* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 14 Selenium reagents
Physical organic chemistry wikipedia , lookup
Metal carbonyl wikipedia , lookup
George S. Hammond wikipedia , lookup
Kinetic resolution wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Elias James Corey wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
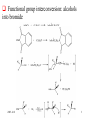
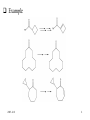
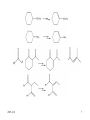
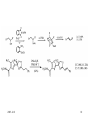
Chapter 14 Selenium reagents Functional group interconversion: alcohols into bromide syn-Elimination from selenoxides Allylic selenoxide and selenide -Selenoaldehydes Hydrogenolysis of carbon-selenium bonds Selenium(Ⅳ) reagents find use as oxidizing agents 2005-12-8 1 General features • A multitalented element: selenium reagents offer numerous possibilities in organic synthesis • Commercially available selenium reagents including: – Potassium selenocyanate, KSeCN – Areneselenols, ArSeH – Diary diselenides, ArSeSeAr – Areneselenyl halides, ArSeX (X = Cl, Br or I) 2005-12-8 2 Functional group interconversion: alcohols into bromide 2005-12-8 3 syn-Elimination from selenoxides Selenoxide can be obtained by oxidation of corresponding selenide. The oxidants may be hydrogen peroxide, peroxy acids, sodium periodate and ozone. Selenoxides with a -hydrogen can readily undergo thermal eliminationreaction to generate alkene. Using this procedure, we can achieve conversion of ketones to enones and synthesis of allylic alcohols. The variants of the procedure is in the preparation of the selenide rather than in the oxidation-elimination stage. 2005-12-8 4 Conversion of Carbonyl compounds to ,-unsaturated Carbonyl compounds by Selenoxide Syn Elimination Preparation of selenides From an electrophilic selenium reagent and a carbon nucleophile. From a nucleophilic selenium reagent and a carbon electrophile From a simpler selenid 2005-12-8 5 Example 2005-12-8 6 2005-12-8 7 Preparation of allylic alcohols and -halogenoalkenes by selenoxide syn-elimination Preparation of selenide From addition of benzeneselenenic acid to alkene. From addition of arylselenenyl halide to alkene. For synthesis of allylic alcohols, the overall reaction amounts to an allylic oxidation, with a rearrangement of the double bond. Selenide from addition of aryselenenyl halide to alkene can also react with nucleophilic functional groups. For alkenes containing suitably positioned nucleophilic functional groups may undergo cyclization. 2005-12-8 8 2005-12-8 9 2005-12-8 10 Unimolecular syn-Eliminations ( Pyrolytic syn-elimination) 2005-12-8 11 2005-12-8 12 Allylic selenoxide and selenide Preparation of allylic selenides Reaction of an allyl halide with a selenide anion Alkylation of an allylselenide anion By a wittig reaction The allyl selenoxide rearrangement Reaction with trialkylboranes: synthesis of -hydrogen alkene Reaction with alkyl-lithium reagents: selenium-lithium exchange 2005-12-8 13 2005-12-8 14 Hydrogenolysis of carbon-selenium bonds • Reagents for hydrogenolysis of carbon-selenium bonds – Raney nickel – Lithium in ethylamine – Triphenyltin(Ⅳ) hydride, Ph3SnH: expensive and air sensitive – Nickel boride, produced in situ by reaction of nickel chloride and sodium borohydride. • Synthetic applications: – Reductive alkylation of aldehydes and ketones – Formation of reduced heterocycles – Oxidation of alkenes to ketones 2005-12-8 15 Selenium(Ⅳ) reagents find use as oxidizing agents Selenium dioxide as oxidizing reagent Ketones containing an -methyllene are oxidized to diketones Elimination of 1,2,3-Selenadiazole Oxidation using benzeneseleninic acid Oxidation using benzeneseleninic anhydride 2005-12-8 16 Summary • Alcohols are converted into alkyl aryl selenides by reaction with aryl selenocyanates, ArSeCN. These react with bromine in the presence of a base, giving alkyl bromides: the overall reaction is ROH RBr with retention of configuration. • Aryl alkyl selenides are preparable either (as above) from electrophilic selenium reagents and carbon ncleophiles or from nucleophilic selenium reagents, e.g. ArSe-Na+, and carbon electrophiles. On oxidation they give selenoxides; if these contain a -hydrogen, they may undergo spontaneous syn-addition at ambient temp. to give alkenes. Allyl selenoxides undergo rearrangement to allyl selenenates, which are hydrolysable to allylic alcohols. • -selenoaldehydes undergo condensation reactions, and a double bond may then be introduced in the product by oxidation at the selenium atom followed by elimination. 2005-12-8 17 Allylic selenides are convertible into allyl-lithium reagents for further reactio with electrophiles. Hydrogenolysis of carbon-selenium bonds is achievable using catalytic methods, dissolving metals, triaryltin hydrides and ‘nickel boride’. 1,2,3-Selenadiazole undergo elimination, giving alkynes, either on heating or treatment with organolithium reagents. Highly reactive cycloalkynes are preparagble in this way. Selenium(Ⅳ) reagents find use as oxidizing agents, such as selenium(Ⅳ) oxide, benzeneseleninic acid (in combination with hydrogen peroxide) and benzeneseleninic anhydride. 2005-12-8 18