* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biochemistry Ch 35 663-676 [4-20
G protein–coupled receptor wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical synapse wikipedia , lookup
Butyric acid wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Peptide synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Signal transduction wikipedia , lookup
Paracrine signalling wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
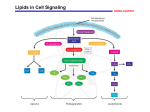
Biochemistry Ch 35 663-676 Metabolism of Eicosanoids Arachidonic acid, a polyunsaturated 20C compound is the most common source of eicosanoids, and is normally found esterified to phospholipids in the plasma membrane -must be obtained in the diet as arachidonic acid or linoleate (plant oils) -AA is released from bilayer through activation of phospholipase A2 (PLA2) or PLC and occurs after a stimulus such as histamine/cytokines interact with membrane receptor LinoleateAA (bound)AA unbound epoxides (cyt450), leukotrienes (lipoxygenase), prostaglandins (cyclooxygenase) -Most obvious symptom of fatty acid deficiencies is red/scaly dermatitis -enzymes that make eicosanoids differ in tissues, sometimes producing PGE2, PGI2, or TxA2, 12HETE -3 pathways are cyclooxygenase pathway, lipoxygenase pathway, and Cyt P450 pathway Cyclooxygenase Pathway: Synthesis of Prostaglandins and Thromboxanes 1. Structure of Prostaglandins – 20C fatty acids with 5C ring. Nomenclature is a PG with an E for structure different with a subscript of 1, 2, or 3 for number of double bonds 2. Structure of Thromboxanes – closely resemble prostaglandins except they have 6 membered ring with an O atom 3. Biosynthesis of Prostaglandins and Thromboxanes – AA (COX) PGG2 (Peroxidase) PGH2 PGD2, E2, I2, and TxA2 (PGD synthase etc..)-Functions of prostaglandins: -PGI2, E2, and D2 – increase vasodilation and cAMP; decreases platelet aggregation, WBC aggregation, IL-1 and 2, T cell proliferation, and lymphocyte migration -PGF2a – increases vasoconstriction, bronchoconstriction, and smth muscle contraction -TxA2 – produced by platelets to stimulate aggregation and vasoconstriction -cyclooxygenase enzymes were found to exist in 2 isoformes: COX1 and COX2. COX1 is constitutive while COX2 is inducible through growth factors and cytokines, and COX2 mRNA is elevated in inflamed tissue -COX1 involved in platelet aggregation and stomach cytoprotection -COX2 involved in inflammation and hyperalgesia -COX enzymes blocked by all NSAID drugs like aspirin, which is irreversible. -other NSAIDs are reversible (Tylenol, ibuprofen) -COX-2 selective inhibitors would get rid of GI ulcers and antiplatelet activity (celecoxib and rofecoxib) 4. Inactivation of Prostaglandins and Thromboxanes – these molecules have short half lives (seconds-minutes). -prostaglandins inactivated by oxidation of 15-OH group to a ketone and double bond at C13 reduced, allowing urine excretion -TxA2 is metabolized to TxB2 by cleavage of O2 bridge between C9 and 11 to form 2 OH groups; TxB2 has no activity Lipoxygenase Pathway – Synthesis of leukotrienes, hydroxyeicosatetraenoic acid, lipoxins -LOX catalyzes incorporation of O2 molecule into a carbon of double bond in AA, and may act on carbons 5, 12, and 15 -type of LOX varies from tissue to tissue: polymorphonuclear leukocytes have 5-LOX, whereas platelets have 12-LOX and eosinophilic leukocytes have 15-LOX 1. Leukotriene synthesis – begins with formation of HPETEs which are later reduced to HETEs or metabolized into leukotrienes or lipoxins -major leukotrienes produced by 5-LOX. -AA + 5-LOX 5-HPETE LTA4 + glutathione S-transferase LTC4 + g glutamyl transpeptidase LTD4 LTE4 - LTA4 also converted to LTB4 Functions of LTB4: increases vascular permeability, t-cell proliferation, leukocyte aggregation, INF-g, IL-1, 2 Functions of LTC4 and LTD4: increases bronchoconstriction vascular permeability, INF-g 2. Lipoxin Synthesis and Actions – lipoxins are formed through 15-LOX followed by 5-LOX on arachidonic acid followed by a series of reductions to lipoxins such as LXA4 -lipoxins induce chemotaxis ad stimulate superoxide anion production in leukocytes Cytochrome P450 Pathway: synthesis and actions of epoxides, HETEs and diol HETEs -AAcytochrome P450 to yield epoxides, HETEs, and diHETEs and are implicated in ocular, vascular, endocrine, and renal systems Isoprostane Synthesis – derived from AA by lipid peroxidation and initiated by free radicals. There is no enzymatic mechanism for their production. -AA undergoes free radical damage; PLA2 removes it from membrane and releases into circulation. Levels can be measured in the urine and can indicate oxidative stress -best studied isoprostane is similar to PGF2a, and has similar effects on cultured cells as does PGF2 Endocannabinoid Synthesis – endogenous ligands or cannabinoid receptors (CB1 and CB2) with effects primarily in the nervous system -anandamide – synthesized in neurons from phosphatidylethanolamine and is unique in transferring an AA group from 2 position to free amino group on ethanolamine, and then using a unique phospholipase D to cleave the modified ethanolamine from the phospholipid -Synthesis is regulated by agonists that cause Ca influx into the neuron -it acts as a retrograde messenger, binding to receptors on presynaptic membrane that later ion fluxes such that neurotransmitter release from presynaptic neuron can be increased and an analgesic effect obtained -degraded by the enzyme fatty acid amide hydrolase, and inhibiting this enzyme can prolong analgesic effects induced by anandamide Mechanism of Action of Eicosanoids – target eicosanoid receptors on plasma membrane of target cell, which activates adenylyl cyclase cAMP- protein kinase A system or causes an influx of Ca2+ into the cytosol of target cells -in some systems, eicosanoids modulate degree of adenylyl cyclase activation in response to other stimuli, where eicosanoid may bind regulatory subunit of GTP binding proteins within plasma membrane of target cell -if eicosanoid binds stimulatory subunit, effect of stimulus is amplified, and vice versa -Eicosanoids can work in a paracrine fashion, such as contraction of vascular smooth muscle by TxA2, as well as autocrine by TxA2 to exhibit platelet aggregation