations on visual projections to claustrum and amygdala. J Comp Neurol 327:159–193