* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slides - Brown Computer Science
Histone acetylation and deacetylation wikipedia , lookup
Endomembrane system wikipedia , lookup
Magnesium transporter wikipedia , lookup
Purinergic signalling wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Phosphorylation wikipedia , lookup
Tyrosine kinase wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Biochemical cascade wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
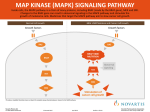
Signal transduction for mathematicians: examples from the JAK-STAT and Ras-Raf-Mek-Erk pathways. Terry Speed, WEHI BioInfoSummer 2006 1 Background and motivation In multicellular organisms, cells must communicate with each other and work together. Messages from outside cells have to be conveyed to headquarters - the nucleus in order to be acted upon through transcription. This talk is about that: intracellular signalling. This subject is important for cancer, immunology and development, and so of interest in biology generally. (Herceptin is a humanized monoclonal antibody to ErbB2, which in some breast cancers has a mutation which makes its tyrosine kinase is constitutively active.) This is often seen as an area where systems biology may contribute, and that’s one reason why I’ve become involved in it. Our examples are active in most mammalian tissues. 2 Intracellular signalling (aka signal transduction) These are the processes by which a cell converts one kind of signal or stimulus to another. They often involve a sequence of biochemical reactions inside the cell, which are carried out by enzymes and linked through second messengers (particular molecules). Such processes take place in as little time as a millisecond or as long as a few seconds. Slower processes are rarely referred to as signal transduction. (Wikipedia) 3 JAK-STAT signalling pathway http://www.genome.ad.jp/kegg/pathway/hsa/hsa04630.html 4 MAPK signalling pathway http://www.genome.ad.jp/dbget-bin/show_pathway?hsa04010+5594 5 MAPK signalling pathway (blow-up of a bit) http://www.genome.ad.jp/dbget-bin/show_pathway?hsa04010+5594 6 Cartoon indicating some steps and players JAK 7 Figure 5.1 The Biology of Cancer (© Garland Science 2007) My plan in these two talks In the first, to introduce the key notions and to some extent explain the nature of the Jak-Stat and Ras-Raf-Mek-Erk pathways, as background to the topic of computational models for them and similar pathways, and then In the second lecture, to describe some of the computational modelling involving these pathways with which I’ve been involved. 8 Growth factor a protein that is able to stimulate the growth and/or proliferation of a cell by binding to a cell surface receptor displayed by that cell. Examples include the Epidermal Growth Factor (EGF), Platelet Derived Growth Factor (PDGF), the Fibroblast Growth Factor (FGF), the Granulocyte-colony stimulating factor (G-CSF), and the Granulocyte-macrophage colony stimulating factor (GM-CSF). 9 Cytokines are secreted proteins that regulate a variety of biological functions by associating with transmembrane receptors at the cell’s surface to activate signal transduction pathways. Examples include the Interleukins: literally, between leukocytes (white blood cells); abbreviated IL-, e.g. IL1, etc; and the Interferons, proteins found to interfere with reproduction of viruses in cells, abbreviated IFN-, e.g. IFNγ, IFNα. Another famous cytokine is erythropoietin (EPO). 10 Receptors A receptor is a protein found on the plasma membrane or within a cell that is capable of specifically binding a signaling molecule (its ligand). Most types of receptors emit signals, such as those inducing cell proliferation in response to such binding. Examples include the estrogen receptor (ER), the PDGF receptor (PDGF-R) and the Epidermal Growth Factor receptor (EGF-R), The EGF-R family of receptors consists of four distinct proteins ErbB1 (EGF-R), ErbB2 (HER2, Neu), ErbB3 (HER3), and ErbB4 (HER4). They often bind ligands as heterodimeric receptors. 11 Phosphorylation is the covalent attachment of a phosphate group (PO3) to a substrate, usually a protein. This typically results in a functional change in the target protein by changing enzyme activity, cellular location or interaction with other proteins. A protein can be phosphorylated at a number of residues including serine (S), threonine (T) and tyrosine (Y). Many proteins have Phosphorylated serine multiple phosphorylation sites. 12 Kinases Are enzymes that covalently attaches phosphate groups to substrate molecules, often proteins, i.e. phosphorylate the substrate. Up to 30% of proteins may be modified by kinases, and the human genome contains about 500 kinases. Kinases act by removing a phosphate group (PO3) from ATP and covalently attaching it to one of the 3 amino acids (S, T, Y) that have a free hydroxyl group. Examples include the JAKs (see later), RAF (see later), AKT, ERK (also called MAPK, see later). Receptor tyrosine kinases (RTKs) have a transmembrane receptor with a tyrosine kinase domain protruding into the cytoplasm. Example: the EGFR. 13 Phosphatases enzymes that catalyse the removal of phosphate groups from phosphorylated substrates. More than 100 phosphotyrosine phosphatases have been found in the human genome, which remove the phosphate groups of the 90 tyrosine kinases present in our cells. Examples include alkaline phosphatase, PTEN, PP1, PP2A and the SHPs. 14 Relevance of the foregoing to intracellular signalling The presence or absence of the phosphate group on proteins, especially enzymes, is known to play a regulatory role in many biochemical and signal transduction pathways. Hence together, specialized kinases and phosphatases regulate enzymatic activity. 15 SH2 domains Src (“sarc”) Homology 2 (SH2) domains are protein modules of about 100 amino acids in size which are found in a large number of proteins involved in signal transduction. The function of SH2 domains is to specifically recognize the phosphorylated state of tyrosine residues, thereby allowing SH2 domain-containing proteins to localize to tyrosine-phosphorylated sites. It was established several years ago that specificity is conferred by the sequence context of the phosphotyrosine within the tyrosine-phosphorylated site, more specifically, by the three residues immediately C-terminal to the phosphotyrosine. 16 Attraction of signal-transducing proteins by phosphorylated receptors A constellation of phosphotyrosines can be formed after the EGFR binds to its ligand. Listed to the right are the different cytoplasmic proteins that can bind to these via their SH2 domain. 17 Figure 6.9b The Biology of Cancer (© Garland Science 2007) Receptor dimerization & transphosphorylation following ligand binding (EGFR works this way) 18 Figure 5.15 The Biology of Cancer (© Garland Science 2007) SH3 domains Src homology 3 domains bind specifically to certain proline-rich sequence domains in partner proteins. These proline-rich sequences thus serve as the ligands of the SH3 domains. An example of a protein with SH3 domains is the Growth factor receptor binding protein 2 (Grb2 “grab two”), which has two. 19 Adaptors As we will see later, certain proteins play a key role in signalling pathways by interacting with phosphorylated receptors or derived molecules. The do so by forming physical bridges between growth factor receptors and other key molecules, e.g Son of sevenless (Sos, see later). Such proteins are known as adaptors. Examples include the Src homology and collagen domain protein (Shc, “shick”) and Grb2 . Both contain SH2 domains, and as just noted, Grb2 contains two SH3 domains. 20 Transcription factors Proteins that are involved in regulating the transcription of genes, often by associating with sequences in the promoter region of the gene. Examples below include AP1, SP1, STAT1, MYC, JUN, FOS, ERG. These are the proteins that make things happen (growth, differentiation, migration, death, etc) though there are several other ways in which gene expression is regulated. 21 Quick general review before we turn to specifics JAK 22 Figure 5.1 The Biology of Cancer (© Garland Science 2007) The JAK-STAT pathway 23 The main players Initially JAK meant “Just Another Kinase” but these days the more sedate name “JAnus-like (tyrosine) Kinases” is given as the explanation of the acronym. There are 4 Jak genes: Jak1, Jak2, Jak3, Tyk2. They associate non-covalently with certain receptors, including those for interferon (IFN), erythropoietin (EPO) and thrombopoietin (TPO). STAT abbreviates: Signal Transduction and Activator of Transcription. These are latent transcription factors with SH2 domains that are activated by receptor associated JAK-mediated phosphorylation. The family includes STAT1, STAT2, and STAT 3 (and several others). 24 The JAK-STAT pathway 25 Figure 6.22 The Biology of Cancer (© Garland Science 2007) Caption to preceding figure Activation of signalling occurs when a cytokine binds to its receptor at the cell surface, leading to dimerisation of the receptor and activation of receptor-associated JAKs. Activated JAK proteins transphosphorylate one another as well as the C-terminal tails of the receptor. The resulting phosphotyrosines attract STAT proteins, which bind via their SH2 domains and become phosphorylated by the JAKs. Once phosphorylated, STAT proteins can dimerise, each using its SH2 domain to bind to the phosphotyrosine of its partner, and, upon transport into the nucleus, operate as transcription factors to induce the transcription of many cytokine-regulated genes. Here there are no adaptors and no signalling cascades. 26 Some other players SOCS: suppressor of cytokine signalling; SOCS1, SOCS2, … PIAS: protein inhibitors of activated STATs; PIAS1, etc SUMO: small ubiquitin-like modifier SHPs = SH2 containing phosphatases 27 Beyond transcription: negative feedback A) B) 28 Caption to right half of preceding figure JAK-STAT signal transduction is repressed by three distinct mechanisms. SHP-1 is constitutively expressed, and can dephosphorylate activated JAKs or receptors. PIAS proteins, also constitutively expressed, ligate SUMOs to STATs, thus inhibiting transcriptional activation. SOCS proteins are induced in response to cytokine signalling, and can inhibit JAK activity, or target signalling components for ubiquitination and subsequent proteolysis. 29 Interferons and their receptors β τ Type II γ γ ω IFNγ-R1 IFNγ-R2 IFNα-R1 IFNα-R2 α Type I δ ε κ ISGF-3 STAT1 STAT2 STAT1 P STAT2 P IRF-9 STAT1 STAT1 P 30 JAK-STAT signalling pathway http://www.genome.ad.jp/kegg/pathway/hsa/hsa04630.html 31 The Ras-Raf-Mek-Erk pathway 32 RAS Ras is the name of a protein, the gene that encodes it, and the family and superfamily of proteins to which it belongs. Proteins in the Ras family are very important molecular switches for a wide variety of signalling pathways. Ras is a G-protein: a regulatory GTP hydrolase that cycles between two conformations - an activated or inactivated form, RAS-GTP and RAS-GDP. It is activated by guanine exchange factors (GEFs, e.g. Sos), which are themselves activated by mitogenic signals and through feedback from Ras itself. It is inactivated by GTPase-activating proteins (GAPs, the most common being RasGAP), which increase the rate of GTP hydrolysis, returning RAS to its GDP-bound form. Ras is attached to the cell membrane by prenylation. 33 The Ras signalling sycle 34 Figure 5.30 The Biology of Cancer (© Garland Science 2007) Sos As just noted, Sos is a guanine nucleotide exchange protein capable of activating Ras. Important to our story is that it contains two distinct proline rich regions which can bind to an SH3 domain. Grb2 has two such domains, and serves as an adaptor linking phosphorylated EGFR to Ras as in the next figure. 35 How the Receptor-Sos-Ras cascade operates 36 Figure 6.12 The Biology of Cancer (© Garland Science 2007) RAF (also called c-RAF or Raf-1) is a protein kinase functioning in the MAPK/ERK pathway. RAF activation is a multi-step process that involves the dephosphorylation of inhibitory sites by Protein Phosphatase 2A (PP2A) as well as the phosphorylation of activating sites by PAK (p21rac/cdc42-activated kinase), Src-family and yet unknown kinases. We assume RAF phosphorylation is caused directly by a free Ras-GTP molecule. Activated RAF has an intrinsic kinase activity and it phosphorylates the MAPK/ERK Kinase, MEK. 37 MEK MAPK/ERK Kinase (MEK) is another mitogen activated protein kinase in the EGFR-ERK pathway. In its doubly phosphorlyated form, it can phosphorylate ERK. The requirement of double phosphorylation here and with ERK seems to be a device which permits activation to be switch-like. Although RAF is the predominant MEK activator, MEK can also be phosphorylated by other kinases, including MEKK. The interaction between Raf-1 and MEK is also regulated by RKIP (Raf kinase inhibitor protein), a protein that binds to Raf and MEK preventing their interaction. 38 ERK (extracellular signal-regulated kinase) = MAPK (mitogen-activated kinase) As just noted, doubly phosphorylated MEK phosphorylates ERK. In turn, ERK needs to be doubly phosphorylated to be activated. Activated ERK has many substrates in the cytosol, e.g. cytoskeletal proteins, phospholipase A2, and signalling proteins including tyrosine kinase receptors, estrogen receptors, Sos, STATs and others. ERK can also enter the nucleus to control gene expression by phosphorylating transcription factors such as Elk-1 and other Ets-family proteins, c-myc, c-Fos and c-Jun, the last two dimerising to form AP-1 (activation protein 1). There is a negative feedback loop from doubly phosphorylated ERK to Sos which causes the dissociation of Grb2-Sos from 39 the receptor complex. Simplified view of the pathway W. Kolch et al. / FEBS Letters 579 (2005) 1891–1895 40 Present animation here http://www.bio.davidson.edu/courses/Immunology/Flash/MAPK.html 41 MAPK signalling pathway http://www.genome.ad.jp/dbget-bin/show_pathway?hsa04010+5594 42 MAPK Pathway II 43 MAPK Pathway III 44 MAPK Pathway IV 45 MAPK Pathway V Nat Rev Mol Cell Biol, 2005 46 Cautionary notes (after RA Weinberg) 1. Although already complicated, this was the simplified view. Omitting details helps initially, but details usually matter. E.g. many of the players in this pathway have more than one alternative form (isozymes), and many are part of small families each slightly different from the others (splice variants). “In most cases. we are ignorant of the functional differences between the superficially similar proteins operating…” 2. Complex positive- and negative-feedback loops serve to amplify or dampen the signals fluxing through each of these pathways. Each component is a complex signal processing device that amplifies, attenuates and/or integrates the signals it receives from upstream in a pathway before passing them on to downstream targets. What is known is likely to be the tip of a much larger iceberg. 47 Cautionary notes (cont.) 3. The ability of proteins to transduce signals to one another is affected by their intracellular concentrations, postranslational modifications (such as phosphorylation) and intracellular localization. We have only begun to plumb the depths of this complexity. 4. None of these intracellular pathways operates in isolation. Instead, each is influenced by cross connections with other pathways, see next slide. 5. Even if these extensive cross connections did not exist, the endpoints of signaling - specific changes in cell phenotype - are the results of combinatorial interactions between multiple converging signalling pathways. The dimension of this complexity is baffling. 48 Two-dimensional signaling maps 49 Figure 6.32 The Biology of Cancer (© Garland Science 2007) Cautionary notes (end) 6. Our depiction of how signals are transmitted through a cell is likely to be fundamentally flawed. Each signaling cascade is likely to operate in a finely tuned dynamic equilibrium, where positive and negative regulators continuously counterbalance on another. Signalling input, e.g a mitogenic stimulus may operate like the plucking of a fibre in one part of a spider web, which results in small reverberations at distant parts throughout the web. Neither our language nor our mathematical representations suffice to clarify our understanding. 50 Acknowledgements WEHI Sam Wormald & many others UCB Sach Mukherjee ICGP project at LBNL Rich Neve, Paul Spellman & many others Robert A Weinberg’s fantastic book the biology of Cancer Garland Science, 2007 51