* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Answer Key for Final Exam
Kinetic resolution wikipedia , lookup
Metal carbonyl wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
2-Norbornyl cation wikipedia , lookup
Discodermolide wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Bottromycin wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Homoaromaticity wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
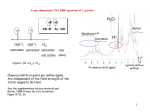
CHM 292 Final Exam Answer Key May 7, 2013 1. Predict the product(s) of the following reactions (5 points each; 35 points total). Acid‐catalyzed elimination to form the most highly substituted alkene possible SN2 substitution to attack the primary halide and form a new S‐C bond Acidic hydroxyl proton will inactivate the Grignard reagent to form a hydrocarbon and the alkoxide salt. Treatment with acid then regenerates the starting material. No actual Grignard addition occurs. Wittig reaction forms an alkene where the carbonyl used to be Conjugate addition of the amino group gives the saturated ketone with a new C‐N bond between the β‐ carbon and the nucleophile. Acid‐catalyzed Fischer esterification between the carboxylic acid and the alcohol to generate the new ester product Aldol reaction followed by elimination to generate the α,β‐unsaturated carbonyl. 2. Which of the following compounds would react most readily by an SN2 mechanism? Briefly explain your choice (8 points). None of these will react by an SN2 mechanism. A and B are sp2 hybridized carbons that cannot undergo SN2 reactions. Structure C cannot form a planar carbocation at the bridge position of the two rings. 3. Draw a mechanism that explains the formation of product B from starting material A. Your mechanism must explain the observed stereochemistry (8 points) Overall retention hints that the mechanism probably proceeded via a double inversion reaction via neighboring group participation. 4. Show two possible ways to make the alkene shown below from a Wittig reaction. For each option, draw the structures of the ylide and carbonyl compound (8 points). Disconnect right across the alkene double bond. In each case, add a carbonyl back to one side and a ylide on the other side. Option A: Option B: 5. Devise a synthesis of product B from starting material A. Indicate all reagents and draw the structures of all intermediates. More than one step is required (8 points). To convert the aryl bromide to a primary alcohol group, you need to make a Grignard that adds to formaldehyde. You first need to protect the ketone, though, so that it does not get attacked by a Grignard reagent on the other molecule. Ketone protection requires an acetal group. 6. Draw a mechanism to explain the formation of product C from starting materials A and B (8 points). There are a number of potential variations on this mechanism. The main idea is shown below: 7. Fill in the structures of compounds A, B, and C (8 points) Dess‐Martin periodinane oxidizes primary alcohols to aldehydes. The Wittig reagent converts the aldehyde to an alkene. H+ then hydrolyzes the acetal protecting group to re‐form the diol and acetone. 8. Draw two different pathways to synthesize phenylacetic acid from benzyl bromide (8 points) ‐ Path A: SN2 substitution using CN as a nucleophile, followed by hydrolysis of the nitrile Path B: Formation of a Grignard followed by Grignard attack on CO2. 9. How would you synthesize the compound shown below from an aldol reaction? Draw the structures of both precursors (8 points). We have a procedure for this: break the double bond, put a hydroxyl on the β‐carbon, and then break the bond between the α and β carbon to form two carbonyl starting materials. 10. How would you synthesize the compound shown below using a malonic ester synthesis? Show the full sequence, including mechanistic details for each step (15 points). I am going to do this both retrosynthetically and in the forward direction. Retrosynthesis: Forward direction: 11. Consider all the data shown below, and determine the structure of the unknown molecule. Assign every peak in the 1H NMR and 13C NMR to atoms in the molecule. Also identify key peak(s) in the IR spectrum and MS spectrum (20 points). Molecular formula: C11H14O2 MS spectrum: The mass spectrum confirms the molecular weight of the compound – 178 g/mol. There is a main fragment around 122 g/mol, but you do not need to focus on this for purposes of structure assignment. IR spectrum: ‐1 The IR spectrum has a carbonyl peak at 1893.72 cm . 13 C NMR spectrum: 1 H NMR spectrum: 1 H NMR shows that there is an aldehyde (singlet at 9.8 ppm). There is also a para‐substituted aromatic ring, as shown by the two doublets at 7.8 and 6.9 ppm. There are nine protons in the aliphatic region (3 sets of two protons plus one set of 3), plus another oxygen that needs to be accounted for. We know that there is no alcohol by the IR spectrum. A structure that matches all data so far is shown below: We can assign all the H NMR peaks to this structure: 1 13 And we can also assign the C NMR peaks: