* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Carbonyl The carbonyl function, C=O, exists in a number of organic
Discodermolide wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Elias James Corey wikipedia , lookup
Metal carbonyl wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Asymmetric induction wikipedia , lookup
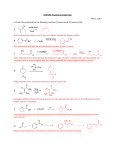
Carbonyl The carbonyl function, C=O, exists in a number of organic functional groups. These groups are: aldehydes, ketones, acids, acid halides and anhydrides, esters and amids. And there are still more. The nitrile group is closely related in its chemistry to the carbonyl group. C O C O C O The important feature of the carbonyl group is that the oxygen atom polarizes the double bond such that the carbon atom has positive character and the oxygen atom has negative character. The positive carbon atom is electrophilic and is responsible for much of the observed chemistry, such as acidity of alpha hydrogens and nucleophic reactions at the carbonyl. If florine atoms are located near the carbonyl function then their electronegativity increases the positive character on the carbonyl carbon creating an even more electrophilic center. The types of reactions of carbonyl compounds falls into two main catagories. One is the simple additions across the carbonyl observed for aldehydes and ketones to form an alcohol. The reaction can be accelerated by acid-catalysis. The other, exemplified by the ester family, RC=OX, is addition to the carbonyl group followed by elimination of the heteroatom attachment, X, to give overall substitution. These reactions are also accelerated by acid. Aldehyde and Ketone reactions. neutral O O + OH H2O Nu Nu Nu acid-catalyzed O H+ OH OH OH + Nu Nu Reactions of RXC=O groups; Acid, ester, acid halide, anhydride, amide The characteristic reaction of this class of carbonyl compounds is the first step of addition of the nucleophile followed by elimination of the X function to give the final product. In acid catalysis the carbonyl is first protonated to give a more electrophilic carbon that adds the nucleophile. These functional groups have a great deal of difference in their overall reactions depending on the relative ease of the loss of X. Thus in leaving group ability, nuch like in nucleophilic substitution, Halide> OAc, > OH, OR, > NR2. Of course if X = H or R then you have an aldehyde or ketone and there is no leaving group and only addition occurse as shown above. neutral O O X + Nu O -X- substitution Nu X Nu addition elimination tetrahedral intermediate acid-catalyzed O H+ OH + Nu X -X- OH OH X OH X -H Nu X Nu O substitution Nu Aldehyde and Ketone Examples Oxygen, Nitrogen, Sulfur Nucleophiles Water reacts with aldehydes or ketones under neutral or acid-catalyzed conditions to form hydrates. neutral H2O PhCH=O OH2 OH PT PhCH Hydrate PhCH O OH acid catalyzed H+ Ph 2C=O Ph 2C-OH Ph 2C-OH Ph 2C=OH H2O H2O H+ OH2 Ph 2C-OH OH Hydrate formation is greater with low molecular weight compounds. Addition of fluorine to the system greatly increses the hydrate content because the fluorine atoms make the carbonyl carbon much more positive. Thus water adds more readily to the fluorinated carbonyl group. H+ Ph2C=O HO PT HO OH Ph2C-OH 2 O OH - H2O Ph2C-OH Ph2C-OH Ph2C=OH H O Ph2 O -H OH HO OH O Ph2 O acetal The dithiane molecules used as a source for carbanions can be seen to be thioacetals of aldehydes or ketones from thiols, RSH. Glucose is a common compound which is a hemiacetal (half-acetal). If glucose is treated with acid and alcohol it is converted into an acetal. K = hydrate/ketone CH2O 100 CH3C=O FCH2 C=OCH3 .11 PhCH=O 1 .008 (CH3)2 C=O .001 (CF3)2 C=O .00001 PhC=OCF3 PhC=OCH3 CF3C=OCH3 35 1,200,000 78 Alcohols react in a similar fashion to form acetals. The acetal derived from ethylene glycol is very useful for protecting the aldehyde carbonyl. Of course the carbonyl group can be recovered from the acetal by treatment with acid and water. These reactions are reversible. HO S S HO O HO OH HO dithane glucose hemi-acetal Reactions with ammonia, hydrazine: Wolff Kishner reduction Aldehydes and ketones undergo addition reaction with ammonia and with hydrazine derivatives. These reactions do not produce alcohols, but instead product imines and hydrazones from loss of water. O NH3 O HO -OH PT + NH3 NH2 NH2 NH -H+ imine Reaction of an aldehyde or ketone with ammonia in the presence of hydrogen and a catalyst results in hydrogenation of the intermediate imine to give an amine. This process is known as reductive amination. reductive amination O NH2 NH H2 + NH3 Pd Aldehydes and ketones readily form hydrazones on reaction with hydrazine and hydrazine compounds. The example below is with 2,4-dinitrophenylhydrazine because the final product hydrazone is always a nice solid. O2N PhCH=O + NH2NH H+ NO2 O2N PhCH=OH PhCH NH2NH NO2 OH O2N O2N PhCH NHNH NO2 PhCH NHNH NO2 OH2 O2N - H+ PhCH NNH NO2 2,4-dinitrophenylhydrazone An old but useful reaction, known as Wolff Kishner reduction, is the reduction of the carbonyl group to a CH2 by reaction of the carbonyl with hydrazine and KOH at high temperature in ethylene glycol. The intermediate hydrazone is converted to intermediate carbanions that are protonated by the water formed in the reaction. Wolff-Kishner Reduction O Ph Ph N=NH Ph NNH 2 NH2 NH2 KOH Ph ethylene glycol Ph -H2O N=NH H2O Ph KOH Ph H Ph -H2O H H2O KOH Ph H Ph Ph H Ph Addition of Carbon Nucleophiles The addition of Grignard reagents, RMgX, and organolithium reagents, RLi, to carbonyl groups to form alcohols constitute a major process in organic chemical synthesis. These reagents add easily to the carbonyl group but as strong bases they can also form alphacarbanions that give aldol reactions. The aldol reactions are minimized by working at cold temperatures. The reaction is exemplified with cyclohexanone. All of these addition reactions require a second step reaction with dilute acid to neutralize the alkoxide formed in the first step addition. Ketones give secondary alcohol, and aldehydes give primary alcohols. O O PhMgBr Ph or PhLi H3O OH Ph Whan the carbonyl component contains a chiral center, diasteriomeric products are possible. The size of the groups attached to the chiral center have significant influence on the diastereomeric mixture. RS RS OH RS CH3 O RL RL RL CH3 OH CH3MgBr + RM R H3O RM R RM R Cornforth Nu Nu O RS Cl OM RM RS RM Cl RX RX For alphal-halo compounds. Halogen and carbonyl are anti. Nu attacks over the Rs. Karabotsos RM RL O RL RS O RX RS RS O RM RM RX RX RL Favored Of the three conformations possible, the one with the carbonyl and Rm eclipsed is the most stable. Nu attacks over the Rs. Felkin-Ahn O RL Nu RX RS RM RS O RL Nu RM RX Nu attacks between Rm and Rs. Least interaction between Rx and Rm or Rs is preferred. Cram Nu O RS Nu RM OM RM RS RL R X RL R X RL and Rs are eclipsed. Nu attacks over the Rs group. Cram Chelate Model R O M O OR OM Nu Nu RS RM RS RX RM RX For alpha-oxygenated carbonyl compounds. Bothy oxygens are eclipsed in complex with a metal. Nu attacks over the Rs. The case is illustrated with 3-phenylbutanone below. Addition of ethyl magnesium bromide follows the model of Felkin-Ahn to give 88% of the diasterioisomer shown. The Cram model gives the same result. CH3 O H3 C Ph CH3CH2MgBr H3O H CH3 Et OH 88% Ph H CH3 CH3CH2MgBr There are many other carbon nucleophiles that add to the carbonyl of aldehydes and ketones, and these reactions makeup a major synthetic arsenal in organic chemistry. Thus reactions that are known as the aldol condensation, the Reformatsky reaction, Knovenagel Condensation are among the many reactions. Another important reaction shown below is the Wittig reaction. The Wittig reaction is very useful because the position of the double bond in the product is determined from the position of the carbonyl group. Cis and trans isomers constitution can be influenced by the addition of other reagents. Wittig and Related Reactions A very nice procedure for the preparation of alkenes from aldehydes and ketones is known as the Wittig reaction, or Wittig-olefination. Phosphonium salt, prepared from a triarylphosphine or a trialkylphosphine and an alkyl halide, is treated with a moderately strong base to form a zwitterion known as an ylide. The ylide is formed in the presence of the carbonyl compound and the resulting product is an alkene. The position of the alkene function is always at the spot of the carbonyl. Thus problems associated with the isomers found in elimination reactions are not present here. The Wittig olefiniation has complete regioselectivity. Ph3P+-CH3 IPh3P + CH3I NaH Ph3P+-CH2- O Ph3P=CH2 Ylide CH2 The mechanism of the reaction is shown below. The ylide adds to the carbonyl group much like many other nucleophiles, but the newly formed intermediate called a betaine cyclizes to a oxphospetane that collapses to the alkene. The driving force for this process is the ultimate formation of the very strong phosphorus to oxygen bond. An alternative to this mechanism has the oxaphosphetane being formed first. _ Ph3P+-CH2- Ph3 P O O + Ph3P-CH 2 O Oxaphosphetane betaine A related alternative to the Wittig olefination is know as the Horner-Wadsworth-Emmons olefination. The reaction utilizes a phosphine oxide, formed from an Arbusov reaction involving an alpha haloester, as a carbanion source. The carbanion adds to the carbonyl group much in the same manner as shown above. Arbusov Reaction OCH2 CH3 (EtO)2 PCH2 CO2 Et + Br- (EtO)3 P + BrCH2CO2Et O (EtO)2 PCH2 CO2 Et Horner-Wadsworth-Emmons Olefination O (EtO)2 PCH2 CO2 Et O (EtO)2 PCHCO2Et n-BuLi O=CHPh O (EtO)2 PCHCO2Et O (EtO)2 P-CHCO2Et O-CHPh O (EtO)2 P-CHCO2Et O-CHPh PhCH=CHCO2 Et + (EtO)2 P=O O The stereochemistry of Wittig reactions is very interesting, and the detailed mechanisms involved are somewhat cloudy. As shown below the reagents add in a fast step to form an eclipsed betaine (or goes directly to the oxaphosphetane). The oxaphosphetane breaks down by syn elimination to give the trans (E) product. In a slower step the staggered more stable betaine is formed that leads to the cis (Z) isomer. Most Wittig reactions give the trans alkene (E isomer), but in some cases the cis isomer is formed (Z isomer). A number of parameters affect the outcome, such as added metal ions (Li+ ), solvents, the size of the R groups, and the functions attached the phosphorus atom (aryl, alkyl, alkylcarbonyl). R3 P+ CH- R + R'CHO slow fast R3 P O H O H R' R R H H R' PR3 R3 P O H R R3 P O H R' H R R' H R R cis trans R' R' An interesting case with a fluorine attachment is shown below. The organic substituents are in the trans position in the absence of fluorine but are in the cis position in the presence of fluorine. Is it possible that the small fluorine atom is large enough to inhibit the formation of the eclipsed betaine. O (EtO)2 PCHCO2Et EtO2 C OHC + O O O O F O (EtO)2 PCFCO2Et + OHC O O EtO2 C O O The Wittig reaction with α− fluoro α,β unsaturated aldehydes is a convenient method for the production of fluorinated dienes. CHO + Ph P=CHOCH 3 3 F F 55 % Sulfur Ylides Sulfur ylides contain a carbanion next to a positively charged sulfur atom. The ylides that contain a sulfur to oxygen bond are called sulfoxonium ylides and those without the oxygen are called sulfonium ylides. O CH3-S-CH2 CH3 CH3 sulfoxonium ylide SPh2 CH3-S-CH2 sulfonium ylide (CH3)2S-CH-CO2Et Ph2S-C(CH3)2 These ylides add to the carbonyl group of aldehydes and ketones to give epoxides as shown below. The larger sulfoxonium ylide adds from the equitorilal direction on a cyclohexanone ring to leave the carbon atom equatorial and the oxygen atom axial. The smaller sulfonium ylide adds to form an expoxide from the axial direction. axial (H3C)2S O t-Bu CH3-S-CH2 t-Bu O CH3 O t-Bu + 15% equitorial 85% O CH3-S-CH2 0% + 100% CH3 The cyclopropyl sulfonium ylide adds to carbonyl groups to give cyclopropyl epoxides. On treatment with acid the epoxides rearrange to cyclobutanones. (Trost reaction). O O SPh2 + O H+ The addition of sulfur ylides to α, β-unsaturated systems offers an excellent procedure for the preparation of cyclopropane compounds. In these reactions the ylide may also add to the carbonyl but the thermodynamics of the addition favor the 1,4 addition. O O CO2Et (CH3)2S-CH-CO2Et O O S(CH3)2 CO2Et CO2Et Simple dimethyl cyclopropanes my be prepared as well. CN CN + Ph2S-C(CH3)2 + Ph2S O CH3-S-CH 2 CH3-S-CH 2 CH3 CH3 sulfonium ylide sulfoxonium ylide (CH3)2 S-CH-CO2Et SPh2 Ph2S-C(CH 3)2 axial (H 3C) 2S O t-Bu CH3-S-CH 2 t-Bu O CH3 O t-Bu + 15% equitorial 85% O CH3-S-CH 2 0% + 100% CH3 The reduction of the carbonyl group of aldehydes or ketones is usually a simple matter. The most common reagents are sodium borohydride (or it derivatives) and lithium aluminum hydride (or it derivatives). The derivatives are reagents in which the reducing power has been reduced by replacing a hydrogen atom by a cyano or alkoxy group. In most cases sodium borohydride is the reagent of choice because of its ease in handling and simple reaction workup. PhCH=O NaBH4 or LiAlH4 2) H3O+ RCH2OH Reaction of Carboxylic Acid Derivatives The types of molecules in this class have the general structure of R(C=O)L, where L is a leaving groups such as Cl, OAc, OR, NR2 These substrates all follow the additionelimination path mostly to give the substitution product. But the rate of the reaction and the final outcome depend largely on the ability of the leaving group, L, to leave. The reactions being considered are hydrolysis or alcoholysis, reaction with grignard or RLi, and reduction. Hydrolysis and Alcohlysis The example below shows the hydrolysis (reaction with water) of an acid chloride under both neutral and acid-catalyzed reactions. Acid chlorides (and acid anhydrides) react very fast with water because of the highly positive carbonyl group and by the good leaving avilibility of the chloride (or OAc in the case of acetic anhydride). acid chloride O O + R Cl OH2 O -Cl- R R Cl O OH2 R OH OH2 acid-catalyzed O R H+ Cl OH R Cl OH R + H2O Cl OH R Cl OH2 O -H -HCl R OH OH substitution R OH Esters are hydrolyzed in the presence of acid catalysts to give carboxylic acids. The addition-elimination mechanism accounts for the process. The steps are all equilibrium steps and the reaction is thus reversible. Thus a carboxylic acid reacts with an alcohol in the presence of an acid catalyst to give an ester. The process is the important Fischer esterification. By using an excess of alcohol (about 10 fold) the reaction is driven to the ester product in very good yield. Ester Hydrolysis (Fischer Esterification) O R H+ OH OH OR' R OR' + H2O R OH2 OH O OH R OHR' R OH PT OR' OH + R'OH R OH + H+ Basic hydrolysis of esters occurs on reaction of the ester with hydroxide. The mechanism is addition -elimination to give the carboxylate salt and an alcohol. Acidification of the reaction mixture gives the free carboxylic acid. Saponification O R O OH OR' R O OR' OH + R OH OR' O R O- + HOR' Acid hydrolysis of amides occurs at low pH. During the elimination step enough acid must be present to give the quaternary nitrogen ion which is lost as ammonia. Basic hydrolysis is very difficult because the leaving group in the elimination step is amide ion, a very poor leaving group. To achieve basic hydrolysis long times and high temperatures are required. Amide Hydrolysis O H+ R NH2 OH OH R NH2 + H2O O OH NH3 R OH NH2 OH2 OH R PT R OH + NH3 R NH4 + + OH Saponification O O OH R NH2 R O + NH2 R OH OH NH2 Nitriles produce amides on hydrolysis. Acid hydrolysis goes further to the carboxylic acid while basic hydrolysis gives the amide. Grignard reagents and organolithium reagents react with acid chlorides, anhydrides and esters in the same manner. First an addition-elimination sequence occurs to give an aldehyde or ketone, depending on R, and then the Grignard reagent does a simple addition to the ketone. The product is a tertiary alcohol that contains two molecules of the Grignard reagent. The reaction is very difficult to control to get just the aldehyde or ketone because the Grignard reagent is very reactive and reacts with both the acid chloride or ketone. O Mg+Br O R Cl + R'MgBr R Cl O R R'MgBr R' R' O R R' R' OH 2) H3O R R' R' An organocadmium reagent, prepared from a Grignard reagent and cadmium chloride, adds to an acid chloride to give a ketone. The cadmium reagent is not reactive enough to add to the ketone, and thus the final product in this procedure is the ketone. CdCl2 R'MgBr O R O R'2 Cd Cl R R'2 Cd NR R' Amides and nitriles undergo addition reactions with Grignard reagents but not the elimination step because the amide fuction, -NR2, is a poor leaving group. Thus after hydrolysis of the reaction mixture an aldehyde or ketone is produced. Since the carbonyl compound is produced after hydrolysis there is no further reaction with the Grignard reagent, as it has also been hydrolyzed. amide O R O NR2 + R'MgBr OH 2) H3O+ R R NR2 NHR 2 R' R' O -HNR2 R R' nitrile R C N R C + R'MgBr N 2) H3O+ R R' O R' Thus very common reagents can be used to deliver a carbonyl group from an amide or a nitrile. N,N-dimethylformamide is equivalent to an aldehyde function, and acetonitrile is equivalent to an acetyl group. O O H-C-N(CH3)2 H-C-R O H3 C C N H3 C R' Trifluoromethyl amides react in the Wittig reaction to produce enamines. CHPh O F3C + N O Ph3P=CHPh F3C N 60 % O