* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plant Cell - Wesleyan College Faculty
Survey
Document related concepts
SNARE (protein) wikipedia , lookup
Cell growth wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Microtubule wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Extracellular matrix wikipedia , lookup
Signal transduction wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell membrane wikipedia , lookup
Cytokinesis wikipedia , lookup
Transcript
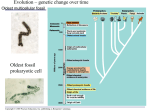
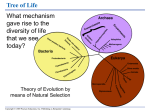
Chapter 6 A Tour of the Cell PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (d) Differential-interference-contrast (Nomarski). Like phase-contrast microscopy, it uses optical modifications to exaggerate differences in density, making the image appear almost 3D. (e) Fluorescence. Shows the locations of specific molecules in the cell by tagging the molecules with fluorescent dyes or antibodies. These fluorescent substances absorb ultraviolet radiation and emit visible light, as shown here in a cell from an artery. 50 µm (f) Confocal. Uses lasers and special optics for “optical sectioning” of fluorescently-stained specimens. Only a single plane of focus is illuminated; out-of-focus fluorescence above and below the plane is subtracted by a computer. A sharp image results, as seen in stained nervous tissue (top), where nerve cells are green, support cells are red, and regions of overlap are yellow. A standard fluorescence micrograph (bottom) of this relatively thick tissue is blurry. 50 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.3 Research Method Light Microscopy TECHNIQUE RESULTS (a) Brightfield (unstained specimen). Passes light directly through specimen. Unless cell is naturally pigmented or artificially stained, image has little contrast. [Parts (a)–(d) show a human cheek epithelial cell.] 50 µm (b) Brightfield (stained specimen). Staining with various dyes enhances contrast, but most staining procedures require that cells be fixed (preserved). (c) Phase-contrast. Enhances contrast in unstained cells by amplifying variations in density within specimen; especially useful for examining living, unpigmented cells. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.1 A cell and its skeleton viewed by fluorescence microscopy 10 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.2 The size range of cells 10 m Human height Length of some nerve and muscle cells Unaided eye 1m 0.1 m Chicken egg 1 cm Frog egg Most plant and animal cells 10 µm 1 µm 100 nm nucleus Nucleus Most bacteria Most bacteria Mitochondrion Smallest bacteria Viruses Ribosomes 10 nm Electron microscope 100 µm Light microscope 1 mm Proteins Lipids 1 nm 0.1 nm Small molecules Atoms Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Measurements 1 centimeter (cm) = 102 meter (m) = 0.4 inch 1 millimeter (mm) = 10–3 m 1 micrometer (µm) = 10–3 mm = 106 m 1 nanometer (nm) = 10–3 µm = 10 9 m Figure 6.6 A prokaryotic cell Pili: attachment structures on the surface of some prokaryotes Nucleoid: region where the cell’s DNA is located (not enclosed by a membrane) Ribosomes: organelles that synthesize proteins Plasma membrane: membrane enclosing the cytoplasm Cell wall: rigid structure outside the plasma membrane Capsule: jelly-like outer coating of many prokaryotes 0.5 µm (a) A typical rod-shaped bacterium Flagella: locomotion organelles of some bacteria Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (b) A thin section through the bacterium Bacillus coagulans (TEM) Figure 6.7 Geometric relationships between surface area and volume Surface area increases while total volume remains constant 5 1 1 Total surface area (height width number of sides number of boxes) 6 150 750 Total volume (height width length number of boxes) 1 125 125 Surface-to-volume ratio (surface area volume) 6 12 6 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.8 The plasma membrane Outside of cell Carbohydrate side chain Hydrophilic region Inside of cell 0.1 µm Hydrophobic region (a) TEM of a plasma membrane. The plasma membrane, here in a red blood cell, appears as a pair of dark bands separated by a light band. Hydrophilic region Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Phospholipid Proteins (b) Structure of the plasma membrane Figure 6.9 Exploring Animal and Plant Cells Animal Cell ENDOPLASMIC RETICULUM (ER) Rough ER Smooth ER Nuclear envelope Nucleolus NUCLEUS Chromatin Flagelium Plasma membrane Centrosome CYTOSKELETON Microfilaments Intermediate filaments Ribosomes Microtubules Microvilli Golgi apparatus Peroxisome Mitochondrion Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Lysosome In animal cells but not plant cells: Lysosomes Centrioles Flagella (in some plant sperm) Animal and Plant Cells: Plant Cell Nuclear envelope Nucleolus Chromatin NUCLEUS Centrosome Rough endoplasmic reticulum Smooth endoplasmic reticulum Ribosomes ( small brown dots ) Central vacuole Tonoplast Golgi apparatus Microfilaments Intermediate filaments CYTOSKELETON Microtubules Mitochondrion Peroxisome Plasma membrane Chloroplast Cell wall Plasmodesmata Wall of adjacent cell Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings In plant cells but not animal cells: Chloroplasts Central vacuole and tonoplast Cell wall Plasmodesmata Figure 6.10 The nucleus and its envelope Nucleus Nucleus 1 µm Nucleolus Chromatin Nuclear envelope: Inner membrane Outer membrane Nuclear pore Pore complex Rough ER Surface of nuclear envelope. TEM of a specimen prepared by a special technique known as freeze-fracture. 0.25 µm Ribosome 1 µm Close-up of nuclear envelope Pore complexes (TEM). Each pore is ringed by protein particles. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Nuclear lamina (TEM). The netlike lamina lines the inner surface of the nuclear envelope. Figure 6.11 Ribosomes Ribosomes ER Cytosol Endoplasmic reticulum (ER) Free ribosomes Bound ribosomes Large subunit 0.5 µm TEM showing ER and ribosomes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Small subunit Diagram of a ribosome Figure 6.12 Endoplasmic reticulum (ER) Smooth ER Rough ER Nuclear envelope ER lumen Cisternae Ribosomes Transport vesicle Smooth ER Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Transitional ER Rough ER 200 µm Figure 6.13 The Golgi apparatus Golgi apparatus cis face (“receiving” side of Golgi apparatus) 1 Vesicles move 2 Vesicles coalesce to 6 Vesicles also from ER to Golgi form new cis Golgi cisternae transport certain Cisternae proteins back to ER 3 Cisternal maturation: Golgi cisternae move in a cisto-trans direction 5 Vesicles transport specific proteins backward to newer Golgi cisternae 0.1 0 µm 4 Vesicles form and leave Golgi, carrying specific proteins to other locations or to the plasma membrane for secretion trans face (“shipping” side of Golgi apparatus) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings TEM of Golgi apparatus Figure 6.14 Lysosomes Nucleus 1 µm Lysosome containing two damaged organelles 1µm Mitochondrion fragment Peroxisome fragment Lysosome Lysosome contains Food vacuole fuses Hydrolytic active hydrolytic enzymes digest with lysosome enzymes food particles Digestive enzymes Lysosome fuses with vesicle containing damaged organelle Lysosome Plasma membrane Lysosome Lysosome Hydrolytic enzymes digest organelle components Digestion Food vacuole (a) Phagocytosis: lysosome digesting food Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Digestion Vesicle containing damaged mitochondrion (b) Autophagy: lysosome breaking down damaged organelle Figure 6.15 The plant cell vacuole Central vacuole Cytosol Tonoplast Nucleus Central vacuole Cell wall Chloroplast 5 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.16 Review: relationships among organelles of the endomembrane system 1 Nuclear envelope is connected to rough ER, which is also continuous with smooth ER Nucleus Rough ER Smooth ER Nuclear envelope 3 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.16 Review: relationships among organelles of the endomembrane system 1 Nuclear envelope is connected to rough ER, which is also continuous with smooth ER Nucleus Rough ER 2 Membranes and proteins produced by the ER flow in the form of transport vesicles to the Golgi Smooth ER cis Golgi Nuclear envelope Transport vesicle 3 Golgi pinches off transport vesicles and other vesicles that give rise to lysosomes and vacuoles trans Golgi 4 Lysosome available 5 Transport vesicle carries for fusion with another proteins to plasma vesicle for digestion membrane for secretion Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.16 Review: relationships among organelles of the endomembrane system 1 Nuclear envelope is connected to rough ER, which is also continuous with smooth ER Nucleus Rough ER 2 Membranes and proteins produced by the ER flow in the form of transport vesicles to the Golgi Smooth ER cis Golgi Nuclear envelope Transport vesicle 3 Golgi pinches off transport vesicles and other vesicles that give rise to lysosomes and vacuoles trans Golgi Plasma membrane 4 Lysosome available 5 Transport vesicle carries 6 Plasma membrane expands for fusion with another proteins to plasma by fusion of vesicles; proteins vesicle for digestion membrane for secretion are secreted from cell Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.17 The mitochondrion, site of cellular respiration Mitochondrion Intermembrane space Outer membrane Free ribosomes in the mitochondrial matrix Inner membrane Cristae Matrix Mitochondrial DNA Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 100 µm Figure 6.18 The chloroplast, site of photosynthesis Chloroplast Ribosomes Stroma Chloroplast DNA Inner and outer membranes Granum 1 µm Thylakoid Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.19 Peroxisomes Chloroplast Peroxisome Mitochondrion 1 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.20 The cytoskeleton Microtubule 0.25 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Microfilaments Figure 6.21 Motor proteins and the cytoskeleton ATP Vesicle Receptor for motor protein Motor protein (ATP powered) Microtubule of cytoskeleton (a) Motor proteins that attach to receptors on organelles can “walk” the organelles along microtubules or, in some cases, microfilaments. Vesicles Microtubule 0.25 µm (b) Vesicles containing neurotransmitters migrate to the tips of nerve cell axons via the mechanism in (a). In this SEM of a squid giant axon, two vesicles can be seen moving along a microtubule. (A separate part of the experiment provided the evidence that they were in fact moving.) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Table 6.1 The Structure and Function of the Cytoskeleton Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.22 Centrosome containing a pair of centrioles Centrosome Microtubule Centrioles 0.25 µm Longitudinal section Microtubules Cross section of one centriole of the other centriole Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.23 A comparison of the beating of flagella and cilia (a) Motion of flagella. A flagellum usually undulates, its snakelike motion driving a cell in the same direction as the axis of the flagellum. Propulsion of a human sperm cell is an example of flagellate locomotion (LM). Direction of swimming 1 µm (b) Motion of cilia. Cilia have a backand-forth motion that moves the cell in a direction perpendicular to the axis of the cilium. A dense nap of cilia, beating at a rate of about 40 to 60 strokes a second, covers this colpidium, a freshwater protozoan (SEM). Direction of organism’s movement Direction of active stroke Direction of recovery stroke 15 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.24 Ultrastructure of a eukaryotic flagellum or cilium Outer microtubule doublet Dynein arms 0.1 µm Central microtubule Outer doublet cross-linking proteins Microtubules Plasma membrane Basal body Radial spoke (b) A cross section through the cilium shows the ”9 + 2“ arrangement of microtubules (TEM). The outer microtubule doublets and the two central microtubules are held together by cross-linking proteins (purple in art), including the radial spokes. The doublets also have attached motor proteins, the dynein arms (red in art). 0.5 µm (a) A longitudinal section of a cilium shows microtubules running the length of the structure (TEM). 0.1 µm Triplet (c) Basal body: The nine outer doublets of a cilium or flagellum extend into the basal body, where each doublet joins another microtubule to form a ring of nine triplets. Each triplet is connected to the next by non-tubulin proteins (blue). The two central microtubules terminate above the basal body (TEM). Cross section of basal body Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Plasma membrane Figure 6.25 How dynein “walking” moves flagella and cilia Microtubule doublets ATP Dynein arm (a) Powered by ATP, the dynein arms of one microtubule doublet grip the adjacent doublet, push it up, release, and then grip again. If the two microtubule doublets were not attached, they would slide relative to each other. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings ATP Outer doublets cross-linking proteins Anchorage in cell (b) In a cilium or flagellum, two adjacent doublets cannot slide far because they are physically restrained, so they bend. (Only two of the nine outer doublets in Figure 6.24b are shown here.) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 1 3 2 (c) Localized, synchronized activation of many dynein arms probably causes a bend to begin at the base of the Cilium or flagellum and move outward toward the tip. Many successive bends, such as the ones shown here to the left and right, result in a wavelike motion. In this diagram, the two central microtubules and the cross-linking proteins are not shown. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.26 A structural role of microfilaments Microvillus Plasma membrane Microfilaments (actin filaments) Intermediate filaments 0.25 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.27 Microfilaments and motility Muscle cell Actin filament Myosin filament Myosin arm (a) Myosin motors in muscle cell contraction. Cortex (outer cytoplasm): gel with actin network Inner cytoplasm: sol with actin subunits Extending pseudopodium (b) Amoeboid movement . Nonmoving cytoplasm (gel) Chloroplast Streaming cytoplasm (sol) Parallel actin filaments (b) Cytoplasmic streaming in plant cells. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Cell wall Figure 6.28 Plant cell walls Central vacuole of cell Plasma membrane Secondary cell wall Primary cell wall Central vacuole of cell Middle lamella 1 µm Central vacuole Cytosol Plasma membrane Plant cell walls Plasmodesmata Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 6.29 Extracellular matrix (ECM) of an animal cell Collagen fibers are embedded in a web of proteoglycan complexes. A proteoglycan complex consists of hundreds of proteoglycan molecules attached noncovalently to a single long polysaccharide molecule. EXTRACELLULAR FLUID Fibronectin attaches the ECM to integrins embedded in the plasma membrane. Plasma membrane Integrin Microfilaments Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings CYTOPLASM Integrins are membrane proteins that are bound to the ECM on one side and to associated proteins attached to microfilaments on the other. This linkage can transmit stimuli between the cell’s external environment and its interior and can result in changes in cell behavior. Polysaccharide molecule Carbohydrates Core protein Proteoglycan molecule Figure 6.30 Plasmodesmata between plant cells Cell walls Interior of cell Interior of cell 0.5 µm Plasmodesmata Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Plasma membranes Figure 6.31 Exploring Intercellular Junctions in Animal Tissues TIGHT JUNCTIONS At tight junctions, the membranes of neighboring cells are very tightly pressed against each other, bound together by specific proteins (purple). Forming continuous seals around the cells, tight junctions prevent leakage of extracellular fluid across a layer of epithelial cells. Tight junction Tight junctions prevent fluid from moving across a layer of cells 0.5 µm DESMOSOMES Desmosomes (also called anchoring junctions) function like rivets, fastening cells together into strong sheets. Intermediate filaments made of sturdy keratin proteins anchor desmosomes in the cytoplasm. Tight junctions Intermediate filaments Desmosome Gap junctions Space between Plasma membranes cells of adjacent cells 1 µm Extracellular matrix Gap junction 0.1 µm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings GAP JUNCTIONS Gap junctions (also called communicating junctions) provide cytoplasmic channels from one cell to an adjacent cell. Gap junctions consist of special membrane proteins that surround a pore through which ions, sugars, amino acids, and other small molecules may pass. Gap junctions are necessary for communication between cells in many types of tissues, including heart muscle and animal embryos. 5 µm Figure 6.32 The emergence of cellular functions from the cooperation of many organelles Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings