* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Eicosanoid Synthesis
Basal metabolic rate wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Citric acid cycle wikipedia , lookup
Lipid signaling wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
12-Hydroxyeicosatetraenoic acid wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
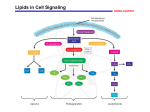
Eicosanoids Deficiency symptoms in the absence of essential fatty acids from the diet: Nonlipid diet plus vitamins A and D Rat –––––––––––––––––––––––––––––––– Reduced growth rate and reproductive deficiency • Deficiency syndrome was cured by the addition of linoleic, a-linolenic, and arachidonic acids to the diet. • Essential fatty acids are found in structural lipids of the cell and are concerned with structural integrity of mitochondrial membrane. • Arachidonic acid is present in membranes and accounts for 5-15% of the fatty acids in phospholipids. SOME MAJOR POLYUNSATURATED FATTY ACIDS Name Linoleic Structure 18:2(9,12) Type ω-6 Significance Essential FA Linolenic 18:3(9,12,15) ω-3 Essential FA Arachidonic 20:4(5,8,11,14) ω-6 Prostaglandin precursor METABOLISM OF LINOLEIC VERSUS LINOLENIC INTO POLYUNSATURATED FATTY ACIDS (PUFAS): Linoleic (18:2) (ω-6) arachidonic (AA) (20:4) (ω-6) Linolenic (18:3)(ω-3) eicosapentanoic acid (EPA) (20:5) (ω-3) and docosahexanoic acid (DHA) (22:6) (ω-3) Dietary linoleic acid is the precursor. It is elongated and further desaturated to 20-carbon, 3, 4, or 5 double bond FAs Omega-3 fatty acids • EPA & DHA are precursors for different eicosanoids than arachidonic acid • Fish oils have high content of ω-3 FA METABOLISM OF UNSATURATED FATTY ACIDS AND EICOSANOIDS • Humans have limited ability in desaturating fatty acids. • Dietary intake of certain polyunsaturated fatty acids derived from a plant source is necessary. • These essential fatty acids give rise to eicosanoic (C20) fatty acids, from which are derived families of compounds known as eicosanoids. • Eicosanoids include prostaglandins, thromboxanes, leukotrienes, and lipoxins. prostacyclins, Eicosanoids • The eicosanoids are considered "local hormones.“ • Eicosanoids have strong hormone-like actions in the tissues where they are produced They have specific effects on target cells close to their site of formation. They are rapidly degraded, so they are not transported to distal sites within the body. • Eicosanoids are not stored and are very unstable Biological actions of eicosanoids • Biologic actions of eicosanoids are diverse in various organs: – vasodilation, constriction, platelet aggregation, contraction of smooth muscle, chemotaxis of leukocytes, release of lysosomal enzymes Biological actions of eicosanoids ……. 2 Also, they have roles in: regulation of blood pressure, blood clotting and immune system modulation Excess production symptoms: pain, inflammation, fever, nausea, vomiting • Arachidonic acid, 20:4 (5, 8, 11, 14), is the precursor of many eicosanoids • Arachidonic acid is normally part of membrane phospholipids (especially phosphatidylinositol). • Arachidonic acid is released by a specialized phospholipase A2 The fatty acid arachidonic acid is often esterified to OH on C2 of glycerophospholipids, especially phosphatidyl inositol. Site of cleavage by Phospholipase A2 O R2 C O O H2C O C C H O CH2O P R1 H OH O Site of cleavage by OH Phospholipase C H OH OH H OH OH H H Phosphatidyl inositol Arachidonic acid is released from phospholipids by hydrolysis catalyzed by Phospholipase A2. This enzyme hydrolyzes the ester linkage between a fatty acid and the OH at C2 of the glycerol backbone, releasing the fatty acid & a lysophospholipid as products. O Phosphatidyl inositol signal cascades may lead to release of arachidonic acid. O R1 C H2C O O C CH H2C cleavage by Phospholipase C R2 O O P O O H OPO32 OH H OH PIP2 OH H H H phosphatidylinositol4,5-bisphosphate H OPO32 After PI is phosphorylated to PIP2, cleavage via Phospholipase C yields diacylglycerol (and IP3). COOH Arachidonic acid O COOH HO OH PGE2 Prostaglandins all have a cyclopentane ring. A letter code is based on ring modifications (e.g., hydroxyl or keto groups). A subscript refers to the number of double bonds in the two side-chains. Thromboxanes are similar but have instead a 6-member ring. CLINICAL ASPECTS: • Thromboxanes are similar but have instead a 6-member ring. • Thromboxanes are synthesized in platelets and upon release cause vasoconstriction and platelet aggregation. Their synthesis is inhibited by low-dose aspirin. Prostaglandin H2 Synthase catalyzes the committed step in the “cyclic pathway” that leads to production of prostaglandins, prostacyclins, & thromboxanes. Different cell types convert PGH2 to different compounds. Mast cells attracts immune cells Corticosteroids are anti-inflammatory because they prevent inducible Phospholipase A2 expression, reducing arachidonic acid release. There are multiple Phospholipase A2 enzymes, subject to activation via different signal cascades. The inflammatory signal platelet activating factor is involved in activating some Phospholipase A2 variants. arachidonic acid may give rise to inflammatory or anti-inflammatory eicosanoids in different tissues. Non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and derivatives of ibuprofen, inhibit cyclooxygenase activity of PGH2 Synthase. They inhibit formation of prostaglandins involved in fever, pain, & inflammation. They inhibit blood clotting by blocking thromboxane formation in blood platelets. Ibuprofen and related compounds block the hydrophobic channel by which arachidonic acid enters the cyclooxygenase active site. H3C CH3 CH CH2 CH H3C COOH Ibuprofen There are at least two isozymes of PGH2 Synthase (COX-1 and COX2) COX-1 is constitutively expressed at low levels in many cell types Specifically, COX-1 is known to be essential for maintaining the integrity of the gastrointestinal epithelium. COX-2 expression is stimulated by growth factors, cytokines, and endotoxin COX-2 levels increase in inflammatory disease states such as arthritis and cancer Up-regulation of COX-2 is responsible for the increased formation of prostaglandins associated with inflammation Next generation NSAIDs • Older NSAIDs inhibit both COX-1 & COX-2: – acetylsalicylate (Aspirin®, Disprin®, etc.) – ibuprofen ( Brufen®, etc.) • Newer generation drugs are specific COX-2 inhibitors: – Celebrex® – Vioxx® Thromboxane A2 stimulates blood platelet aggregation, essential to the role of platelets in blood clotting. Many people take a daily aspirin for its anti-clotting effect, attributed to inhibition of thromboxane formation in blood platelets. This effect of aspirin is long-lived because platelets lack a nucleus and do not make new enzyme. LIPOXYGENASE PATHWAY: • –Produces leukotrienes from eicosanoic acids in leukocytes, mast cells, platelets, and macrophages in response to both immunologic and nonimuunologic stimuli. SYNTHESIS OF LEUKOTRIENES FROM ARACHIDONIC ACID • Leukotrienes are produced from arachidonic acid via a different enzyme: lipoxygenase • HPETE Hydroxyperoxytetraenoic LEUKOTRIENES HAVE ROLES IN INFLAMMATION. They are produced in areas of inflammation in blood vessel walls as part of the pathology of atherosclerosis. Leukotrienes are also implicated in asthmatic constriction of the bronchioles. ANTI-ASTHMA MEDICATIONS INCLUDE: inhibitors of 5-Lipoxygenase, e.g., Zyflo (zileuton) drugs that block leukotriene-receptor interactions e.g., Singulair (montelukast) & Accolate (zafirlukast) block binding of leukotrienes to their receptors on the plasma membranes of airway smooth muscle cells. Therapeutic Uses of Eicosanoids • • • • • Induction of midtrimester abortion Treatment of peptic ulcer Maintain patency of ductus arteriosus (?) Ischemic disease . Impotence (intracavernous injection of PGE2) leukotrienes Linear pathway Lipoxyganase phospholipids arachidonate diacylglycerol Cyclic pathway PGH2 Synthase Cyclic pathway: Prostacyclin prostaglandin H2 Thromboxane Synthase Synthase prostacyclins thromboxanes other prostaglandins Prostaglandin H2 Synthase catalyzes the committed step in the “cyclic pathway” that leads to production of prostaglandins, prostacyclins, & thromboxanes. Different cell types convert PGH2 to different compounds.