* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Pyruvate Kinase
Signal transduction wikipedia , lookup
Photosynthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Paracrine signalling wikipedia , lookup
Mitochondrion wikipedia , lookup
Biochemical cascade wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Electron transport chain wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Blood sugar level wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
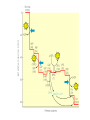
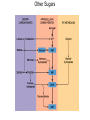
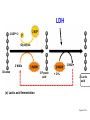
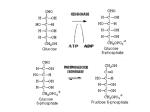
glyceraldehyde-3-phosphate NAD+ + Pi Glyceraldehyde-3-phosphate Dehydrogenase NADH + H+ Recall that there are 2 G3P per glucose. 1,3-bisphosphoglycerate ADP Phosphoglycerate Kinase ATP 3-phosphoglycerate Phosphoglycerate Mutase 2-phosphoglycerate Enolase H2O phosphoenolpyruvate ADP Pyruvate Kinase ATP pyruvate Glyceraldehyde-3-phosphate Dehydrogenase H O NAD+ 1C H 2 C OH + Pi 2 CH OPO 2 3 3 glyceraldehyde3-phosphate OPO32 + H+ O NADH 1C H C 2 OH 2 CH OPO 2 3 3 1,3-bisphosphoglycerate Exergonic oxidation of the aldehyde in glyceraldehyde-3phosphate, to a carboxylic acid, drives formation of an acyl phosphate, a "high energy" bond (~P). This is the only step in Glycolysis in which NAD+ is reduced to NADH. Phosphoglycerate Kinase O OPO32 ADP ATP O O 1C H 2C OH 2 3 CH2OPO3 1,3-bisphosphoglycerate C 1 Mg 2+ H 2C OH 2 3 CH2OPO3 3-phosphoglycerate 7. Phosphorylation by Phosphoglycerate Kinase: 1,3-bisphosphoglycerate + ADP 3-phosphoglycerate + ATP The enzyme undergoes substrate-induced conformational change similar to that of Hexokinase. (hinge) Pi is transferred from substrate to ATP Phosphoglycerate Kinase O OPO32 ADP ATP O O 1C H 2C OH 2 3 CH2OPO3 1,3-bisphosphoglycerate C 1 Mg 2+ H 2C OH 2 3 CH2OPO3 3-phosphoglycerate 1, 3 BPG has higher phosphoryl potential that ATP such that Pi can be transferred to ATP. This is called substrate level phosphorylation: since Pi donor is a substrate with high phosphoryl potential. (differs from ETC making ATP.) Phosphoglycerate Mutase O O C 1 O O C 1 H 2C OH 2 3 CH2OPO3 H 2C OPO32 3 CH2OH 3-phosphoglycerate 2-phosphoglycerate 8. Isomerization by Phosphoglycerate Mutase: 3-phosphoglycerate 2-phosphoglycerate Phosphate is shifted from the OH on C3 to the OH on C2. isomerase enzyme that catalyzes the structural rearrangement of isomers. Mutase: enzyme that catalyzes the shifting of a functional group from one position to another within the same molecule EC 5 Isomerases EC 5.4 Intramolecular transferases EC 5.4.2 Phosphomutases that transfer phospho groups EC 5.4.2.1 D-phosphoglycerate 2,3-phosphomutase Phosphoglycerate Mutase O O C 1 H 2C OH 2 3 CH2OPO3 3-phosphoglycerate histidine O O H C 1 H 2C OPO3 3 CH2OH 2 2-phosphoglycerate An active site histidine side-chain participates in Pi transfer, by donating & accepting phosphate. The process involves a 2,3bisphosphate intermediate. Enz-His-Pi + 3PG < Enz-His + 2,3BPG Enz-His + 2,3BPG < Enz-His-Pi + 2PG 2, 3 BPG Pi donor and regenerated H3N+ COO C CH2 C HN HC CH NH O O C 1 H 2C OPO32 2 3 CH2OPO3 2,3-bisphosphoglycerate Enolase O O C 1 H 2 C OPO32 3 CH2OH H O O C C OH O O 1 OPO32 CH2OH C 2C OPO32 3 CH2 2-phosphoglycerate enolate intermediate phosphoenolpyruvate 9. Dehydration by Enolase: 2-phosphoglycerate phosphoenolpyruvate + H2O This dehydration reaction is Mg++-dependent. 2 Mg++ ions interact with oxygen atoms of the substrate carboxyl group at the active site. The Mg++ ions help to stabilize the enolate anion intermediate that forms when a Lys extracts H+ from C #2. Enolase O O C 1 H 2 C OPO32 3 CH2OH H O O C C OH O O 1 OPO32 CH2OH C 2C OPO32 3 CH2 2-phosphoglycerate enolate intermediate phosphoenolpyruvate 9. Dehydration by Enolase: 2-phosphoglycerate phosphoenolpyruvate + H2O enolate anion http://www.biochem.wisc.edu/faculty/rayment/lab/gallery_jpgs/enolase.jpg Pyruvate Kinase O O C 1 C 2 ADP ATP O O C 1 OPO32 3 CH2 phosphoenolpyruvate C 2 O 3 CH3 pyruvate 10. Phosphorylation by Pyruvate Kinase: phosphoenolpyruvate + ADP pyruvate + ATP Again a substrate-level phosphorylation with PEP have a high phosphoryl potential. Pyruvate Kinase O O C 1 C 2 ADP ATP O O C 1 OPO32 3 CH2 phosphoenolpyruvate C 2 O 3 CH3 pyruvate 10. Phosphorylation by Pyruvate Kinase: phosphoenolpyruvate + ADP pyruvate + ATP Pyruvate Kinase is highly regulated also! Allosterically regulated by ATP, if there is enough then we don’t need to go through glycolysis to make more! BUT it is also regulated by.. Alanine Pyruvate Kinase it is also regulated by.. Alanine transaminase So if there is a lot of alanine in the cell, it tells the cell that there is a lot of pyruvate, therefore enough ATP!!!! Pyruvate Kinase Pyruvate Kinase, the last step Glycolysis, is controlled in liver partly by modulation of the amount of enzyme. O O C 1 C 2 ADP ATP O O C 1 OPO32 3 CH2 phosphoenolpyruvate C 2 O 3 CH3 pyruvate High [glucose] within liver cells causes a transcription factor to activate transcription of the gene for Pyruvate Kinase. This facilitates converting excess glucose to pyruvate, which is metabolized to acetyl-CoA, the main precursor for synthesis of fatty acids, for long term energy storage. Pyruvate kinase Pyruvate kinase has at least three isozymes and one of them is liver-specific. The liver pyruvate kinase is being regulated differently than other tissue type. Pyruvate Kinase 3 isozymes ATP, AcSCoA, long chain fatty acids inhibit all isozymes L inhibited by phosphorylation by glucagon –activated cAMP-dependent protein kinase (low blood sugar cAMP) M activated by cAMP in response to epinephrine (Gprotein system) Regulation of pyruvate kinase cAMP dependent Flux through the Glycolysis pathway is regulated by control of 3 enzymes that catalyze spontaneous reactions: Hexokinase, Phosphofructokinase & Pyruvate Kinase. Local control of metabolism involves regulatory effects of varied concentrations of pathway substrates or intermediates, to benefit the cell. Global control is for the benefit of the whole organism, & often involves hormone-activated signal cascades. Liver cells have major roles in metabolism, including maintaining blood levels various of nutrients such as glucose. Thus global control especially involves liver. glyceraldehyde-3-phosphate NAD+ + Pi Glyceraldehyde-3-phosphate Dehydrogenase NADH + H+ Recall that there are 2 G3P per glucose. 1,3-bisphosphoglycerate ADP Phosphoglycerate Kinase ATP 3-phosphoglycerate Phosphoglycerate Mutase 2-phosphoglycerate Enolase H2O phosphoenolpyruvate ADP Pyruvate Kinase ATP pyruvate Glycolysis Balance sheet for ~P bonds of ATP: 2 How many ATP ~P bonds expended? ________ How many ~P bonds of ATP produced? (Remember there 4 are two 3C fragments from glucose.) ________ 2 Net production of ~P bonds of ATP per glucose: ________ http://www.youtube.com/watch?v=mmACA_eVLTE Balance sheet for ~P bonds of ATP: 2 ATP expended 4 ATP produced (2 from each of two 3C fragments from glucose) Net production of 2 ATP per glucose. Glycolysis - total pathway, omitting H+: glucose + 2 NAD+ + 2 ADP + 2 Pi 2 pyruvate + 2 NADH + 2 ATP In aerobic organisms: pyruvate produced in Glycolysis is oxidized to CO2 via Krebs Cycle (can also be stored as fatty acids) NADH produced in Glycolysis & Krebs Cycle is reoxidized via the respiratory chain, with production of much additional ATP. Glycolysis Enzyme/Reaction DGo' kJ/mol DG kJ/mol Hexokinase Phosphoglucose Isomerase -20.9 +2.2 -27.2 -1.4 Phosphofructokinase Aldolase Triosephosphate Isomerase -17.2 -25.9 +22.8 -5.9 +7.9 negative Glyceraldehyde-3-P Dehydrogenase & Phosphoglycerate Kinase -16.7 -1.1 Phosphoglycerate Mutase Enolase Pyruvate Kinase +4.7 -3.2 -23.0 -0.6 -2.4 -13.9 net -44.2 *Values in this table from D. Voet & J. G. Voet (2004) Biochemistry, 3rd Edition, John Wiley & Sons, New York, p. 613. Other Sugars Other Fates 2 Pyruvic acid Glucose Figure 6.8 Glycolysis, omitting H+: glucose + 2 NAD+ + 2 ADP + 2 Pi 2 pyruvate + 2 NADH + 2 ATP Fermentation, from glucose to lactate: glucose + 2 ADP + 2 Pi 2 lactate + 2 ATP Anaerobic catabolism of glucose yields only 2 “high energy” bonds of ATP. What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? NADH is energy - two possible fates: If O2 is available: NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation If O2 is not available (anaerobic conditions): NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? Pyruvate is also energy - two possible fates: If O2 is available pyruvate enters the mitochondria, where it undergoes further breakdown If O2 is not available (anaerobic conditions) fermentation occurs and pyruvate undergoes reduction Fermentation is an anaeorbic process and does not require oxygen. In humans, pyruvate is reduced to lactic acid during fermentation. What happens when oxygen is not available? Cells turn to fermentation. During fermentation, pyruvate formed by glycolysis is reduced to lactate. The reduction of pyruvate to lactate regenerates NAD+ from NADH. The NAD+ is free to pick up more electrons during early steps of glycolysis This keeps glycolysis going. Fermentation in Human Muscle Cells Human muscle cells can make ATP with and without oxygen They have enough ATP to support activities such as quick sprinting for about 5 seconds A secondary supply of energy (creatine phosphate) can keep muscle cells going for another 10 seconds To keep running, your muscles must generate ATP by the anaerobic process of fermentation Pyruvate is reduced by NADH, producing NAD+, which keeps glycolysis going In human muscle cells, lactic acid is a by-product 2 Pyruvate Glucose LDH 2 ADP+ 2 Glycolysis 2 NAD 2 NAD Glucose 2 Pyruvic acid + 2 H 2 Lactic acid (a) Lactic acid fermentation Figure 6.15a Lactate Dehydrogenase O O C C NADH + H+ NAD+ O O O C HC OH CH3 CH3 pyruvate lactate Skeletal muscles ferment glucose to lactate during exercise. Lactate released to the blood may be taken up by other tissues, or by skeletal muscle after exercise, and converted via Lactate Dehydrogenase back to pyruvate, which may be oxidized in Krebs Cycle or (in liver) converted to back to glucose via gluconeogenesis Lactate Dehydrogenase O O C C NADH + H+ NAD+ O O O C HC OH CH3 CH3 pyruvate lactate Lactate serves as a fuel source for cardiac muscle as well as brain neurons. Astrocytes, which surround and protect neurons in the brain, ferment glucose to lactate and release it. Lactate taken up by adjacent neurons is converted to pyruvate that is oxidized via Krebs Cycle. Pyruvate Decarboxylase Alcohol Dehydrogenase CO2 NADH + H+ NAD+ O O C C O CH3 pyruvate H O C CH3 acetaldehyde H H C OH CH3 ethanol Some anaerobic organisms metabolize pyruvate to ethanol, which is excreted as a waste product. NADH is converted to NAD+ in the reaction catalyzed by Alcohol Dehydrogenase. Advantages and Disadvantages of Fermentation Fermentation can provide a rapid burst of ATP in muscle cells, even when oxygen is in limited supply. Lactate, however, is toxic to cells. Initially, blood carries away lactate as it forms; eventually lactate builds up, lowering cell pH, and causing muscles to fatigue. Oxygen debt occurs, and the liver must reconvert lactate to pyruvate. Efficiency of Fermentation Two ATP produced during fermentation are equivalent to 14.6 kcal. Complete oxidation of glucose to CO2 and H2O represents a yield of 686 kcal per molecule of glucose. Thus, fermentation is only 2.1% efficient compared to cellular respiration. (14.6/686) x 100 = 2.1%