* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
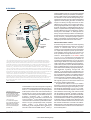
Download FOCAL ADHESION KINASE: IN COMMAND AND CONTROL OF
G protein–coupled receptor wikipedia , lookup
Cell membrane wikipedia , lookup
Cell encapsulation wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Endomembrane system wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Tyrosine kinase wikipedia , lookup
Phosphorylation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cytokinesis wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Paracrine signalling wikipedia , lookup
REVIEWS FOCAL ADHESION KINASE: IN COMMAND AND CONTROL OF CELL MOTILITY Satyajit K. Mitra, Daniel A. Hanson and David D. Schlaepfer Abstract | A central question in cell biology is how membrane-spanning receptors transmit extracellular signals inside cells to modulate cell adhesion and motility. Focal adhesion kinase (FAK) is a crucial signalling component that is activated by numerous stimuli and functions as a biosensor or integrator to control cell motility. Through multifaceted and diverse molecular connections, FAK can influence the cytoskeleton, structures of cell adhesion sites and membrane protrusions to regulate cell movement. INTEGRINS A large family of heterodimeric transmembrane proteins that function as receptors for celladhesion molecules. EXTRACELLULAR MATRIX (ECM). The complex, multimolecular material that surrounds cells. The ECM comprises a scaffold on which tissues are organized, it provides cellular microenvironments and it regulates various cellular functions. The Scripps Research Institute, Department of Immunology, IMM21 10550 North Torrey Pines Road, La Jolla, California 92037, USA. Correspondence to D.D.S. e-mail: [email protected] doi:10.1038/nrm1549 56 Cell migration is a coordinated process that involves rapid changes in the dynamics of actin filaments, together with the formation and disassembly of cell adhesion sites1. A complex interplay between the actin cytoskeleton and cell adhesion sites leads to the generation of membrane protrusions and traction forces2. External stimuli that control cell migration are transduced into intracellular biochemical signals through the interactions of transmembrane INTEGRINS that bind to EXTRACELLULAR MATRIX (ECM) proteins, growth factors that bind to their cognate cell-surface receptors, or mechanical stimuli such as shear stress that promote deformation of the actin cytoskeleton. For a cell to process these different environmental motility-promoting stimuli correctly, there must be essential intracellular signalling proteins that function as ‘integrators’ — that is, proteins that are stimulated by multiple extracellular inputs and that function to regulate multiple signalling pathway outputs3. Here, we describe the unique molecular connections of focal adhesion kinase (FAK) that allow this tyrosine kinase to function as an important receptor-proximal regulator of cell shape, adhesion and motility. The complexities of FAK FAK was independently identified in 1992 by Steve Hanks, Jun-Lin Guan and Michael Schaller as a substrate of the viral Src oncogene and, in normal cells, as a | JANUARY 2005 | VOLUME 6 highly tyrosine-phosphorylated protein that localized to integrin-enriched cell adhesion sites that are known as focal contacts (BOX 1). Focal contacts are formed at ECM–integrin junctions that bring together cytoskeletal and signalling proteins during the processes of cell adhesion, spreading and migration. Early studies found that FAK could be activated by either ECM or growth factors, and that tyrosine phosphorylation of FAK was a rapid event that was associated with the formation of focal contacts4. Subsequent studies using knockout mice revealed that null mutation of FAK resulted in defective developmental morphogenesis5. As FAK-null fibroblasts show excessive, rather than decreased (as was initially predicted), formation of focal contacts, FAK signalling has been associated with the disassembly of integrin-based adhesion sites6. The loss of FAK expression also disrupts microtubule polarization within cells7, and this phenotype, as well as the defect in focal contact turnover, has been linked to the FAK-mediated regulation of RHO-FAMILY GTPases in cells8. Rho-family GTPases are molecular ‘switches’ within cells, which control the formation and disassembly of actin cytoskeletal structures (STRESS FIBRES, LAMELLIPODIA and filopodia) and that function to provide the molecular framework that supports directed cell motility. In both normal and transformed cells, FAK signalling can promote increased cell motility. The genomic designation of human FAK is protein-tyrosine kinase-2 www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS Box 1 | Molecular architecture of focal contacts α -A c tin in c tin in in in FAK p130 p130 Src Cas Cas Src in ul nc Plasma membrane illin Pax in a Tl α -A Vi FAK c tin in ul nc α -A Vi in ul nc Vi Zyxin α -A c tin in My My os os in My os in Actin stress fibres Focal contact proteins P ax ill Tali in n Integrins α β β α Extracellular matrix The extracellular matrix, integrins (α- and β-transmembrane heterodimeric proteins) and the cell cytoskeleton interact at sites called focal contacts. Focal contacts are dynamic groups of structural and regulatory proteins that transduce external signals to the cell interior and can also relay intracellular signals to generate an activated integrin state at the cell surface113. The integrin-binding proteins paxillin and talin recruit focal adhesion kinase (FAK) and vinculin to focal contacts (see figure). α-Actinin is a cytoskeletal protein that is phosphorylated by FAK, binds to vinculin and crosslinks actomyosin stress fibres and tethers them to focal contacts. Zyxin is an α-actinin- and stress-fibrebinding protein that is present in mature contacts. Although the aforementioned proteins are found in most focal contacts, the membrane-associated protein tyrosine kinase Src and the ADAPTOR PROTEIN p130Cas associate with focal contacts following integrin clustering. Integrin-mediated FAK activation is mediated in part by matrix binding or by force-dependent changes in cytoskeletal linkages. Several other proteins such as extracellular signal-regulated kinase 2 (ERK2) and calpain are known to be transiently present at focal contacts (not shown). The composition of a focal contact is therefore constantly varying depending on external cues and cellular responses. RHO-FAMILY GTPases A subfamily of small (~21 kDa) GTP-binding proteins that are related to Ras and that regulate the cytoskeleton. The nucleotide-bound state is regulated by GTPase-activating proteins, which catalyse hydrolysis of the bound GTP, and guanine nucleotideexchange factors, which catalyse GDP–GTP exchange. (PTK2) and it is located at human chromosome 8q24term, a locus that is subject to amplification in human cancer cells9. Furthermore, elevated levels of PTK2 mRNA have been found in studies of human carcinoma tumours and in acute lymphoblastic leukaemias, as detected by large-scale gene expression profiling10,11. FAK protein expression is elevated in many highly malignant human cancers12, and studies have shown that FAK signalling can promote changes in cell shape13,14 and the formation of podosomes or invadopodia15, which leads to an invasive cell phenotype16,17. Whereas NATURE REVIEWS | MOLECUL AR CELL BIOLOGY many studies have shown that FAK inhibition blocks the response to cell motility cues18, recent and provocative studies have shown that inhibition of FAK expression or activity resulted in increased carcinoma cell migration through the dissolution of N-cadherin-mediated cell–cell contacts in HeLa CELLS19. It is possible that this latter observation might be a cell-type-specific signalling event. However, we speculate that the ability of FAK to promote both the maturation and turnover of focal contacts is related to its role as both a signalling kinase and as an adaptor/scaffold protein, which places FAK in a position to modulate various intracellular signalling pathways (FIG. 1). Namely, it is the association of FAK with both activators and/or inhibitors of various small GTPase proteins (Rho, Rac, Cdc42 and Ras) that enables changes in FAK activity to be connected to alterations in the polymerization or stabilization of actin and microtubule filaments. Additionally, because migrating cells experience changes in forces through integrin contacts that link the ECM with the cytoskeleton, FAK is important in the ‘sensing’ of mechanical forces that are either generated internally or exerted on cells20. FAK activation is therefore involved in modulating ‘corrective’ cell responses to environmental stimuli. FAK does this through signal-mediated effects on actin polymerization, the assembly or disassembly of focal contacts, and the regulation of protease activation or secretion16,21,22. The FERM and FAT domains of FAK FAK is a ubiquitously expressed 125-kDa protein tyrosine kinase that is composed of an N-terminal FERM (protein 4.1, ezrin, radixin and moesin homology) domain, a central kinase domain, proline-rich regions and a C-terminal focal-adhesion targeting (FAT) domain (FIG. 2). The FERM domain of FAK facilitates a signalling linkage from receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR) and the platelet-derived growth factor receptor (PDGFR)23. In analysing cell-motility-promoting signals that are initiated by G-PROTEIN-COUPLED RECEPTORS (GPCRs), overexpression of the FAK FERM domain blocked FAK activation and resulted in the inhibition of G-protein-stimulated cell migration24. It is the FAK FERM domain that can bind to and promote the integrin- and FAK-mediated activation of other non-receptor tyrosine kinases such as ETK25. Additionally, actin- and membrane-associated adaptor proteins such as ezrin can bind to the FAK FERM domain and facilitate increased FAK activation in an integrin-independent manner26. How the FAK FERM domain associates with various targets is an active area of research. FAK can become post-translationally modified by the covalent addition of a small ubiquitin-related modifier (SUMO) at the ε-amino position of Lys152 (REF. 27). In most instances, sumoylation is associated with the nuclear import of proteins and, correspondingly, sumoylated FAK was enriched in the nuclear fraction of cells27. Although blocking nuclear export using leptomycin B promotes the nuclear accumulation of FAK, and exogenous expression of the FAK FERM domain exhibits strong VOLUME 6 | JANUARY 2005 | 5 7 © 2005 Nature Publishing Group REVIEWS Extracellular matrix directly to the cytoplasmic tails of integrins33, accumulated evidence supports an indirect association of FAK with integrins through binding to integrinassociated proteins such as paxillin and talin18. The FAK FAT domain also binds directly to an activator of Rho-family GTPases that is known as p190 RhoGEF, and FAK-mediated tyrosine phosphorylation of p190 RhoGEF might be a direct link to RhoA activation34. Growth factor receptors α β Integrins Assembly Cadherins Focal contacts FAK Disassembly ? Rho-family GTPases mDia Microtubule stabilization RhoA Rac Cdc42 Stress fibres Lamellipodia Filopodia Cell migration Figure 1 | Focal adhesion kinase integrates signals to promote cell migration. Focal adhesion kinase (FAK) is activated by growth factors and integrins during migration, and functions as a receptor-proximal regulator of cell motility. At contacts between cells and the extracellular matrix, FAK functions as an adaptor protein to recruit other focal contact proteins or their regulators, which affects the assembly or disassembly of focal contacts. FAK activity and downstream signalling can promote changes in actin and microtubule structures, and FAK signalling can affect the formation and disassembly of cell–cell (cadherin-based) contacts. The Rho-family GTPases (RhoA, Rac and Cdc42) direct local actin assembly into stress fibres, lamellipodia and filopodia, respectively. FAK can influence the activity of Rho-family GTPases through a direct interaction with, or phosphorylation of, protein activators or inhibitors of Rho GTPases. RhoA can also influence the stability of microtubules through its effector Diaphanous (mDia). STRESS FIBRES Also termed ‘actinmicrofilament bundles’, these are bundles of parallel filaments that contain F-actin and other contractile molecules, and often stretch between cell attachments as if under stress. LAMELLIPODIA Broad, flat protrusions at the leading edge of a moving cell that are enriched with a branched network of actin filaments. HeLa CELLS An established tissue-culture strain of human epidermoid carcinoma cells, containing 70–80 chromosomes per cell. These cells were originally derived from tissue taken from a patient named Henrietta Lacks in 1951. 58 nuclear localization28, it is not known whether these events are dependent on sumoylation. As sumoylated FAK showed elevated activity27, and FAK signalling has been linked to enhanced gene transcription29 and cellcycle progression30, it is possible that sumoylation of FAK might facilitate a direct signalling route between focal contacts and the nucleus. The C-terminal domain of FAK contains two proline-rich regions that function as binding sites for SRC-HOMOLOGY (SH)3-DOMAIN-containing proteins (FIG. 2). SH3-domain-mediated binding of the adaptor protein p130Cas to FAK is important in promoting cell migration through the coordinated activation of Rac at membrane extensions31,32. The SH3-mediated binding of other proteins, such as GRAF (GTPase regulator associated with FAK) and ASAP1 (Arf GTPase-ACTIVATING PROTEIN (GAP) containing SH3, ankyrin repeat and pleckstrin homology (PH) domains-1), connects FAK to the regulation of cytoskeletal dynamics and focal contact assembly. However, the downstream connections of GRAF and ASAP1 remain undefined4. The C-terminal domain of FAK also encompasses the FAT region, which promotes the colocalization of FAK with integrins at focal contacts (BOX 1). Whereas it was first hypothesized that FAK might bind | JANUARY 2005 | VOLUME 6 FAK activation and phosphorylation The best-characterized FAK phosphorylation event is AUTOPHOSPHORYLATION at Tyr397, which can occur in either cis or trans 35. Phosphorylation of FAK at Tyr397 creates a motif that is recognized by various SH2-DOMAINcontaining proteins, such as SRC-FAMILY KINASES (SFKs), phospholipase Cγ (PLCγ), suppressor of cytokine signalling (SOCS), growth-factor-receptor-bound protein-7 (GRB7), the Shc adaptor protein, p120 RasGAP, and the p85 subunit of phosphatidylinositol 3-kinase (PI3K)4,18,31,33 (FIG. 2). It is not known whether these different signalling proteins differentially bind to Tyr397-phosphorylated FAK in response to particular cell stimuli or whether simultaneously there are different complexes with a larger pool of activated FAK. In this respect, we favour a sequential association model whereby the binding of cellular Src (hereafter referred to as Src) to FAK initiates signalling (discussed below) and the association of SOCS with FAK is a terminal event that leads to ubiquitin-mediated degradation of FAK36. For integrin-, growth factor- and G-protein-linked stimuli that promote cell motility, it is the transient recruitment of SFKs into a signalling complex with FAK that is one of the first events associated with FAK activation18. Proline-rich tyrosine kinase-2 (PYK2) is related to FAK and shares a similar domain structure (FERM, kinase, proline-rich and FAT domains) as well as common phosphorylation sites (BOX 2). The binding of SFKs to PYK2 that is phosphorylated at Tyr402 is also associated with PYK2 activation. However, FAK and PYK2 possess distinct signalling roles in cells, partly owing to differential binding of target proteins to the FERM and FAT domains of FAK and PYK2, respectively. Additionally, PYK2 is preferentially expressed in cells of the endothelium, central nervous system and haematopoietic lineages; PYK2 activation is sensitive to intracellular Ca2+ signals; and PYK2 is only weakly activated in response to the binding of α5β1-integrin to fibronectin, whereas FAK is strongly activated37. The difference in α5β1-mediated activation of FAK versus PYK2 is directly related to the FAT-mediated localization of FAK at focal contacts compared with a perinuclear distribution of PYK2 in cells38. Although PYK2 and FAK can bind SFKs and can activate common signalling pathways, the differential binding activities of the FERM and FAT domains might limit the functional redundancy of these PTKs in cells. The activity of FAK is dependent on integrinmediated cell adhesion. Models of integrin-mediated intermolecular FAK activation are based on the fact that FAK mutants can compete for integrin association and www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS p120 RasGAP, GRB7, Shc, PLCγ, p85, Src, SOCS Ezrin PDGF receptor, ETK, EGF receptor P Tyr397 FERM FAK Lys152 GRB2, p190 RhoGEF, talin, paxillin P P P P Tyr861 Tyr925 Tyr576 Tyr577 Kinase domain PRR1 FIP200 SUMO FAT PRR2 PRR3 p130Cas, ASAP1, GRAF Figure 2 | Focal adhesion kinase domain structure and phosphorylation sites. Focal adhesion kinase (FAK) contains a FERM (protein 4.1, ezrin, radixin and moesin homology) domain, a kinase domain and a focal adhesion targeting (FAT) domain. The FERM domain mediates interactions of FAK with the epidermal growth factor (EGF) receptor, platelet-derived growth factor (PDGF) receptor, the ETK tyrosine kinase and ezrin, and the FERM domain can be conjugated to SUMO (small ubiquitin-related modifier) at Lys152. The FAT domain recruits FAK to focal contacts by associating with integrin-associated proteins such as talin and paxillin. It also links FAK to the activation of Rho GTPases by binding to guanine nucleotide-exchange factors (GEFs) such as p190 RhoGEF. FAK contains three proline-rich regions (PRR1–3), which bind Srchomology-3 (SH3) domain-containing proteins such as p130Cas, the GTPase regulator associated with FAK (GRAF) and the Arf-GTPase-activating protein ASAP1. FAK is phosphorylated (P) on several tyrosine residues, including Tyr397, 407, 576, 577, 861 and 925. Tyrosine phosphorylation on Tyr397 creates a Src-homology-2 (SH2) binding site for Src, phospholipase Cγ (PLCγ), suppressor of cytokine signalling (SOCS), growth-factor-receptorbound protein 7 (GRB7), the Shc adaptor protein, p120 RasGAP and the p85 subunit of phosphatidylinositol 3-kinase (PI3K). Phosphorylation of Tyr576 and Tyr577 within the kinase domain is required for maximal FAK catalytic activity, whereas the binding of FAK-family interacting protein of 200 kDa (FIP200) to the kinase region inhibits FAK catalytic activity. FAK phosphorylation at Tyr925 creates a binding site for GRB2. ADAPTOR PROTEINS Proteins that augment cellular responses by recruiting other proteins to a complex. They usually contain several protein–protein interaction domains. G-PROTEIN-COUPLED RECEPTOR A seven-helix membranespanning cell-surface receptor that signals through heterotrimeric GTP-binding and GTP-hydrolysing G-proteins to stimulate or inhibit the activity of a downstream enzyme. SRC-HOMOLOGY (SH)3-DOMAIN A protein sequence of 50 amino acids that recognizes and binds sequences that are rich in proline. GTPase-ACTIVATING PROTEIN (GAP). A protein that stimulates the intrinsic ability of a GTPase to hydrolyse GTP to GDP. Therefore, GAPs negatively regulate GTPases by converting them from active (GTP-bound) to inactive (GDP-bound). can inhibit endogenous FAK activity4 and that kinaseinactive FAK can become transphosphorylated on Tyr397 in cells23. FAK Tyr397 phosphorylation promotes Src binding, which leads to the conformational activation of Src and results in a dual-activated FAK–Src signalling complex18. Within this FAK–Src complex, Src phosphorylates FAK at Tyr861, and this is associated with an increase in SH3-domain-mediated binding of p130Cas to the FAK C-terminal proline-rich regions39. Activated Src also phosphorylates FAK at Tyr925, which creates an SH2-binding site for the GRB2 adaptor protein. GRB2 binding to FAK is one of several connections that lead to the activation of Ras and the extracellular signal-regulated kinase-2 (ERK2)/mitogen-activated protein kinase (MAPK) cascade18. ERK2 phosphorylation and the subsequent activation of myosin light chain kinase can modulate focal contact dynamics in motile cells3, as well as generate both proliferative and survival signals inside cells31. thereby reinforced the role of integrins in the regulation of FAK signalling. Results showing that FAK catalytic activity can be modulated by either posttranslational or mutational changes in activation-loop residues are consistent with crystal structure analysis of the ATP-bound kinase domain of FAK, which shows a disordered activation-loop conformation41. As the crystal structure of the kinase domain of FAK also showed the presence of an unusual disulphide bond between Cys456 and Cys459 in a regulatory region, conformational changes or protein-binding interactions might also function to modulate the activation state of FAK. This model is supported by findings that cellular proteins such as FAK-interacting protein of 200 kDa (FIP200) bind to the kinase domain of FAK and inhibit FAK activity42. Additionally, evidence is accumulating that intramolecular constraints also have a role in the regulation of FAK activity. There are alternatively spliced isoforms of FAK in which amino-acid additions surrounding the Tyr397 site promote a change in the kinetics of FAK activation (as measured by Tyr397 phosphorylation) from a primarily trans-intermolecular reaction to a cis-intramolecular reaction35. Although alternative splicing of FAK does not alter the FERM domain residues of FAK, truncation or removal of the FAK FERM domain does result in enhanced FAK catalytic activity35. As binding of proteins such as ezrin or the GUANINE NUCLEOTIDE-EXCHANGE FACTOR (GEF) TRIO to the FAK FERM domain result in enhanced FAK activity26,43, and as the FAK FERM domain can bind in trans to the FAK catalytic domain, resulting in the inhibition of FAK activity44, it is possible that binding interactions or conformational changes in the FAK FERM domain might function to release cis-inhibitory constraints on FAK catalytic activation. The activity of FAK can also be modulated positively45 or negatively 46 by the action of protein-tyrosine phosphatases (PTPs). Studies using PTPα-deficient fibroblasts showed that this phosphatase was required for maximal stimulation of Src catalytic activity by β1-integrins, and that PTPα functioned as an upstream regulator of FAK Tyr397 phosphorylation45. This result is consistent with the potential intermolecular activation of FAK by Src. As Src can also become activated through direct interaction with the cytoplasmic domains of β-integrins47, these types of result reinforce the fact that Tyr397 phosphorylation of FAK might not always reflect FAK catalytic activity that is mediated by autophosphorylation. Regulation of FAK catalytic activity Src-mediated transphosphorylation of FAK within the kinase domain ACTIVATION LOOP at Tyr576 and Tyr577 promotes maximal FAK catalytic activation31. Mutation of FAK within this loop produces FAK mutants with either enhanced or refractory activities. One such mutant, ‘superFAK’, contains a Lys to Glu substitution at residues 578 and 581, and results in a FAK protein with adhesion-independent activity40. However, the phosphorylation of downstream targets in superFAKexpressing cells remained adhesion dependent, which NATURE REVIEWS | MOLECUL AR CELL BIOLOGY p130Cas and paxillin as targets of FAK In addition to promoting maximal FAK activation, the recruitment of Src into a FAK–Src signalling complex functions to facilitate the phosphorylation of various FAK-associated proteins, as many FAK targets are also independent binding partners and phosphorylation targets of Src. Two of the best-characterized target proteins of FAK–Src-mediated phosphorylation are p130Cas and paxillin31,32,48. SH3-mediated binding of p130Cas to FAK is linked to enhanced tyrosine phosphorylation of VOLUME 6 | JANUARY 2005 | 5 9 © 2005 Nature Publishing Group REVIEWS Box 2 | The FAK-related kinase PYK2 Src ↑ Tyr397 Tyr576 Tyr577 FAK FERM Kinase domain PRR1 Sequence <10% similarity PYK2 GRB2 ↑ Tyr861 Tyr925 ~40% FERM FAT PRR2 ~60% PRR3 ~40% Kinase domain FAT PRR1 Tyr402 Tyr579 Tyr580 PRR2 ↓ Src PRR3 Tyr881 ↓ GRB2 Proline-rich tyrosine kinase-2 (PYK2) shares a similar domain arrangement with focal adhesion kinase (FAK) (see figure), with 60% sequence identity in the central kinase domain, conservation of proline-rich regions (PRRs), and identical positions of four tyrosine phosphorylation sites. PYK2 tyrosines 402, 579, 580 and 881 correspond to FAK tyrosines 397, 576, 577 and 925, respectively. Phosphorylation of PYK2 Tyr402 and Tyr881 create Src-homology-2 (SH2) binding sites for Src and growth-factor-receptorbound-2 (GRB2), respectively. PYK2 contains a C-terminal focal adhesion targeting (FAT) domain that binds to paxillin53. However, PYK2 shows perinuclear distribution and is not strongly localized to focal contacts in many cells37. The substitution of the FAK C-terminal domain to PYK2 facilitated the colocalization of this PYK2–FAK chimaera to β1-integrin-containing focal contacts38, which indicates that there are biologically relevant binding differences between FAK and PYK2. For instance, the FAK C-terminal domain uniquely binds the integrin-associated protein talin114, and PYK2 — but not FAK — binds the actin-associated protein gelsolin115. Although PYK2 can be activated by integrins, this is dependent on integrin-mediated activation of Src-family kinases116,117. The 40% sequence similarity between the N-terminal FERM (protein 4.1, ezrin, radixin and moesin homology) domains of PYK2 and FAK also accounts for differential association with target proteins118.What remains unknown is why PYK2 activity is highly dependent on intracellular Ca2+ levels and how PYK2 associates with members of the Janus kinase family37,119 — properties that are not shared by FAK.As PYK2 regulates several signalling events that are crucial for macrophage120 and monocyte morphology121, and the Pyk2-null phenotype results in a MARGINAL ZONE B-cell developmental defect122, there is probably a unique role for PYK2 in mediating haematopoietic cell responses to chemokine stimuli. AUTOPHOSPHORYLATION The transfer of a phosphate group by a protein kinase either to a residue in the same kinase molecule (cis) or to a residue in a different kinase molecule but of the same type (trans). SH2 DOMAIN A protein motif that recognizes and binds tyrosinephosphorylated sequences, and thereby has a key role in relaying cascades of signal transduction. SRC-FAMILY KINASES Kinases that belong to the Src family of tyrosine kinases, the largest of the non-receptortyrosine-kinase families. 60 p130Cas at multiple sites, which promotes SH2-mediated binding of the Crk adaptor protein to p130Cas. Signalling downstream of p130Cas results in increased activity of Rac, enhanced MEMBRANE RUFFLING or lamellipodia formation, and the promotion of cell motility or invasion17,49,50 (FIG. 3). Paxillin is phosphorylated by FAK–Src on Tyr31 and Tyr118, and this can also promote SH2-mediated binding of Crk to paxillin48,51. Overexpressing paxillin that is mutated at these phosphorylation sites inhibits the turnover of focal contacts6 and cell motility52, which therefore supports the presence of multiple routes for FAK–Src-mediated signalling in modulating the dynamics of cell adhesion sites. Regulated targeting of FAK to focal contacts It is the C-terminal FAT domain of FAK that facilitates the linkage to integrins and focal contacts. The FAT domain adopts a four-helix bundle structure that contains binding sites for integrin-associated proteins such as paxillin53. Point mutations in the FAT domain of FAK that disrupt paxillin binding also prevent the association | JANUARY 2005 | VOLUME 6 of FAK with β1-integrin and the localization of FAK to focal contacts38. Paxillin binding is mediated by two leucine-rich peptide regions in paxillin that are known as LD MOTIFS, which interface with hydrophobic surface grooves on the FAT domain54,55. Interestingly, the SH2 binding site for GRB2 at FAK Tyr925 partially overlaps with one of the two paxillin LD-motif binding sites in the FAT domain54, and localization studies of phosphorylated FAK have shown that Tyr925-phosphorylated FAK might be selectively excluded from focal contact sites56. Overexpression of a Tyr925Phe mutant of FAK resulted in strong focal contact distribution56, and in activated Src-expressing cells, Tyr925Phe FAK blocks the turnover of focal contacts (V. Brunton, personal communication; see note added in proof). As NMR analyses have shown that the FAT domain can undergo conformational rearrangements that might selectively promote either Tyr925 phosphorylation and/or paxillin binding57, it is possible that Src-mediated phosphorylation of FAK on Tyr925, and subsequent GRB2 binding, could displace paxillin, promote the dissociation of FAK from focal contacts, and subsequently lead to focal contact turnover through undefined mechanisms (FIG. 3). Ser910 within the FAT domain is phosphorylated during mitosis58 and after growth factor stimulation of cells. Ser910 is phosphorylated by ERK2 and this is also associated with reduced paxillin binding to FAK59. So, Src-mediated phosphorylation of Tyr925 on FAK and GRB2 binding leading to ERK2 activation, coupled with the feedback of ERK2-mediated Ser910 phosphorylation, could potentiate the release of FAK from focal contacts. Alternatively, FAK–Src-mediated phosphorylation of paxillin at Tyr118 promotes the binding of ERK2 to paxillin60, and ERK2-mediated phosphorylation of paxillin can facilitate FAK binding to paxillin and can enhance FAK activation60,61. Therefore, we speculate that there might be a regulatory cycle in which FAK–Src activation and signalling to ERK2 can function first to promote FAK release from existing focal contacts and then, through ERK2-mediated phosphorylation of paxillin, to promote FAK re-binding and activation at new or different focal contacts in a migrating cell (FIG. 3). Regulation of focal contact dynamics Lessons from FAK–/– and SHP2 –/– cells. In analyses monitoring the formation of focal contacts, FAK was found to be one of the first signalling proteins to be recruited to these sites62. Although FAK recruitment to focal contacts is associated with increased FAK tyrosine phosphorylation63, focal contacts readily form in FAK-null (FAK–/–) fibroblasts, which indicates that FAK activity is not essential for the process of focal-adhesion formation5. However, focal contacts in FAK–/– cells form primarily around the cell periphery, enmeshed in a cortical actin ring, and do not undergo a normal maturation cycle64. In normal fibroblasts, peripheral immature focal contacts become connected to longitudinal stress fibres in cells and undergo actin contractility-mediated maturation during cell polarization63. FAK re-expression in www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS α β Migration Membrane Integrins Rac Crk Src Focal contacts FAK SH2 P P P Tyr397 Tyr576 Tyr577 FERM p130Cas P SH3 P-X-X-P Kinase domain Tyr925 Paxillin ERK2 FAT P Tyr118 P Release ERK2 P GRB2 Tyr925 FAK FERM Kinase domain FAT Ser910 P Figure 3 | Focal adhesion kinase (FAK)–Src signals that regulate cell motility and focal contact localization. Integrin clustering promotes FAK autophosphorylation (P) at Tyr397, which creates a binding site for the Src-homology (SH)2 domain of Src. Src-mediated phosphorylation of FAK at Tyr576 and Tyr577 promotes maximal FAK catalytic activity. Active FAK–Src facilitates SH3-mediated binding of p130Cas to FAK and its subsequent phosphorylation. Crk binding to phosphorylated p130Cas facilitates Rac activation, lamellipodia formation and cell migration. Paxillin binding to the FAK focal adhesion targeting (FAT) domain is important for FAK focal contact localization. Src-mediated phosphorylation of FAK at Tyr925 creates an SH2 binding site for the growth-factor-receptor-bound protein 2 (GRB2) adaptor protein, which leads to the activation of Ras and the extracellular signal-regulated kinase-2 (ERK2) cascade. The GRB2 and paxillin binding sites within the FAT domain overlap and Tyr925-phosphorylated FAK might be selectively released from focal contacts. ERK2 activation promotes FAK phosphorylation at Ser910, which is also associated with decreased paxillin binding to FAK. Within focal contacts, FAK–Src-mediated phosphorylation of paxillin at Tyr118 promotes ERK2 binding. ERK2-mediated phosphorylation of paxillin can facilitate FAK binding to paxillin and enhances FAK activation. So, there might be a cycle whereby Src- and ERK2-mediated phosphorylation of FAK promotes its release from focal contacts and ERK2-mediated phosphorylation of paxillin promotes the association of unphosphorylated FAK with paxillin at new or growing focal contact sites. MARGINAL ZONE A region in the spleen in which white blood cell precursors such as B-cells, granulocytes, macrophages and plasma-cells reside or transit through during primary or secondary immune responses. ACTIVATION LOOP A conserved structural motif in kinase domains, which needs to be phosphorylated for full activation of the kinase. GUANINE NUCLEOTIDEEXCHANGE FACTOR A protein that facilitates the exchange of GDP for GTP in the nucleotide-binding pocket of a GTP-binding protein. FAK–/– cells promotes the reorganization of the ‘immature’ focal contacts, which allows for their connection to actin stress fibres, therefore mediating cell contractility and cell polarization64. Mechanistically, these alterations in focal contacts and actin structures involve the regulation of the activity of α-actinin, a protein that promotes actin crosslinking and that has an important role in maintaining the linkage between focal contacts and stress fibres1,65 (FIG. 4). FAK phosphorylates α-actinin at Tyr12, which results in reduced α-actinin binding to actin66. α-Actinin is not phosphorylated in FAK–/– cells, so this FAK signalling linkage to α-actinin might underlie some of the maturation defects, as well as turnover dynamics, of focal contacts in FAK–/– cells66. It is possible that the lack of α-actinin phosphorylation might be associated with the presence of focal contacts enmeshed in a cortical actin ring at the FAK–/– cell periphery. This hypothesis is further supported by studies of cells that lack the tyrosine NATURE REVIEWS | MOLECUL AR CELL BIOLOGY phosphatase SHP2 (SH2-domain-containing protein tyrosine phosphatase 2). SHP2–/– cells have increased FAK activity, but they also show an accumulation of immature focal contacts and similar refractory migration defects to FAK–/– cells67. Hyperactive FAK in SHP2–/– cells results in an increase in the levels of Tyr12-phosphorylated α-actinin, which thereby reduces the crosslinking of stress fibres and prevents the maturation of focal contacts68. In SHP2–/– cells, there is a high level of focal contact turnover68, whereas in FAK–/– cells, focal contact turnover and maturation are inhibited8. So, both SHP2–/– and FAK–/– cells show an accumulation of immature focal contacts, but through different mechanisms. These studies support the importance of FAK expression and the precise temporal regulation of FAK activity as important factors that control the dynamics of focal contacts. Paxillin–/–, p130Cas–/– and Src –/– cells. In addition to FAK–/– and SHP2–/– cells, fibroblasts that contain null mutations for various other focal-contact-associated proteins also show altered dynamics of focal contact maturation, cell spreading defects and refractory cell motility responses (TABLE 1). Time-lapse analyses showed that the incorporation of labelled paxillin into focal contacts of FAK–/–, paxillin–/–, p130Cas–/– or SYF–/– (Src–/–, Yes–/– and Fyn–/–) cells did not significantly differ compared to normal fibroblasts6. However, the rate of focal contact disassembly was much slower in these null cells. Analyses of FAK–/– cells showed that disassembly was dependent on FAK Tyr397 phosphorylation; SYF–/– cells showed that Src kinase activity was required; and studies with paxillin–/– cells indicated that the integrity of the Tyr31 and Tyr118 paxillin phosphorylation sites were needed to promote focal contact turnover6. As the expression of constitutively active Src in FAK–/– cells can promote focal contact turnover and increased cell motility17,69, these combined analyses support the conclusion that, in normal cells, integrin- and FAK-mediated control of Src activity is a key event that promotes focal contact dynamics. FAK–Src and proteolysis. In addition to signalling events that are associated with the phosphorylation of α-actinin, p130Cas or paxillin, FAK–Src signalling can affect focal contact dynamics through the regulation of both extracellular and intracellular proteolytic events. Inhibition of FAK activity in human carcinoma cells or Src-transformed cells, or stable FAK re-expression in FAK–/– cells, can alter the expression and activation of 16,17,22 MATRIX METALLOPROTEINASES (MMPs) . The influence of FAK on MMP regulation is associated with signalling from Ras to ERK2 and from Rac to Jun N-terminal kinase (JNK). Activation of MMPs at the LEADING EDGE of migrating cells functions to promote matrix proteolysis, which leads to the extracellular release of integrin–matrix contacts and thereby facilitates focal contact turnover70. The intracellular linkage of focal contacts to the actin cytoskeleton is also regulated by calpain-mediated proteolysis71. Calpain can cleave constituents of focal VOLUME 6 | JANUARY 2005 | 6 1 © 2005 Nature Publishing Group REVIEWS Actin stress fibres Actin stress fibres Myosin Myosin Assembly Myosin Myosin ARP2/3 in ctin ROCK in ctin α-A N-WASP α-A in Cdc42 ctin FAK Tyr256 N-WASP MEMBRANE RUFFLE A process that is formed by the movement of lamellipodia that are in the dynamic process of folding back onto the cell body from which they previously extended. LD MOTIF A short sequence found within proteins that has the consensus sequence LDXLLXXL and functions as a protein-binding interface. MATRIX METALLOPROTEINASES Proteolytic enzymes that degrade the extracellular matrix and have important roles in tissue remodelling and tumour metastasis. LEADING EDGE The thin margin of a lamellipodium that spans the area of the cell from the plasma membrane to a depth of about 1 µm into the lamellipodium. 62 Reduced α-actinin crosslinking MLC phosphatase Increased α-actinin crosslinking P Tyr256 MLCK ↑ Rho p190 RhoGEF α-A Cdc42 Tyr12 P Tyr256 N-WASP P Focal contact GRAF Rho p190 RhoGEF FAK Focal contact Nucleus Figure 4 | Focal adhesion kinase promotes cytoskeletal fluidity. Stress fibres and cortical actin are continuously destabilized/stabilized by focal adhesion kinase (FAK)-regulated processes. Normally, the actin cytoskeleton exists in a semi-solid state, owing to a high degree of α-actinin-mediated crosslinking of stress fibres, which are tethered and exert tension at focal contacts (left panel). Conversion to a more soluble state (right panel) is promoted by FAK phosphorylation (P) on Tyr12 of α-actinin, which results in reduced crosslinking and the release of actin stress fibres from focal contacts. Cytoskeletal fluidity is also regulated by the effects of FAK on Rho-family GTPases and on the neuronal Wiskott–Aldrich syndrome protein (N-WASP). FAK phosphorylates Cdc42-activated N-WASP at Tyr256, thereby retaining phosphorylated N-WASP in the cytoplasm where it can affect ARP2/3-mediated actin polymerization. Through associations with Rho GTPase-activating proteins (GAPs) and Rho guanine nucleotide-exchange factors (GEFs), FAK can regulate actomyosin stress fibre polymerization. Reduced tension can be attributed in part to increased RhoGAP activity of GTPase regulator associated with FAK (GRAF). Conversely, FAK can promote cytoskeletal tension through phosphorylation and activation of p190 RhoGEF. Subsequent Rho activation indirectly regulates myosin light chain (MLC) phosphorylation through Rho-associated kinase (ROCK) phosphorylation of MLC phosphatase, which leads to increased MLC kinase (MLCK) activity through the downregulation of MLC phosphatase activity. contacts, such as talin and FAK, and calpain-4–/– cells have an increased number of peripheral focal contacts72. Calpain is not appropriately activated in FAK–/– cells21, and this defect might be due in part to ERK2 activation being required for calpain function73. Notably, FAK re-expression in FAK–/– cells promotes the formation of a complex between calpain, ERK2 and activated Src21. Restoration of calpain activity in FAK–/– cells requires specific FAK phosphorylation events: a form of FAK that is mutated at several phosphorylation sites can form a complex with calpain and ERK2, but it does not restore full calpain activity in contrast to cells in which wild-type FAK is re-expressed74. So, FAK signalling is connected to the increased turnover of focal contacts through calpain activation. As calpain is a Ca2+-dependent protease, and FAK activation is associated with local Ca2+-flux-induced disassembly of focal contacts75, the FAK–calpain linkage might be selectively activated at either cell protrusions or tail retraction sites in motile cells. | JANUARY 2005 | VOLUME 6 FAK effects on GTPases and actin The activity of Ras and the Rho-family GTPases Rho, Rac and Cdc42 is positively regulated by GEFs and negatively regulated by GAPs. As mentioned above, a number of studies have shown that FAK–Src-mediated phosphorylation events can lead to the activation of Ras–ERK2 and Rac–JNK signalling cascades to promote increased cell migration and invasion18. In a recently discovered mechanism, FAK overexpression facilitated the SH2-mediated binding and sequestering of p120 RasGAP, which diminished the association of p120 RasGAP with active Ras76 and thereby led to Ras activation. In FAK –/– cells, the intrinsic GTPase activity of RhoA is elevated8, and pharmacological inhibitors of Rho-associated kinase (ROCK) — a substrate of Rho — partially reverse the polarization defects of FAK–/– cells77. Integrin signalling can suppress RhoA activity by tyrosine phosphorylation of p190 RhoGAP (which increases its GAP activity)78. Likewise, stable www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS Table 1 | Phenotypes associated with null mutations in focal contact proteins Cell phenotypes Embryonic (lethality) day Focal contact formation Focal contact turnover Integrin-stimulated migration FAK tyrosine phosphorylation FAK–/– (p53–/–) 8.5 Increased immature Inhibited Inhibited NA SYF–/– 9.5 No change Inhibited Reduced pTyr397 reduced 123 p130Cas–/– 11.5–12.5 No change Inhibited Reduced No difference 124 Paxillin–/– 9.5 Increased size Decreased Inhibited pTyr397 reduced 125 Vinculin–/– 10.0 Decreased size ND Stimulated Increased activity 126 –/– Reference 5 PTPα None Delayed ND Reduced pTyr397 reduced 45 SHP2–/– 8.5–10.5 Increased immature Elevated Inhibited Increased activity in suspension 67,68 Calpain-4–/– 10.0 Larger Reduced Decreased No change 72 This table summarizes the phenotypes of fibroblasts that are derived from mice that are null for various proteins associated with focal contacts. FAK and paxillin are involved in the formation of focal contacts, whereas FAK, Src-family kinases (Src, Yes and Fyn; SYF), p130Cas and calpain are also involved in focal contact turnover. Except for vinculinnull cells, the lack of any of the above proteins results in impaired integrin-stimulated cell migration. FAK, focal adhesion kinase; NA, not applicable; ND, not determined; PTPα, protein tyrosine phosphatase-α; pTyr, phosphotyrosine; SHP2, Src-homology (SH)2-containing phosphotyrosine phosphatase-2. ARP2/3 COMPLEX A complex that consists of two actin-related proteins ARP2 and ARP3, along with five smaller proteins. When activated, the ARP2/3 complex binds to the side of an existing actin filament and nucleates the assembly of a new actin filament. The resulting branch structure is Y-shaped. FAK re-expression in FAK–/– cells decreased RhoA activity 8 and enhanced p190 RhoGAP tyrosine phosphorylation17. In other cell types, FAK activation and tyrosine phosphorylation are associated with RhoA activation and the formation of stress fibres18. This connection could be mediated by FAK binding to, and phosphorylating, p190 RhoGEF34. In neuronal development, FAK signalling through p190 RhoGEF controls axonal branching and synapse formation79. Although FAK-mediated activation of p190 RhoGEF is a direct route to RhoA activation, the formation of distinct signalling complexes will probably influence whether FAK activation leads to increased or decreased RhoA activity in cells (FIG. 4). In addition to affecting the activity of Ras, Rac and Rho, FAK can influence the function of Cdc42 through binding and phosphorylation of the Cdc42 effector Wiskott–Aldrich syndrome protein N-WASP (neuronal WASP)80. N-WASP, which, in contrast to its name, is ubiquitously expressed, regulates the actin cytoskeleton through activation of the ARP2/3 COMPLEX3. Interestingly, FAK only associates with Cdc42-activated N-WASP, and does not itself activate N-WASP. Although FAK phosphorylation of N-WASP at Tyr256 does not affect N-WASP activity towards ARP2/3, it does seem important for maintaining a cytoplasmic distribution of N-WASP and for promoting cell motility80. As Cdc42 regulates actin dynamics in cellular projections, the interaction of FAK with Cdc42-activated N-WASP might couple actin polymerization with membrane protrusion during cell motility (FIG. 4). FAK and microtubules GANGLIOSIDE An anionic glycosphingolipid that carries, in addition to other sugar residues, one or more sialic acid residues. LIPID RAFTS Lateral aggregates of cholesterol and sphingomyelin that are thought to occur in the plasma membrane. Integrating factors coordinate the regulation of microtubule structures and the actin cytoskeleton during cell motility. Microtubules are important in the establishment and maintenance of cell polarity, and the Rho effector Diaphanous (mDia) functions to stabilize microtubules at the leading edge of migrating cells81. Integrin-mediated activation of FAK is required for microtubule stabilization by the Rho–mDia signalling pathway7 (FIG. 5). This is partly the result of the FAK-regulated localization of a NATURE REVIEWS | MOLECUL AR CELL BIOLOGY lipid-raft marker, GANGLIOSIDE GM1, to the leading edge of motile cells. It is hypothesized that the lipid environment at the leading edge preferentially localizes microtubule capping or bridging proteins, which stabilize the association of microtubules with cortical receptors82. This regulation of a distinct membrane lipid environment by FAK or integrin signalling83 also functions to promote Rac signalling by maintaining a suitable lipid environment that facilitates the interaction of Rac and effectors such as p21-activated kinase (PAK)84. Interestingly, FAK-stimulated phosphorylation of Ser298 in MAPK/ERK kinase-1 (MEK1, also known as MAPK kinase-1) by PAK is a secondary route that leads to ERK2/MAPK activation85. In neuronal cells, a fraction of FAK colocalizes with a distinct microtubule structure that arises from microtubule-organizing centres and that extends around the nucleus in a branched fork-like form termed a microtubule fork86. Microtubule forks are believed to promote nuclear re-positioning in the direction of cell movement. Cyclin-dependent kinase-5 (CDK5) phosphorylates FAK at Ser732 in post-mitotic neurons, and antibodies that recognize Ser732-phosphorylated FAK specifically stain microtubule fork structures near the nucleus86. Neurons that are devoid of CDK5 or that express a FAK mutant in which Ser732 cannot be phosphorylated show a malformed microtubule fork, impaired nuclear movement and altered neuronal development positioning in vivo 86. Whereas the molecular mechanisms that link FAK Ser732 phosphorylation to the localization and organization of microtubule fork structures remain to be defined, this observation is the first of its kind and supports studies that link FAK phosphorylation to enhanced neuronal cell migration87. FAK and membrane composition The polarization of migrating cells requires membrane modification as well as changes in the underlying cytoskeleton. In addition to the role of FAK in the translocation of LIPID RAFT components7, FAK–Src signalling is involved in the modification of phosphatidylinositol lipids, and differentially phosphorylated lipid VOLUME 6 | JANUARY 2005 | 6 3 © 2005 Nature Publishing Group REVIEWS PtdIns α Formation of membrane proximal contacts Integrins β PtdIns(4)P Talin PIPKIγ PtdIns(4,5)P2 Vinculin FAK PI3K Paxillin p85 PtdIns(3,4,5)P3 Membrane component mDia GM1 Phospholipid modification by phosphorylation Stable lamellipodia Rac Lipid rafts Rac New membranes FAK and intercellular contacts Increased membrane fluidity Microtubule stabilization + + + Figure 5 | Focal adhesion kinase influences phospholipid and microtubule structures. The phospholipid kinases that have a role in the modification of phosphatidylinositol (PtdIns) cooperate with focal adhesion kinase (FAK) at several levels. Type I PtdIns phosphate kinase-γ (PIPKIγ) associates with FAK and talin, and promotes the conversion of PtdIns-4-phosphate (PtdIns(4)P) to PtdIns-4,5-bisphosphate (PtdIns(4,5)P2). PIPKIγ is phosphorylated by FAK, which leads to increased PIPKIγ activity and increased generation of PtdIns(4,5)P2. The binding of PtdIns(4,5)P2 to talin and vinculin is associated with the formation of focal contacts. PtdIns(4,5)P2 can be converted to PtdIns-3,4,5-trisphosphate (PtdIns(3,4,5)P3) by PtdIns 3-kinase (PI3K). The regulatory p85 subunit of PI3K binds to FAK at Tyr397, which leads to PI3K activation by FAK112. Directional motility requires the generation of phospholipid components such as PtdIns(4,5)P2 and PtdIns(3,4,5)P3. Integrin and FAK signalling also promote the translocation of specific components of lipid rafts to membranes. The stabilization of lipid rafts through integrin signalling facilitates the coupling of Rac to target proteins. FAK-mediated translocation of the lipid ganglioside GM1 to the membrane, which is mediated through the activation of the Rho GTPase effector Diaphanous (mDia), regulates microtubule polarity at the leading edge of motile cells. Microtubule polarization and Rac activation contribute to the formation of membrane ruffles and stable lamellipodia. ADHERENS JUNCTION A cell–cell adhesion complex that contains classical cadherins and catenins that are attached to cytoplasmic actin filaments. TIGHT JUNCTION A circumferential ring at the apex of epithelial cells that seals adjacent cells to one another. Tight junctions regulate solute and ion flux between adjacent epithelial cells. 64 phosphorylated by a FAK–Src complex, which facilitates increased PIPKIγ activity (and, therefore, increased production of PtdIns(4,5)P2) and increased PIPKIγ association with talin91. In this manner, FAK signalling is connected to the formation of focal contacts and the spatial regulation of PtdIns(4,5)P2 generation. However, the integrin and PIPKIγ binding sites within the talin FERM domain overlap, which implies that PIPKIγ binding might displace talin from integrin tails92. To this end, FAK-enhanced Src-mediated phosphorylation of PIPKIγ on Tyr644 creates a high affinity binding site for the talin FERM domain, which displaces β-integrin binding from talin FERM93. So, although FAK–Src activity could promote the production of PtdIns(4,5)P2 and the formation of focal contacts by enhancing the activity of PIPKIγ, subsequent phosphorylation of PIPKIγ by activated Src might break the talin–integrin linkage and promote the turnover of focal contacts. intermediates function as binding sites for signalling proteins that are involved in the formation of focal contacts (FIG. 5). Phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) binds to and controls the assembly of proteins such as α-actinin, vinculin and talin into focal contacts2. As the binding of the talin FERM domain to β-integrin cytoplasmic tails is enhanced by PtdIns(4,5)P2 (REF. 88), and the talin rod domain binds vinculin and actin89, a link between integrins, focal contact formation and the actin cytoskeleton is established. The type I phosphatidylinositol phosphate kinase-γ (PIPKIγ) is an enzyme that makes PtdIns(4,5)P2 and it is targeted to focal contacts by an association with the talin FERM domain90. PIPKIγ is | JANUARY 2005 | VOLUME 6 Another biological context in which FAK signalling has been associated with the formation or turnover of contacts is cadherin-based cell–cell junctions. Cadherins are transmembrane proteins that mediate Ca2+-dependent homophilic protein–protein attachments between cells and that are also linked to the actin cytoskeleton through interaction with α- or βcatenins94. Downregulation of E-cadherin-based ADHERENS JUNCTIONS is a hallmark of malignant and invasive carcinomas, and the activity of the FAK–Src complex promotes the disruption of colon carcinoma cell homotypic adhesions95. Importantly, expression of a FAK protein that is mutated at five tyrosine phosphorylation sites (Tyr407, 576, 577, 861 and 925) blocked the Src-mediated disruption of colon carcinoma E-cadherin-based contacts, thereby implying that phosphorylation-dependent signalling through FAK was required96. In an opposite manner, overexpression of a kinase-defective mutant of FAK blocked the accumulation of peripheral E-cadherin in endothelial cells that were subjected to a hyperosmolar challenge (a stimulus that promotes increased E-cadherin-based TIGHT97 JUNCTION barrier formation) . These results imply that FAK signalling has a role in both the formation and turnover of E-cadherin-based contacts. As opposed to E-cadherin function, N-cadherin expression in carcinoma cells is generally associated with a scattered morphology and a migratory or invasive phenotype. Antisense and DOMINANT-NEGATIVE inhibition of FAK showed that FAK expression and activity were needed for the formation of N-cadherin-based cell–cell contacts in HeLa cells19. However, in contradiction, the above study also found that cells with less FAK expression and reduced N-cadherin-mediated cell–cell contacts exhibited ‘increased’ motility when plated as individual cells on a collagen matrix. Whereas much remains to be determined about the molecular role of FAK in either the dissolution or formation of cadherinbased contacts, it is intriguing that the findings so far are somewhat similar to the bi-functional role of FAK in focal contact dynamics. www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS FAK as a biosensor Early studies with integrins found that they ‘sensed’ environmental cues and functioned to control anchoragedependent cell proliferation and survival98. Integrins are also intimately involved in the conversion of physical signals, such as contractile forces or external mechanical perturbations, into chemical signalling events, and FAK activation is an important component of this ‘mechanosensing’ by cells20. Early observations showed that FAK could be activated by TANGENTIAL FLUID SHEAR STRESS of endothelial cells and that this was associated with the formation of a FAK–Src signalling complex, FAK Tyr925 phosphorylation, and the downstream activation of ERK2 and JNK18. In a process known as mechanotaxis, endothelial cells that encounter shear stress will initiate focal contact remodelling and cell migration in the direction of the flow. Under these conditions, phosphorylation of FAK is enhanced at the leading edge of motile cells99. Another means of ‘mechanoperception’ is the ability of cells to sense the ‘rigidity’ of the surrounding ECM, as cells will preferentially migrate towards areas of higher substrate rigidity in a process known as durotaxis. Studies have shown that this response requires FAK expression and that FAK–/– cells are insensitive to changes in substrate flexibility100. Interestingly, whereas expression of a Tyr397Phe mutant of FAK does not rescue the overall migration speed or directional motility persistence defects of FAK–/– cells23,64,101, cells expressing this mutant exhibited similar durotaxis responses to wild-type cells100. Although it remains to be determined whether intrinsic FAK catalytic activity or the role of FAK as a scaffolding protein at focal contacts is the determining factor for the durotaxis response, this observation remains one of the few examples in which Tyr397 FAK phosphorylation was not required for a signalling response. Moving in three dimensions DOMINANT NEGATIVE A defective protein that retains interaction capabilities and so competes with normal proteins, thereby impairing protein function. TANGENTIAL FLUID SHEAR STRESS A planar force exerted by the friction of a flowing substance — for example, forces experienced by endothelial cells as blood flows through capillaries. CYTOTROPHOBLAST The inner trophoblastic layer of cells that give rise to the syncytiotrophoblast facing the maternal circulation and constitute a layer through which all substances must pass from the mother to the fetus. As adherent cells in culture readily make focal contacts, these points of cell adhesion undergo a maturation process to form fibrillar adhesions and they will form three-dimensional (3D) adhesions when cells are placed in a 3D environment102. FAK–/– fibroblasts fail to properly remodel focal contacts into fibrillar adhesions103 and FAK–/– endothelial cells do not form tubule structures when grown in a 3D matrix environment13. Interfering with FAK activity prevents tubule formation in human brain microvascular endothelial cells14, and gain-of-function experiments with FAK–/– fibroblasts show that the formation of fibrillar adhesions requires phosphorylation of Tyr397, FAK catalytic activity and FAK scaffolding functions103. Similar to the maturation process of fibrillar and 3D adhesions, FAKdependent matrix organization is also observed during dorsal forebrain development in mice, as glial cells that lack FAK exhibit basement-membrane formation defects104. Although immunostaining of cells that were grown in 3D did not reveal enhanced Tyr397 phosphorylation of FAK compared with the levels found in cells forming NATURE REVIEWS | MOLECUL AR CELL BIOLOGY 2D focal contacts105, functional experiments with human lung fibroblasts106, breast carcinoma cells107 or normal CYTOTROPHOBLASTS108 have supported the importance of FAK Tyr397 phosphorylation in promoting cell survival, cell proliferation and cell invasion, respectively, in 3D cell culture. As increased FAK phosphorylation of Tyr397 is associated with the elevated rigidity of a 3D collagen matrix107, it is likely that factors such as integrin engagement and cell tension are needed to promote FAK activity in 3D. Integrin signalling is also important in the formation of tumour cell invadopodia — 3D cell extensions that are enriched in MMPs and that promote tumour cell invasion through matrix barriers109. FAK–Src signalling has an important role in promoting invadopodia formation110 and lung carcinoma cell invasion22, in part through the induction of Rac activity17,49. FAK–Src signalling leads to the elevated expression and secretion of MMPs and is associated with a metastatic tumour cell phenotype16. As FAK expression is correlated with increased tumour cell malignancy12, the importance of FAK catalytic activity in promoting cell motility and invasion17,64 make it an attractive target for potential therapeutic intervention. Conclusions and perspectives The number of different signalling connections that have been characterized for FAK has expanded greatly over the past 5 years. In this review, we have highlighted the diversity of FAK-signalling inputs and outputs and the molecular mechanisms that connect FAK to both the assembly and disassembly of focal contacts. It is in this unique signalling position that FAK can exert control over cytoskeletal or cell adhesion dynamics and, therefore, cell motility. Continued efforts will be needed to understand the regulatory factors that influence whether FAK–Src signalling is coupled to the assembly or disassembly of cell adhesion sites in 2D and 3D, and how FAK targeting to different cellular locations influences these processes. As such, it is likely that the many interactions and phosphorylation events that are associated with FAK are transient events and possibly occur in a defined sequence as adhesions transit from assembly to disassembly during cell migration. Additionally, as structural studies have provided important information about the function of the FAT domain of FAK, similar studies are needed to understand the role of the FAK FERM domain. The finding that exogenous overexpression of the FAK FERM domain can inhibit cell motility24 might be associated with FERM-mediated inhibition of FAK catalytic activity44, so it is also possible that the FAK FERM domain functions to target FAK to distinct cellular sites that are involved in growth factor signalling or GPCR signalling events23,24. To this end, sumoylation can occur within the FAK FERM domain, which promotes FAK activation27, a fraction of FAK can translocate to the nucleus28,111, and FAK signalling can influence the expression of transcription factors30. How — if at all — these events are related to the ability of FAK to promote cell polarization and motility is the subject of active investigation. VOLUME 6 | JANUARY 2005 | 6 5 © 2005 Nature Publishing Group REVIEWS It is also notable that the FERM domain of FAK can localize to cell–cell junctions111, whereas the FAT domain is associated with focal contacts in epithelial cells. It is possible that the FAK-mediated regulation of cadherin-based cell contacts might be distinguished through the differential FERM- or FAT-mediated targeting of FAK to distinct intracellular sites. As many of the phenotypes that are associated with FAK have been elucidated using overexpression studies, the development of pharmacological inhibitors to FAK and analyses of catalytically-defective FAK mutants in FAK–/– cells will 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 66 Brakebusch, C. & Fassler, R. The integrin–actin connection, an eternal love affair. EMBO J. 22, 2324–2333 (2003). DeMali, K. A., Wennerberg, K. & Burridge, K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15, 572–582 (2003). Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). Parsons, J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 116, 1409–1416 (2003). Provides a good overview of the early studies on FAK. Ilic, D. et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 (1995). Shows that null mutation of FAK results in defects in embryonic morphogenesis, and that FAK-null cells show enhanced focal-contact formation and cell motility defects in culture. Webb, D. J. et al. FAK–Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 6, 154–161 (2004). Palazzo, A. F., Eng, C. H., Schlaepfer, D. D., Marcantonio, E. E. & Gundersen, G. G. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303, 836–839 (2004). Provides evidence that FAK promotes cell polarization through the stabilization of microtubules at leading edges of motile cells. Ren, X. et al. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J. Cell Sci. 113, 3673–3678 (2000). Agochiya, M. et al. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene 18, 5646–5653 (1999). Bhattacharjee, A. et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl Acad. Sci. USA 98, 13790–13795 (2001). Yeoh, E. J. et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell 1, 133–143 (2002). Cance, W. G. et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin. Cancer Res. 6, 2417–2423 (2000). Ilic, D. et al. Focal adhesion kinase is required for blood vessel morphogenesis. Circ. Res. 92, 300–307 (2003). Haskell, H. et al. Focal adhesion kinase is expressed in the angiogenic blood vessels of malignant astrocytic tumors in vivo and promotes capillary tube formation of brain microvascular endothelial cells. Clin. Cancer Res. 9, 2157–2165 (2003). Hauck, C. R., Hsia, D. A., Ilic, D. & Schlaepfer, D. D. v-Src SH3-enhanced interaction with focal adhesion kinase at β1 integrin-containing invadopodia promotes cell invasion. J. Biol. Chem. 277, 12487–12490 (2002). Hauck, C. R., Hsia, D. A., Puente, X. S., Cheresh, D. A. & Schlaepfer, D. D. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 21, 6289–6302 (2002). Hsia, D. A. et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160, 753–767 (2003). This reference, together with reference 69, shows that constitutively active Src can bypass the need for FAK in promoting the turnover of focal contacts. probably yield valuable insights into the role of FAK as an essential scaffolding protein or as an active contributor to the formation of a FAK–Src signalling complex. Note added in proof V. Brunton’s personal communication has now been accepted for publication: Brunton, V. G. et al. Identification of Src-specific phosphorylation site on FAK: dissection of the role of Src SH2 and catalytic functions and their consequences for tumour cell behaviour. Cancer Res. (in the press). 18. Schlaepfer, D. D., Mitra, S. K. & Ilic, D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta 1692, 77–102 (2004). Provides a solid review on the role of FAK during embryonic development. 19. Yano, H. et al. Roles played by a subset of integrin signaling molecules in cadherin-based cell–cell adhesion. J. Cell Biol. 166, 283–295 (2004). 20. Katsumi, A., Orr, A. W., Tzima, E. & Schwartz, M. A. Integrins in mechanotransduction. J. Biol. Chem. 279, 12001–12004 (2004). 21. Carragher, N. O., Westhoff, M. A., Fincham, V. J., Schaller, M. D. & Frame, M. C. A novel role for FAK as a proteasetargeting adaptor protein. Regulation by p42 ERK and Src. Curr. Biol. 13, 1442–1450 (2003). 22. Hauck, C. R. et al. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factorstimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 61, 7079–7090 (2001). 23. Sieg, D. J. et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nature Cell Biol. 2, 249–256 (2000). 24. Streblow, D. N. et al. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J. Biol. Chem. 278, 50456–50465 (2003). Together with reference 23, this paper shows that the FAK FERM domain has important roles in promoting growth-factor-stimulated and G-proteinstimulated cell motility. 25. Chen, R. et al. Regulation of the PH-domain-containing tyrosine kinase Etk by focal adhesion kinase through the FERM domain. Nature Cell Biol. 3, 439–444 (2001). 26. Poullet, P. et al. Ezrin interacts with focal adhesion kinase and induces its activation independently of cell–matrix adhesion. J. Biol. Chem. 276, 37686–37691 (2001). 27. Kadare, G. et al. PIAS1-mediated sumoylation of focal adhesion kinase activates its autophosphorylation. J. Biol. Chem. 278, 47434–47440 (2003). Shows that sumoylation of FAK within the FERM domain is associated with catalytic activation and preferential nuclear localization. 28. Jones, G. & Stewart, G. Nuclear import of N-terminal FAK by activation of the FcεRI receptor in RBL-2H3 cells. Biochem. Biophys. Res. Comm. 314, 39–45 (2004). 29. McKean, D. M. et al. FAK induces expression of Prx1 to promote tenascin-C-dependent fibroblast migration. J. Cell Biol. 161, 393–402 (2003). 30. Zhao, J. et al. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol. Cell 11, 1503–1515 (2003). 31. Hanks, S. K., Ryzhova, L., Shin, N. Y. & Brabek, J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. 8, 982–996 (2003). 32. Chodniewicz, D. & Klemke, R. L. Regulation of integrinmediated cellular responses through assembly of a CAS/Crk scaffold. Biochim. Biophys. Acta. 1692, 63–76 (2004). 33. Schaller, M. D. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta. 1540, 1–21 (2001). 34. Zhai, J. et al. Direct interaction of focal adhesion kinase with p190RhoGEF. J. Biol. Chem. 278, 24865–24873 (2003). | JANUARY 2005 | VOLUME 6 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. Together with reference 79, shows that FAK can directly activate Rho through binding and phosphorylation of a GEF, and that this activation regulates axonal branching. Toutant, M. et al. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol. Cell. Biol. 22, 7731–7743 (2002). Liu, E., Cote, J. F. & Vuori, K. Negative regulation of FAK signaling by SOCS proteins. EMBO J. 22, 5036–5046 (2003). This paper established a link between FAK activation, phosphorylation of Tyr397 and subsequent degradation of FAK. Avraham, H., Park, S. Y., Schinkmann, K. & Avraham, S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal 12, 123–133 (2000). Klingbeil, C. K. et al. Targeting Pyk2 to β1-integrincontaining focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J. Cell Biol. 152, 97–110 (2001). Lim, Y. et al. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts. J. Biol. Chem. 279, 29060–29065 (2004). Gabarra-Niecko, V., Keely, P. J. & Schaller, M. D. Characterization of an activated mutant of focal adhesion kinase: ‘SuperFAK’. Biochem. J. 365, 591–603 (2002). Nowakowski, J. et al. Structures of the cancer-related Aurora-A, FAK, and EphA2 protein kinases from nanovolume crystallography. Structure (Camb.) 10, 1659–1667 (2002). Abbi, S. et al. Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell 13, 3178–3191 (2002). Medley, Q. G. et al. Signaling between focal adhesion kinase and Trio. J. Biol. Chem. 278, 13265–13270 (2003). Cooper, L. A., Shen, T. L. & Guan, J. L. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol. Cell. Biol. 23, 8030–8041 (2003). Zeng, L. et al. PTPα regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J. Cell Biol. 160, 137–146 (2003). Chiarugi, P. et al. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 161, 933–944 (2003). Arias-Salgado, E. G. et al. Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl Acad. Sci. USA 100, 13298–13302 (2003). Shows that selected β-integrin subunits can bind and activate Src in the absence of a contribution from FAK. Turner, C. E. Paxillin and focal adhesion signalling. Nature Cell Biol. 2, 231–236 (2000). Cho, S. Y. & Klemke, R. L. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J. Cell Biol. 156, 725–736 (2002). Brabek, J. et al. CAS promotes invasiveness of Src-transformed cells. Oncogene 23, 7406–7415 (2004). Schaller, M. D. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20, 6459–6472 (2001). Subauste, M. C. et al. Vinculin modulation of paxillin–FAK interactions regulates ERK to control survival and motility. J. Cell Biol. 165, 371–381 (2004). www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group REVIEWS 53. Hayashi, I., Vuori, K. & Liddington, R. C. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nature Struct. Biol. 9, 101–106 (2002). 54. Liu, G., Guibao, C. D. & Zheng, J. Structural insight into the mechanisms of targeting and signaling of focal adhesion kinase. Mol. Cell. Biol. 22, 2751–2760 (2002). 55. Gao, G. et al. NMR solution structure of the focal adhesion targeting domain of focal adhesion kinase in complex with a paxillin LD peptide: evidence for a two-site binding model. J. Biol. Chem. 279, 8441–8451 (2004). 56. Katz, B. Z. et al. Targeting membrane-localized focal adhesion kinase to focal adhesions: roles of tyrosine phosphorylation and SRC family kinases. J. Biol. Chem. 278, 29115–29120 (2003). 57. Prutzman, K. C. et al. The focal adhesion targeting domain of focal adhesion kinase contains a hinge region that modulates tyrosine 926 phosphorylation. Structure (Camb.) 12, 881–891 (2004). References 53, 54, 55 and 57 provide structural analyses of the FAK FAT domain and the paxillin LD peptide binding, and show that Tyr925 phosphorylation might require conformational alterations in the FAT domain. 58. Ma, A., Richardson, A., Schaefer, E. M. & Parsons, J. T. Serine phosphorylation of focal adhesion kinase in interphase and mitosis: a possible role in modulating binding to p130Cas. Mol. Biol. Cell 12, 1–12 (2001). 59. Hunger-Glaser, I., Fan, R. S., Perez-Salazar, E. & Rozengurt, E. PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J. Cell Physiol. 200, 213–222 (2004). 60. Liu, Z. X., Yu, C. F., Nickel, C., Thomas, S. & Cantley, L. G. Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin–focal adhesion kinase association. J. Biol. Chem. 277, 10452–10458 (2002). 61. Ishibe, S., Joly, D., Zhu, X. & Cantley, L. G. Phosphorylation-dependent paxillin–ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell 12, 1275–1285 (2003). 62. Kirchner, J., Kam, Z., Tzur, G., Bershadsky, A. D. & Geiger, B. Live-cell monitoring of tyrosine phosphorylation in focal adhesions following microtubule disruption. J. Cell Sci. 116, 975–986 (2003). 63. Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116, 4605–4613 (2003). 64. Sieg, D. J., Hauck, C. R. & Schlaepfer, D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112, 2677–2691 (1999). 65. Rajfur, Z., Roy, P., Otey, C., Romer, L. & Jacobson, K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nature Cell Biol. 4, 286–293 (2002). 66. Izaguirre, G. et al. The cytoskeletal/non-muscle isoform of α-actinin is phosphorylated on its actin-binding domain by the focal adhesion kinase. J. Biol. Chem. 276, 28676–28685 (2001). 67. Yu, D. H., Qu, C. K., Henegariu, O., Lu, X. & Feng, G. S. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J. Biol. Chem. 273, 21125–21131 (1998). 68. Von Wichert, G., Haimovich, B., Feng, G. S. & Sheetz, M. P. Force-dependent integrin–cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22, 5023–5035 (2003). Together with reference 67, this study shows that null mutation of SHP2 results in FAK hyperactivation, elevated α-actinin phosphorylation, and the failure to promote the maturation of integrin–cytoskeletal linkages. 69. Moissoglu, K. & Gelman, I. H. v-Src rescues actin-based cytoskeletal architecture and cell motility and induces enhanced anchorage independence during oncogenic transformation of focal adhesion kinase-null fibroblasts. J. Biol. Chem. 278, 47946–47959 (2003). 70. Visse, R. & Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92, 827–839 (2003). 71. Bhatt, A., Kaverina, I., Otey, C. & Huttenlocher, A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci. 115, 3415–3425 (2002). 72. Dourdin, N. et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem. 276, 48382–48388 (2001). 73. Cuevas, B. D. et al. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 22, 3346–3355 (2003). 74. Westhoff, M. A., Serrels, B., Fincham, V. J., Frame, M. C. & Carragher, N. O. Src-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol. Cell. Biol. 24, 8113–8133 (2004). 75. Giannone, G. et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J. Biol. Chem. 279, 28715–28723 (2004). 76. Hecker, T. P., Ding, Q., Rege, T. A., Hanks, S. K. & Gladson, C. L. Overexpression of FAK promotes Ras activity through the formation of a FAK/p120RasGAP complex in malignant astrocytoma cells. Oncogene 23, 3962–3971 (2004). 77. Chen, B. H., Tzen, J. T., Bresnick, A. R. & Chen, H. C. Roles of Rho-associated kinase and myosin light chain kinase in morphological and migratory defects of focal adhesion kinase-null cells. J. Biol. Chem. 277, 33857–33863 (2002). 78. Arthur, W. T., Petch, L. A. & Burridge, K. Integrin engagement suppresses RhoA activity via a c-Srcdependent mechanism. Curr. Biol. 10, 719–722 (2000). 79. Rico, B. et al. Control of axonal branching and synapse formation by focal adhesion kinase. Nature Neurosci. 7, 1059–1069 (2004). 80. Wu, X., Suetsugu, S., Cooper, L. A., Takenawa, T. & Guan, J. L. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J. Biol. Chem. 279, 9565–9576 (2004). 81. Palazzo, A. F., Cook, T. A., Alberts, A. S. & Gundersen, G. G. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nature Cell Biol. 3, 723–729 (2001). 82. Gundersen, G. G., Gomes, E. R. & Wen, Y. Cortical control of microtubule stability and polarization. Curr. Opin. Cell Biol. 16, 106–112 (2004). 83. del Pozo, M. A. et al. Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842 (2004). 84. del Pozo, M. A., Price, L. S., Alderson, N. B., Ren, X. D. & Schwartz, M. A. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19, 2008–2014 (2000). 85. Slack-Davis, J. K. et al. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. J. Cell Biol. 162, 281–291 (2003). 86. Xie, Z. et al. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114, 469–482 (2003). 87. Ivankovic-Dikic, I., Gronroos, E., Blaukat, A., Barth, B.-U. & Dikic, I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nature Cell Biol. 2, 574–581 (2000). 88. Calderwood, D. A. Integrin activation. J. Cell Sci. 117, 657–666 (2004). 89. Papagrigoriou, E. et al. Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. EMBO J. 23, 2942–2951 (2004). 90. Di Paolo, G. et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1γ by the FERM domain of talin. Nature 420, 85–89 (2002). 91. Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002). 92. Barsukov, I. L. et al. Phosphatidylinositol phosphate kinase type 1γ and β1-integrin cytoplasmic domain bind to the same region in the talin FERM domain. J. Biol. Chem. 278, 31202–31209 (2003). 93. Ling, K. et al. Tyrosine phosphorylation of type Iγ phosphatidylinositol phosphate kinase by Src regulates an integrin–talin switch. J. Cell Biol. 163, 1339–1349 (2003). Together with references 91 and 92, this reference shows that FAK–Src phosphorylation events function to control the composition of membrane lipids and the dynamics of focal contacts. 94. Wheelock, M. J. & Johnson, K. R. Cadherins as modulators of cellular phenotype. Ann. Rev. Cell Dev. Biol. 19, 207–235 (2003). 95. Irby, R. B. & Yeatman, T. J. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human NATURE REVIEWS | MOLECUL AR CELL BIOLOGY colon cancer and transformed rodent cells. Cancer Res. 62, 2669–2674 (2002). 96. Avizienyte, E. et al. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nature Cell Biol. 4, 632–638 (2002). 97. Quadri, S. K., Bhattacharjee, M., Parthasarathi, K., Tanita, T. & Bhattacharya, J. Endothelial barrier strengthening by activation of focal adhesion kinase. J. Biol. Chem. 278, 13342–13349 (2003). 98. Miranti, C. K. & Brugge, J. S. Sensing the environment: a historical perspective on integrin signal transduction. Nature Cell Biol. 4, E83–E90 (2002). 99. Li, S. et al. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc. Natl Acad. Sci. USA 99, 3546–3551 (2002). 100. Wang, H. B., Dembo, M., Hanks, S. K. & Wang, Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc. Natl Acad. Sci. USA 98, 11295–11300 (2001). Shows that FAK functions as an important environmental biosensor in promoting directional motility signals in response to changes in substrate flexibility. 101. Owen, J. D., Ruest, P. J., Fry, D. W. & Hanks, S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 19, 4806–4818 (1999). 102. Cukierman, E., Pankov, R. & Yamada, K. M. Cell interactions with three-dimensional matrices. Curr. Opin. Cell Biol. 14, 633–639 (2002). 103. Ilic, D. et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J. Cell Sci. 117, 177–187 (2004). 104. Beggs, H. E. et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40, 501–514 (2003). References 103 and 104 show that FAK has crucial roles in promoting 3D-matrix assembly and/or remodelling during development and in cell culture model systems. 105. Cukierman, E., Pankov, R., Stevens, D. R. & Yamada, K. M. Taking cell–matrix adhesions to the third dimension. Science 294, 1708–1712 (2001). 106. Xia, H., Nho, R. S., Kahm, J., Kleidon, J. & Henke, C. A. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a β1 integrin viability signaling pathway. J. Biol. Chem. 279, 33024–33034 (2004). 107. Wozniak, M. A., Desai, R., Solski, P. A., Der, C. J. & Keely, P. J. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 163, 583–595 (2003). 108. Ilic, D. et al. Plasma membrane-associated pY397FAK is a marker of cytotrophoblast invasion in vivo and in vitro. Am. J. Pathol. 159, 93–108 (2001). 109. Bowden, E. T., Coopman, P. J. & Mueller, S. C. Invadopodia: unique methods for measurement of extracellular matrix degradation in vitro. Methods Cell Biol. 63, 613–627 (2001). 110. Hauck, C. R., Hunter, T. & Schlaepfer, D. D. The v-Src SH3 domain facilitates a cell adhesion-independent association with focal adhesion kinase. J. Biol. Chem. 276, 17653–17662 (2001). 111. Stewart, A., Ham, C. & Zachary, I. The focal adhesion kinase amino-terminal domain localises to nuclei and intercellular junctions in HEK 293 and MDCK cells independently of tyrosine 397 and the carboxy-terminal domain. Biochem. Biophys. Res. Comm. 299, 62–73 (2002). 112. Chen, H. C., Appeddu, P. A., Isoda, H. & Guan, J. L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3kinase. J. Biol. Chem. 271, 26329–26334 (1996). 113. Calderwood, D. A. & Ginsberg, M. H. Talin forges the links between integrins and actin. Nature Cell Biol. 5, 694–697 (2003). 114. Zheng, C. et al. Differential regulation of Pyk2 and focal adhesion kinase (FAK). J. Biol. Chem. 273, 2384–2389 (1998). VOLUME 6 | JANUARY 2005 | 6 7 © 2005 Nature Publishing Group REVIEWS 115. Wang, Q. et al. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol. 160, 565–575 (2003). 116. Sieg, D. J. et al. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectinstimulated signaling events but Pyk2 does not fully function to enhance FAK– cell migration. EMBO J. 17, 5933–5947 (1998). 117. Lakkakorpi, P. T., Bett, A. J., Lipfert, L., Rodan, G. A. & Duong, L. T. Pyk2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 278, 11502–11512 (2003). 118. Lev, S. et al. Identification of a novel family of targets of Pyk2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell. Biol. 19, 2278–2288 (1999). 119. Benbernou, N., Muegge, K. & Durum, S. K. Interleukin (IL)-7 induces rapid activation of Pyk2, which is bound to Janus kinase 1 and IL-7Rα. J. Biol. Chem. 275, 7060–7065 (2000). 120. Okigaki, M. et al. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl Acad. Sci. USA 100, 10740–10745 (2003). 68 121. 122. 123. 124. 125. 126. Shows that null mutation of the FAK-related kinase PYK2 results in integrin and chemokinestimulated motility defects of macrophages that are not functionally compensated by FAK expression. Watson, J. M. et al. Inhibition of the calcium-dependent tyrosine kinase (CADTK) blocks monocyte spreading and motility. J. Biol. Chem. 276, 3536–3542 (2001). Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. V. Absence of marginal zone B cells in Pyk2-deficient mice defines their role in the humoral response. Nature Immunol. 1, 31–36 (2000). Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A. & Soriano, P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 18, 2459–2471 (1999). Honda, H. et al. Cardiovascular anomaly, impaired actin bundling and resistance to Src- induced transformation in mice lacking p130Cas. Nature Genet. 19, 361–365 (1998). Hagel, M. et al. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22, 901–915 (2002). Xu, W., Baribault, H. & Adamson, E. D. Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–337 (1998). | JANUARY 2005 | VOLUME 6 Acknowledgements S. Mitra is supported by a fellowship from the California Tobacco-Related Disease Research Program and D. Schlaepfer is supported by grants from the National Cancer Institute. This is manuscript 16827-IMM from The Scripps Research Institute. Competing interests statement The authors declare no competing financial interests. Online links DATABASES The following terms in this article are linked online to: Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene PTK2 Swiss-Prot: http://www.expasy.ch calpain | CDK5 | Diaphanous | E-cadherin | ezrin | FAK | FIP200 | Fyn | N-cadherin | N-WASP | p130Cas | paxillin | PTPα | RhoA | SHP2 | Src | talin| TRIO | Yes FURTHER INFORMATION The Schlaepfer laboratory: http://www.scripps.edu/imm/schlaepfer/index1.htm Access to this interactive links box is free online. www.nature.com/reviews/molcellbio © 2005 Nature Publishing Group