* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein
NMDA receptor wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Magnesium transporter wikipedia , lookup
Phosphorylation wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein domain wikipedia , lookup
Tyrosine kinase wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Proteolysis wikipedia , lookup
Paracrine signalling wikipedia , lookup
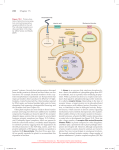
Chapter 15: Signal transduction Know the terminology: Enzyme-linked receptor IP3+DAG G-protein linked receptor cAMP nuclear hormone receptor Ca2+ G-protein adaptor protein scaffolding protein protein kinase SH2 domain MAPK Ras phosphodiesterase phospholipase protein phosphatase crosstalk Chapter 15: Signal transduction Outline: General principles of signal transduction Overview of: – – – – Signaling Receptors Transducers Targets Major types of cell-surface receptors - RTK signaling - G-protein signaling General Principles of Signal Transduction • Communication usually involves (i) a signaling molecule, (ii) a receptor, (iii) intracellular signal transducers and (iv) targets General Principles of Signal Transduction 2. Each cell responds to a complex profile of signaling molecules (crosstalk) General Principles of Signal Transduction 3. Different cells respond differently to a particular signaling molecule General Principles of Signal Transduction 4. Cells can remember the effects of some signals 5. Cells can adjust their sensitivity to a signal General Principles of Signal Transduction 4. Cells can remember the effects of some signals 5. Cells can adjust their sensitivity to a signal General Principles of Signal Transduction 6. Signal can exhibit complex responses to signal concentration Signaling molecules Signaling molecules come in many chemical forms: • Proteins: insulin, glucagon • Steroids et al.: testosterone, estradiol, cortisol • Amines: thyroxine, catecholamines, acetylcholine • Gases: nitric oxide Signaling pathways require molecules with rapid rates of synthesis and degradation Typically released from one cell and recognized by another cell Signaling molecules Secretory signals: • Autocrine-signals affect same cell or cell type • Paracrine-signals affect neighbouring cell • Endocrine-signals affect distant cells Contact-dependent signals: -signals are not released but affect other cells in contact through protein-protein interactions Autocrine signaling Signals released by one cell affect other cells in the immediate vicinity Amplify a response by inducing many “like-cells” to respond in the same way Allows cells to exhibit a coordinated response (a community effect) Autocrine signaling Paracrine signaling Signals released by one cell affect different cells in the immediate vicinity Synaptic transmission resembles paracrine stimulation but the response is limited to cells in very close proximity The outward propagation of the signal is limited by -cellular uptake, -extracellular degradation -& binding Endocrine signaling Signals released by one cell affect different cells far away Endocrine signaling often exerts multiple effects on the organism by affecting many different tissues Receptors = Proteins that bind signals and initiate a signaling cascade Cell membrane receptors -integral membrane proteins that bind an extracellular signal and start a signal cascade Intracellular receptors -nuclear hormone receptors Nuclear hormone receptors Examples include -steroid hormone receptor and -thyroid hormone receptor -Retinoic acid receptor -Vitamine D receptor NHRs are transcription factors that respond to specific ligands Ligands alter the ability to bind to specific DNA regulatory elements Intracellular signal transduction Once the receptor is activated, the signal is propagated by proteins that act as: •Relay proteins •Messenger proteins •Adaptor proteins •Amplifier proteins •Transducer proteins •Bifurcation proteins •Integrator proteins •Latent gene regulatory proteins Intracellular signal transduction Activated cell membrane receptors can alter the activity of intracellular enzymes including: –Protein modifying enzymes •Kinases (PKA, PKC, PKG)/ phosphatases •acetylases/ deacetylases –Lipid modifying enzymes •Phospholipases (PLCβ ou γ) •Phosphotidyl inositol kinase (PI3K) –Nucleotide modifying enzymes •cyclases/ phosphodiesterases Protein kinases Phospholipases PLC generates DAG and phosphoinositides, such as IP3 (inositol 1, 4, 5- triphosphate) Targets The final targets of signaling cascades are usually proteins: •Regulators of gene expression (transcription factors, histone remodeling enzymes) •Enzymes (metabolic enzymes) •Structural proteins (cytoskeletal proteins) •Effects alter activity (catalytic, DNA binding) or the ability to interact with other proteins (structural proteins, subcellular localization). Cell surface receptors 3 main classes of cell surface receptors: Ion-channel linked receptors Enzyme linked receptors may possess intrinsic enzyme activity or once ligands bind, activate enzyme activity G-protein linked receptors are monomeric (7TM) and activate trimeric G-protein (GTP-binding protein) that regulate downstream proteins Enzyme-linked receptors 5 main classes distinguished by: •type of effector (e.g. kinase vs. phosphatase) •target (serine/threonine, tyrosine, histidine) •type of linkage between receptor and enzyme 1. Receptor tyrosine kinase (RTK) 2. Tyrosine kinase linked receptor 3. Receptor serine/threonine kinase (PKA, PKC, PKG, PK-Ca2+-CAM) 4. Receptor guanylyl cyclase 5. Histidine-kinase associated receptors Receptor tyrosine kinases Most common type of receptor for many common protein hormones including EGF, PDGF, FGF, HGF, IGF-1, VEGF, NGF. Receptor tyrosine kinases Receptor itself possesses intrinsic tyrosine kinase activity Once the ligand binds, the receptor can dimerize and it become an active tyrosine kinase It phosphorylates itself (autophosphorylation), causing: 1. Increase kinase activity 2. Increased affinity for other proteins Once bound, these docking proteins can become phosphorylated Page 684 Figure 19-23 Domain organization in a variety of receptor tyrosine kinase (RTK) subfamilies. Structure of the 2:2:2 complex of FGF2, the D2–D3 portion of FGFR1, and a heparin decasaccharide. D2 Heparin decasaccharide D3 Ligand-dependent autophosphorylation and docking Ligand-dependent autophosphorylation and docking Page 686 Schematic diagrams of RTKs. Page 687 Structure of the PTK domain of the insulin receptor. PTK Domain undergoes major conformation change & autophosphorylation (1 to 3 Tyr residues) Relaying the signal: Binding Modules, Adaptors, GEF, GAP •SH2 domains mediate signal Transduction •PTB domains bind pY-containing peptides •SH3 domains bind Pro-rich peptides SH2 P T B GRB2 SH3 P P P GEF Page 691 Structure of the 104-residue Src SH2 domain in complex with an 11residue polypeptide containing the protein’s pYEEI target tetrapeptide. Src homology (SH2): •present in PTK, PLC-γ, certain GAP, etc •SH2 domain bind specifically phosphoTyr residues in target peptides with high affinity (hydrophobic pocket) •recognizes sequence of target peptide on C-terminal side of pY •does not bind phospho-Ser/phospho-Thr (much more abundant) SH2 P T B SH3 Page 692 PTB domain of Shc in complex with a 12-residue polypeptide from the Shc binding site of a nerve growth factor (NGF) receptor. PTB domain: •Specifically binds phospho-Tyr target peptides •Consensus domain NPXpY •Recognizes the sequence on N-terminal side of pY SH2 P T B SH3 Page 693 SH3 domain from Abl protein in complex with its 10residue target Pro-rich polypeptide (APTMPPPLPP). SH3 domain: Molecular velcro: mediate interactions between kinases & regulatory proteins present in great variety of proteins GF • receptor Tyrosine Kinases P SH2 • non-Receptor Tyrosine Kinases, GRB2 • adaptor proteins (ex. Grb2) SH3 PSOS Ras P • structural proteins (myosin, spectrin) P bind Pro-rich peptides Relaying the signal: Binding Modules, Adaptors, GEF, GAP •SH2 domains mediate signal Transduction •PTB domains bind pY-containing peptides •SH3 domains bind Pro-rich peptides Other Binding Modules WW domain (2 Trp residues) Plekstrin homology domain (PH domain) PDZ domain Relay: Grb2, Shc & IRS: •adaptor proteins •recruit Sos to the vicinity of Ras Ras is activated by RTK via Grb2-SOS complex Page 694 Grb2. Complex between Ras and the GEF-containing region of Sos. Relay: Grb2, Shc & IRS: •adaptor proteins •recruit Sos to the vicinity of Ras Ras is activated by RTK via Grb2-SOS complex •Sos opens Ras’s Nucleotide Binding Site •GAP functions to turn Off Ras-mediated Signals JUST THE TIP OF THE ICEBERG OF PROTEIN DOMAINS USED FOR PROTEIN/PROTEIN INTERACTIONS Docking of intracellular proteins on phosphotyrosines Phosphotyrosine domains are binding sites for many different proteins with SH2 (=PTB) domains These can be enzymes (e.g., PLC, PI3K) or they can act as adaptor molecules to bind other proteins (e.g. Grb2) Linking RTK to Ras and the MAPK cascade Once an adaptor protein binds to the RTK (e.g., Grb2), it attracts another protein - Ras GEF (guanine nucleotide exchange factor) Ras GEF induces Ras to exchange its GDP for GTP (activating Ras). Active Ras then activates MAPKKK, which phosphorylates and activates MAPKK, which phosphorylates and activates MAPK, which phosphorylates many proteins, including transcription factors. Ras GTPase Cycle Ras-GTP H2O GTP Hydrolysis Exchange Pi GDP Ras-GDP Ras GTPase Cycle Ras-GTP GTP GTPase Activating Proteins (GAPs) Guanine Nucleotide Exchange Factors (GEFs) GDP H2O Pi Ras-GDP -GAPs discovered biochemically -GEFs discovered genetically- first in yeast and then drosophila Ras belongs to the larger family of small GTPbinding switch Small GTP-binding proteins: initiation & elongation factors (protein synthesis). Ras (growth factor signal cascades). Rab (vesicle targeting and fusion). ARF (forming vesicle coatomer coats). Ran (transport of proteins into & out of the nucleus). Rho (regulation of actin cytoskeleton) All GTP-binding proteins differ in conformation depending on whether GDP or GTP is present at their nucleotide binding site. Generally, GTP binding induces the active state. Most GTP-binding proteins depend on helper proteins: GAPs, GTPase Activating Proteins, promote GTP hydrolysis. protein-GTP (active) GDP GEF GTP GAP Pi protein-GDP (inactive) A GAP may provide an essential active site residue, while promoting the correct positioning of the glutamine residue of the switch II domain. Frequently a (+) charged arginine residue of a GAP inserts into the active site and helps to stabilize the transition state by interacting with (-) charged O atoms of the terminal phosphate of GTP during hydrolysis. protein-GTP (active) GDP GEF GTP GAP Pi protein-GDP (inactive) Ga of a heterotrimeric G protein has innate capability for GTP hydrolysis. It has the essential arginine residue normally provided by a GAP for small GTP-binding proteins. However, RGS proteins, which are negative regulators of G protein signaling, stimulate GTP hydrolysis by Ga. protein-GTP (active) GDP GEF GTP GAP Pi protein-GDP (inactive) GEFs, Guanine Nucleotide Exchange Factors, promote GDP/GTP exchange. The activated receptor (GPCR) serves as GEF for a heterotrimeric G protein. Mutant Ras in Tumors Can’t Hydrolyze GTP and Accumulates in the Active GTP-Bound State Ras-GTP GTP X Exchange GDP Ras-GDP Ras Superfamily Ras Rho H-Ras N-Ras K-Ras RhoA RhoB RhoC TC21 RhoG RhoE Rap1 Rap2 R-Ras RalA RalB Rab Arf Rab1-N Arf1-6 Ran Ran CDC42 Rac1 Rac2 Growth/ Cytoskeleton Differentiation Vesicle sorting NuclearTranslocation Functions of Ras Proteins 1) Promote Cell Proliferation -fibroblasts, epithelial cells, lymphocytes -mediate actions of growth factors 2) Promote Cell Differentiation -neuronal progenitor cells (PC12) -mediate action of neurotrophins 3) Contribute to Differentiated Cell Functions -CNS neurons -mediate effects of calcium signaling Multiple Regulators of Ras Function Tyrosine Kinases SOS GRB2 Phospholipase C Ras-GRP DAG Ca2+ Calmodulin Ras-GRF Ras Each GEF has motif that connects it to distinct upstream signals but similar catalytic domain that allows it to activate Ras Multiple Effectors of Ras Function Ras Ral-GEF PI3Kinase Raf Ral PDK1 Akt Mek Erk Exocyst Src RalBP Page 697 Structure of the Ras binding domain of Raf (RafRBD; orange) in complex with Rap1A·GDPNP (=homolog of Ras light blue). Page 700 Structure of Src·ADPNP lacking its N-terminal domain and with Tyr 527 phosphorylated. MAP kinase Pathway Activation of Ras Activation of MAPK cascade Page 696 The Ras-activated MAP kinase cascade Page 698 MAP kinase cascades in mammalian cells. Scaffolding proteins help organize MAPKs Page 699 Scaffold proteins that modulate mammalian MAP kinase cascades. (a) JIP-1(JNK-interacting protein). Page 699 Scaffold proteins that modulate mammalian MAP kinase cascades. (b) MEKK1. Insulin signalling • • • • • • Two extracellular alpha chains each with an insulin-binding site, linked to two transmembrane beta chains, each with a cytosolic tyrosine kinase domain. Following insulin binding to the alpha chains, the tyrosine kinase domain of each beta chain catalyzes autophosphorylation of tyrosine residues in the adjacent kinase domain. The tyrosine kinase domains also catalyze the phosphorylation of proteins called insulin-receptor substrates (IRSs). Some of the effects of insulin binding are mediated through the second messenger system of PIP3 which regulates several serine-threonine protein kinases. Binding of insulin in some cell types (e.g., muscle) leads to stimulation of phoshatase cascades leading to inactivation of glycogen phoshorylase in the glycogen degradation pathways Insulin formation of PIP3 • • • • Binding of insulin to its receptor activates the protein tyrosine kinase activity of the receptor, leading to the phosphorylation of insulin-receptor substrates (IRSs). The phosphorylated IRSs interact with the phosphotidylinositide 3-kinase (PI kinase) at the plasma membrane, where the enzyme catalyzes the phophorylation of PIP2 to PIP3. PIP3 acts as a second messenger, carrying the message from extracellular insulin to certain intracellular protein kinases. Recent research has found that many of the effects of insulin on cells are mediated through PIP2. Whereas the inositol-phospholipid signalling system leads to hydrolysis of PIP2, insulin activates phophorylation of PIP2 to PIP3. INSULIN SIGNALLING Page 719 Figure 19-64 Insulin signal transduction. CAP: Cbl-associated protein C3G: a G-nucleotide exchange factor CrkII: a SH2/SH3 adapter protein PDK1: phophoinositide-dependent kinase mTOR: target of rapamycin Insulin Transduction PI3K Page 694 Structure of an insulin receptor substrate protein. Protein Tyr Phosphatases •SHP-2 Protein Ser/Thr Phosphatases •PP1 •PP2A •PP2B (=calcineurin / target of immunosuppressant drugs) •PP2C Page 706 Protein Tyrosine Phosphatase SHP-2. PP2A •Structurally variable •Functionally diverse •Catalytic subunit ©) •Scaffold subunit (A)(PR65) •Four different regulatory subunits (B, B’, B’’, B’’’), bind to A & C subunits A subunit of PP2A. Page 707 Calcineurin. (a) human FKBP12·FK506–CaN. Calcineurin. (b) human CaN with CaNA yellow, its autoinhibitory segment red, and CaNB cyan. Abl-Akt Page 703 Structure of the Abl PTK domain in complex with a truncated derivative of gleevec (anticancer drug). Integrins-Pakcytoskeleton Non-Receptor-Tyrosine kinases Domain organization of the major NRTK subfamilies. Page 701 Model of Src activation. Page 702 The JAK-STAT pathway for the intracellular relaying of cytokine signals. JAKSTAT JakSTAT GPCR = G-protein coupled Receptors G-protein linked receptors Ligand: Diverse ligands, such as epinephrine Receptor: Integral membrane protein with 7TM (7 transmembrane domains) G-protein: trimeric protein (α, β, γ) attached to the cell membrane by lipid anchors Effectors: Target proteins that show altered activity when they interact with activated Gprotein subunits (α, or βγ) G Protein Signal Cascade A hormone (e.g., epinephrine or glucagon) that activates formation of cAMP or IP3, binds at the cell surface to a receptor with 7 transmembrane αhelices. Rhodopsin was the first member of the family of 7-helix receptors to have its structure determined by X-ray crystallography. Rhodopsin PDB 1F88 Cytosolic domains of 7-helix receptors interact with G-proteins (=heterotrimeric GTP-binding protein). A G-protein has 3 subunits, designated α, β, γ. 7-Helix receptors that interact with G-proteins are called GPCR, or G-Protein-Coupled Receptors. Various proteins interact with GPCRs to modulate their activity. Effects of these interactions include: altered ligand affinity receptor dimerization that may enhance or alter activity altered receptor localization Ligand-induced receptor clustering may also regulate receptor function. G-protein linked receptors and G-proteins Receptor G-protein Interaction between receptor and G-protein Once the ligand binds, the activated receptor recruits a G-protein Nucleotide exchange occurs (GTP replaces GDP) and the trimer dissociates into 2 parts: -α subunit -βγ subunit Both parts can regulate downstream pathways Variety of G-proteins • • • • Gs are stimulatory Gi/0 are inhibitory Gq act on PLC G12/13 act on ion channels – 22 α subunits – 5 β subunits – 12 γ subunits Gs proteins are stimulatory Upon dissociation, a Gs protein stimulates an effector enzyme, such as -adenylate cyclase, -phospholipase Cβ or -ion channels (K+ or Ca2+) Adenylate cyclase converts ATP to cAMP Elevated cAMP stimulates cAMP-dependent protein kinase (PKA) by inducing the release of inhibitory subunits A G-protein that is part of a pathway that stimulates Adenylate Cyclase is called Gs & its α subunit Gsα. hormone signal outside GPCR plasma membrane α γ GDP β GTP GDP γ + α AC GTP β cytosol ATP cAMP + PPi The α subunit of a G-protein (Gα) binds GTP, & can hydrolyze it to GDP + Pi. α & γ subunits have covalently attached lipid anchors that bind a G-protein to the plasma membrane cytosolic surface. Adenylate Cyclase (AC) is a transmembrane protein, with cytosolic domains forming the catalytic site. hormone signal outside The complex of β & γ subunits Gβ,γ inhibits Gα. GPCR plasma membrane α γ GDP β GTP GDP γ + α AC GTP β cytosol ATP cAMP + PPi The sequence of events by which a hormone activates cAMP signaling: 1. Initially Gα has bound GDP, and α, β, & γ subunits are complexed together. hormone signal outside GPCR plasma membrane α γ GDP β GTP GDP γ β + α AC GTP cytosol ATP cAMP + PP i 2. Hormone binding to a 7-helix receptor (GPCR) causes a conformational change in the receptor that is transmitted to the G protein. The nucleotide-binding site on Gα becomes more accessible to the cytosol, where [GTP] > [GDP]. Gα releases GDP & binds GTP (GDP-GTP exchange). hormone signal outside GPCR plasma membrane α γ GDP β GTP GDP γ + α AC GTP β cytosol ATP cAMP + PPi 3. Substitution of GTP for GDP causes another conformational change in Gα. Gα-GTP dissociates from the inhibitory βγ complex & can now bind to and activate Adenylate Cyclase. hormone signal outside GPCR plasma membrane α γ GDP β GTP GDP γ + α AC GTP β cytosol ATP cAMP + PPi 4. Adenylate Cyclase, activated by Gα-GTP, catalyzes synthesis of cAMP. 5. Protein Kinase A (cAMP Dependent Protein Kinase) catalyzes phosphorylation of various cellular proteins, altering their activity. G-protein dissociation GTP hydrolysis ends signaling and induces trimerization The stimulatory Gsα, when it binds GTP, activates Adenylate cyclase. An inhibitory Giα, when it binds GTP, inhibits Adenylate cyclase. Different effectors & their receptors induce Giα to exchange GDP for GTP than those that activate Gsα. In some cells, the complex of Gβ,γ that is released when Gα binds GTP is itself an effector that binds to and activates other proteins. Variety of G-proteins • • • • Gs are stimulatory Gi/0 are inhibitory Gq act on PLC G12/13 act on ion channels – 22 α subunits – 5 β subunits – 12 γ subunits VARIETY OF G-PROTEINS: Gα, -β, -γ ADP-Ribosylation Cholera toxin catalyzes covalent modification of Gsα. ADPribose is transferred from NAD+ to an arginine residue at the GTPase active site of Gsα. This ADP-ribosylation prevents Gsα from hydrolyzing GTP. Thus Gsα becomes permanently activated. Pertussis toxin (whooping cough disease) catalyzes ADPribosylation at a cysteine residue of Giα, making the inhibitory Gα incapable of exchanging GDP for GTP. Thus the inhibitory pathway is blocked. ADP-ribosylation is a general mechanism by which activity of many proteins is regulated, in eukaryotes (including mammals) as well as in prokaryotes. Page 680 Figure 19-19 Mechanism of action of cholera toxin . Cholera Toxin composition = AB5 B:103AA / A: 240AA Activation: clivage en A1 (195AA) + A2(45AA) reliés par -S-SA pénètre dans cellule (endocytose) / dirigé dans ER: liaison par séquence KDEL de A2 Effet: ADP-ribosylation de Arg de Gsα ADP-ribosylation de Cys sur Giα ADP-RIBOSILATION BY PERTUSSIS TOXIN ADP ribosylation oO − H o NH2 O C protein o 2 NH +N N o CH2 O CH2 O o o P O H H H H OH OH NH2 NH2 o O N N o O NH2 NH − o P o CH2 O P O CH2 O o H H H H OH OH NH o NH2 O 2 N NN N N N N N N P o CH2 oO P O CH 2 O o o O H H H H + NAD OH OH o P O − o O protein (CH2)3 NH Arg C residue NH2 NH NH C − (nicotinamide adenine dinucleotide) C 2)3 (CH NH2+ o O NH2 O H o C C CH2 CH2 H H OH N N o O + NH2 N N H H OH ADP-ribosylated protein NH2 + N N nicotinamide H VARIETY OF G-PROTEINS: Gα, -β, -γ Structure of G proteins: PDB 1GIA The nucleotide binding site in Gα consists of loops that extend out from the edge of a 6-stranded β-sheet. Three switch domains have been identified, that change position when GTP substitutes for GDP on Gα. GTPγS Inhibitory Gα These domains include residues adjacent to the terminal phosphate of GTP and/or the Mg++ associated with the two terminal phosphates. O O GTP hydrolysis N N H H H O O H O O O O O O − O PP O CH2 O P P O O P P O O C O O O H O− O− O− O O O H OH N N NH N N N NH2 NH2 H H OH GTP hydrolysis occurs by nucleophilic attack of a water molecule on the terminal phosphate of GTP. Switch domain II of Gα includes a conserved glutamine residue that helps to position the attacking water molecule adjacent to GTP at the active site. PDB 1GP2 PDB 1GP2 Gβ - side view of β-propeller Gβ – face view of β-propeller The β subunit of the heterotrimeric G Protein has a β-propeller structure, formed from multiple repeats of a sequence called the WD-repeat. The β-propeller provides a stable structural support for residues that bind Gα. Adenylate Cyclase catalyzes: ATP cAMP + PPi Binding of certain hormones (e.g., epinephrine) to the outer surface of a cell activates Adenylate Cyclase to form cAMP within the cell. Cyclic AMP is thus considered to be a second messenger. NH 2 NH2 cAMP N N N N N N N N H2 5'C 4' O O O O O O H H 3' PP O O O O- H 1' 2' H OH Protein Kinase A (cAMP-Dependent Protein Kinase) transfers Pi from ATP to OH of a Ser or Thr in a particular 5-amino acid sequence. Protein Kinase A in the resting state is a complex of: • 2 catalytic subunits (C) • 2 regulatory subunits (R) : R2C2 Each regulatory subunit (R) of Protein Kinase A contains a pseudosubstrate sequence, like the substrate domain of a target protein but with Ala substituting for the Ser/Thr. The pseudosubstrate domain of (R), which lacks a hydroxyl that can be phosphorylated, binds to the active site of (C), blocking its activity. When each (R) binds 2 cAMP, a conformational change causes (R) to release (C). Each catalytic subunit can then catalyze phosphorylation of Ser or Thr on target proteins. R2C2 + 4 cAMP Æ R2cAMP4 + 2 C R2C2 + 4 cAMP Æ R2cAMP4 + 2 C AKAPs, A-Kinase anchoring proteins, bind to the regulatory subunits (R) of Protein Kinase A. AKAPs localize Protein Kinase A to specific regions of a cell. PKIs, Protein Kinase Inhibitors, modulate activity of the catalytic subunit (C). PKA activation by cAMP PKA activates gene expression CREB = cAMP responsive Elements Binding Protein EXAMPLE _ Glycogen Metbolism Gs & Gi Pathways Inactivation of PKA pathway The G-protein -PKA pathway is inactivated by: –Receptor desensitization (phophorylation by PKA) –GTP hydrolysis in G-protein (GTPase of a-subunit) –cAMP hydrolysis by phosphodiesterase –PKA inhibition –Phosphatase action on PKA targets –Activation of an antagonistic pathway (Gi) Turn off of the signal: 1. Gα hydrolyzes GTP to GDP + Pi. (GTPase). The presence of GDP on Gα causes it to rebind to the inhibitory βγ complex. Adenylate Cyclase is no longer activated. 2. Phosphodiesterase catalyzes hydrolysis of cAMP Æ AMP. Turn off of the signal (cont.): 3. Hormone Receptor desensitization occurs. This process varies with the hormone. Some receptors are phosphorylated via Gprotein-coupled receptor kinases. The phosphorylated receptor may then bind to a protein arrestin that blocks receptor-Gprotein activation & promotes removal of the receptor from the membrane by clathrinmediated endocytosis. 4. Protein Phosphatase catalyzes removal by hydrolysis of phosphates that were attached to proteins via Protein Kinase A. Phosphodiesterase enzymes catalyze: cAMP cAMP + H2O Æ AMP N N N N The phosphodiesterase that cleaves cAMP is activated by phosphorylation catalyzed by Protein Kinase A. Thus cAMP stimulates its own degradation, leading to rapid turnoff of a cAMP signal. NH2 NH2 N N H2 5' C C 4' O O O O O O H H 3' PP O O O O- N N H 1' 2' H OH G-protein coupled Receptor ARRESTIN Model for interaction of β-loop with Arrestin Novel roles of endocytosis and scaffolding in signal propagation H Kholodenko B.N. (2002) Trends Cell Biol. 12: 173. Signal amplification is an important feature of signal cascades: One hormone molecule can lead to formation of many cAMP molecules. Each catalytic subunit of Protein Kinase A catalyzes phosphorylation of many proteins during the life-time of the cAMP. PLCβ & PKC G-proteins and phospholipases Some G-proteins activate PLCβ (phospholipase Cβ), triggering formation of inositol triphosphate (IP3) and diacylglycerol (DAG) DAG, IP3, Ca2+ and signal transduction DAG: •substrate for production of eicosanoids, potent signaling molecules including arachadonic acid •activates PKC IP3: induces release of Ca2+ from ER stores via IP3sensitive Ca-channels Ca2+: Elevated Ca2+ can activates PKC and CamK. Phosphatidylinositol Signal Cascades O O C OH R2 C O O O C R O H2C O R1 C C R O C CH HO H2C C R O P O O P O− O 1 OH HO 2 phosphatidylinositol H H OH 3 H OH OH H 6 OH H 4 OH OH 5 H OH OH Some hormones activate a signal cascade based on the membrane lipid phosphatidylinositol. Page 707 IP3 as second messenger - Phosphatidylinositides Molecular formula of the phosphatidylinositides. Page 714 Flow chart of reactions in the synthesis of phosphoinositides in mammalian cells. Page 714 Domain organization of the 3 classes of PI3Ks. OO O O O OO C C R2OH HH 2C R2 2C R R HO RO R11 CC C O OO O CH C CH H2CC O H2C O R P O P O O O− − O O1 OH OH H HO 2 2 H PIP 2 phosphatidylH phosphatidylinositolinositol-4,5-bis-Ph 4,5-bisphosphate H 6 1 OHH OH 3 OH H 6 OPO32− OH OPO3H OH H OH 5 H 4 H3 OH H 5 H 2− 4 3 OPO H OPO3H OH Kinases sequentially catalyze transfer of Pi from ATP to OH groups at positions 5 & 4 of the inositol ring, to yield phosphatidylinositol-4,5-bisphosphate (PIP2). PIP2 is cleaved by the enzyme Phospholipase C. Page 709 A phospholipase is named according to the bond that it cleaves on a glycerophospholipid. Different isoforms of PLC O O C R O H2C have different regulatory domains, & thus respond to RO1 C C R O C CH different signals. H2C O C R One form of PLC is activated by a G-protein, cleavage by Phospholipase C Gq. O O C C O O R2 O P O O O− O OH 2 H PIP2 phosphatidylinositol4,5-bisphosphate H 1 6 H OH OH 5 H OPO3 3 H 4 OPO3 OPO32− H OPO32− A GPCR (receptor) is activated. GTP exchanges for GDP. Gqa-GTP activates Phospholipase C. Ca++, which is required for activity of Phospholipase C, interacts with negatively charged residues & with phosphate moieties of IP3 at the active site. H OPO32− OPO3 1 OH OPO3 H 2 OH H 3 H 6 OH H 4 O O 2− OPO3 OPO3 5 H OPO32− IP3 inositol-1,4,5-trisphosphate H2C C O O R1 C C HO O R C C OH R2 O R CH C H2C C OH OH diacylglycerol Cleavage of PIP2, by PLC, yields two 2nd messengers: inositol-1,4,5-trisphosphate (IP3) & diacylglycerol (DG). Diacylglycerol, with Ca++, activates Protein Kinase C, which catalyzes phosphorylation of several cellular proteins, altering their activity. Page 708 Role of PIP2 in intracellular signaling. Page 709 Domain organization of the four classes of phosphoinositide-specific PLCs. Page 713 Activation of PKC. Ca++ Ca++-release channel IP3 Ca ATP calmodulin Ca ++ endoplasmic reticulum Ca++-ATPase ++ ADP + Pi IP3 activates Ca++-release channels in ER membranes. Ca++ stored in the ER is released to the cytosol, where it may bind calmodulin, or help activate Protein Kinase C. Signal turn-off includes removal of Ca++ from the cytosol via Ca++-ATPase pumps, & degradation of IP3. OPO32− OPO3 H OH OPO3 H OH OH H OPO32− OPO3 H H H IP3 OH OPO32− (3 steps) OH H OH H OH OH H + 3 Pi H H H OH inositol Sequential dephosphorylation of IP3 by enzymecatalyzed hydrolysis yields inositol, a substrate for synthesis of PI. IP3 may instead be phosphorylated via specific kinases, to IP4, IP5 or IP6. Some of these have signal roles. E.g., the IP4 inositol-1,3,4,5-tetraphosphate in some cells activates plasma membrane Ca++ channels. O O R O1 C R O C H2C C CH C C H2C phosphatidylinositol3-phosphate O O O C R C R2 O O O O O O PP O O− O OH 2 H 1 H 6 OH OH H 5 H OPO32− OPO3 H 3 H 4 OH The kinases that convert PI (phosphatidylinositol) to PIP2 (PI-4,5-P2) transfer Pi from ATP to OH at positions 4 & 5 of the inositol ring. PI 3-Kinases instead catalyze phosphorylation of phosphatidylinositol at the 3 position of inositol ring. O O H2C O O C C OH R2 O C R CH O RO C O C C R 1 O C R O P P O H2C O O O− phosphatidylinositol3-phosphate OH 2 H 1 H 6 OH OH H 5 2− H OPO3 OPO3 H 3 H 4 OH PI-3-P, PI-3,4-P2, PI-3,4,5-P3, & PI-4,5-P2 have signaling roles. These are ligands for particular pleckstrin homology (PH) and FYVE protein domains that bind proteins to membrane surfaces. PKB (Protein Kinase B, also called Akt) becomes activated when it is recruited from the cytosol to the plasma membrane surface by binding to products of PI-3 Kinase, e.g., PI-3,4,5-P3. Other kinases at the cytosolic surface of the plasma membrane then catalyze phosphorylation of PKB, activating it. Activated PKB catalyzes phosphorylation of Ser or Thr residues of many proteins, with diverse effects on metabolism, cell growth, and apoptosis. Downstream metabolic effects of PKB include stimulation of glycogen synthesis, stimulation of glycolysis, and inhibition of gluconeogenesis. Signal cascades may be mediated by complexes of proteins that assemble at the cytosolic surface of the plasma membrane, frequently in areas of distinct lipid composition called lipid rafts. Signal proteins may be recruited into such complexes by • insertion of their lipid anchors in the plasma membrane, • interaction with membrane-associated scaffolding proteins, • or • interaction of their pleckstrin homology domains with transiently formed PI derivatives. Interactions between G-proteins and RTKs Summary on Enzyme-linked receptors Enzyme-linked receptors generate variable cellular responses Multiplicity of players (receptors, kinases etc) arise from gene duplication and divergence Recognize the critical role of phosphorylation/ dephosphorylation control as molecular switches Adaptor molecules allow construction of protein signaling cascades with variable outputs JAK-STAT JAK-STAT cGMP Pathway