* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Weber et al. - 2000
Subventricular zone wikipedia , lookup
Axon guidance wikipedia , lookup
Convolutional neural network wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Multielectrode array wikipedia , lookup
Central pattern generator wikipedia , lookup
Development of the nervous system wikipedia , lookup
Nervous system network models wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Synaptic gating wikipedia , lookup
Circumventricular organs wikipedia , lookup
Electrophysiology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Efficient coding hypothesis wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Superior colliculus wikipedia , lookup
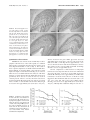
Experimental Glaucoma and Cell Size, Density, and Number in the Primate Lateral Geniculate Nucleus Arthur J. Weber,1 Hao Chen,1 William C. Hubbard,2 and Paul L. Kaufman2 PURPOSE. To examine the effects that elevated intraocular pressure (IOP), a glaucoma risk factor, has on the size, density, and number of neurons in the primate lateral geniculate nucleus (LGN). METHODS. The monkey model of experimental glaucoma was combined with standard histologic staining and analysis techniques. Fourteen animals were examined. RESULTS. Mean IOPs higher than 40 mm Hg for 2.5, 4, 8, and 24 weeks resulted in reductions of 10% to 58% in the cross-sectional areas of LGN neurons receiving input from the glaucomatous eye. Reductions for animals with lower mean IOPs (37 and 28 mm Hg) for 16 and 27 weeks were 16% and 30%, respectively. Neurons receiving input from the normal eye also were reduced in size (4 –26%). No differential effect in cell size was seen for magnocellular versus parvocellular neurons. Elevation of IOP resulted in an increase in cell density in all layers of the LGN. The increase was approximately two times greater in parvocellular (59%) than magnocellular (31%) layers. When corrected for volumetric shrinkage of the LGN, the estimated loss of neurons was approximately four times greater in the magnocellular than parvocellular layers (38% versus 10%). CONCLUSIONS. Elevation of IOP affects the size, density, and number of neurons in the LGN, and the volume of the nucleus itself. Although higher mean pressures (more than 40 mm Hg) reduce the period during which these changes occur, comparable damage can be achieved by even moderate (28 –37 mm Hg) levels of elevated IOP. On the basis of cell loss, elevation of IOP appears to have a more profound degenerative effect on the magnocellular than on the parvocellular regions of the LGN. (Invest Ophthalmol Vis Sci. 2000;41:1370 –1379) I n many vertebrates, the transfer of visual information from the eye to visual cortex involves the relay of signals through the dorsal lateral geniculate nucleus of the thalamus. In primates, the LGN comprises six distinct layers of neurons, each receiving input from a single eye. The two ventral layers contain relatively large neurons and are referred to as the magnocellular (M) layers, whereas the four dorsal layers contain smaller neurons, and are identified as the parvocellular (P) layers.1 Although visual information leaves the eye in several parallel streams, the retinogeniculate projection in primates typically is described as consisting of only two major pathways, the M-pathway representing the projection through the magnocellular layers and the P-pathway representing the projection through the parvocellular layers. Functionally, the M-pathway is thought to be concerned primarily with stimulus movement and form, whereas the P-pathway plays a role in the analysis of detail and color vision.2–5 In the visual system, as in other parts of the brain, neurons depend on connections with other neurons for proper function and survival. Trauma or disease that affects cells in one area typically also has a degenerative effect on neurons in associated areas.6 – 8 In primary open-angle glaucoma, a disease often characterized by elevated IOP, the primary site of damage appears to be the optic nerve in the region of the lamina cribrosa.9 Because the axons of retinal ganglion cells form the optic nerve, one consequence of glaucoma is the retrograde degeneration of ganglion cells within the retina itself. Although the degenerative effects that elevated IOP has on the optic nerve10,11 and retina12–14 are well known, few data are available concerning transynaptic changes within the LGN.15–17 To examine these changes, we compared the size, density, and number of neurons in the M- and P-layers of normal monkeys and monkeys with experimental glaucoma. MATERIALS From the 1Department of Physiology, Neuroscience Program, and Center for Clinical Neuroscience and Ophthalmology, Michigan State University, East Lansing; and the 2Department of Ophthalmology and Visual Sciences, University of Wisconsin, Madison. Supported in part by Alcon Laboratories, Fort Worth, Texas (AJW), American Health Assistance Foundation (AJW), and National Institutes Health of Grants EY-11159 (AJW), EY-02698 (PLK), and RR-00167 to the Wisconsin Regional Primate Research Center. Submitted for publication September 1, 1999; revised December 7, 1999; accepted December 16, 1999. Commercial relationships policy: N. Corresponding author: Arthur J. Weber, Department of Physiology, B-512 West Fee Hall, Michigan State University, East Lansing, MI 48824. [email protected] 1370 AND METHODS Subjects and Procedures Fourteen monkeys (13 rhesus, Macaca mulatta; 1 cynomolgus, Macaca fascicularis), of both sexes, aged 6 to 18 years, were used in this study (Table 1). All had clinically normalappearing eyes (slit lamp biomicroscopy, gonioscopy [Zeiss, Thornwood, NY], and stereoscopic funduscopy by fundus camera [Zeiss]), and all had baseline IOPs, measured by tonometer under ketamine HCl anesthesia (Goldman18 [Goldman, Haag-Streit, Köniz, Switzerland] or Tono-pen XL; Mentor O&O, Norwell, MA), below 21 mm Hg (normal IOP for ketamineInvestigative Ophthalmology & Visual Science, May 2000, Vol. 41, No. 6 Copyright © Association for Research in Vision and Ophthalmology Glaucoma and the Primate LGN IOVS, May 2000, Vol. 41, No. 6 1371 TABLE 1. Summary of Animals Studied Animal Sex Age (y) Mean IOP* (mm Hg) M5559 M5562 M81102 AE96 AA02 M86005 M85128 M87035 M78075 M88081 M80142 G-1† AF02 M78002 F F M F F M M F M M M F M F 7 7 11 15 18 8 9 7 14 6 14 6 8 17 16 18 21/46 ⫾ 17 19/44 ⫾ 21 18/62 ⫾ 9 18/46 ⫾ 9 20/52 ⫾ 9 18/44 ⫾ 12 18/42 ⫾ 17 19/37 ⫾ 10 19/36 ⫾ 9 20/49 ⫾ 10 16/29 ⫾ 5 17/27 ⫾ 3 Peak IOP (mm Hg) Duration (wk) Cup/Disc and Initial/Final Ratios 62 58 72 52 68 65 62 52 61 62 42 32 Normal Normal 2.5 2.5 4 4 8 8 8 16 16 24 27 27 0.2 0.2 0.2/0.9 0.3/0.9 0.3/0.9 0.3/0.8 0.3/1.0 0.4/1.0 0.3/0.8 0.3/0.8 0.3/0.6 0.3/1.0 0.3/0.6 0.2/0.4 * Values are presented as baseline IOP/mean elevated IOP ⫾ 1 SD. † Cynomolgus. anesthetized monkeys, 10 –20 mm Hg). Topical proparacaine HCl (Alcaine 0.5%; Alcon Laboratories, Fort Worth, TX) was used for all procedures involving contact with the cornea, and dilation of the pupils for fundus photography was achieved with 2.5% phenylephrine HCl (Mydfrin; Alcon) and 1% tropicamide HCl (Mydriacyl; Alcon). All procedures were approved by the Animal Care Committees of the University of WisconsinMadison and Michigan State University, and all adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Chronic elevation of IOP was induced by laser scarification of the trabecular meshwork.19,20 Each animal first was anesthetized with ketamine HCl (10 mg/kg intramuscularly, supplemented with 5 mg/kg intramuscularly as needed) and sedated with diazepam (1 mg/kg intramuscularly). A standard clinical argon laser (model 900; Coherent Radiation, Palo Alto, CA) and slit lamp delivery system then was used to produce a series of focal lesions in the trabecular meshwork in one eye (50 –250 spots, 50-m spot diameter, 1–1.5 W, 0.5 second duration). IOP was monitored for 2 to 3 weeks after treatment, and if not consistently 25 mm Hg or more, additional laser treatments were performed until stable ocular hypertension was achieved. The opposite eye served as the normal control. At least once per week after laser treatment, the normal and treated eyes were examined with the slit lamp, and corneal clarity, anterior chamber cells, and flare were noted. After measurement of IOP, the anterior chamber angle and optic nerve head were examined with a goniolens (Zeiss; or model OG3M-13; Ocular Instruments, Inc., San Jose, CA). Fundus photographs were obtained every 3 to 4 weeks, depending on the clinical appearance of the optic disc compared with its appearance in previous eye examinations. After 2.5 to 27 weeks of elevated IOP, the animals were anesthetized deeply with ketamine HCl (15 mg/kg, intramuscularly), followed by intravenous pentobarbital sodium (35 mg/kg). The eyes then were removed for intracellular analysis,14 and the animals were perfused transcardially with 0.5 l of 0.9% saline followed by 1 to 2 l of 10% formol-saline solution. The brains were removed and postfixed for 4 to 6 weeks with several changes of fresh fixative. Tissue Processing and Correction for Shrinkage After fixation, each brain was blocked in the frontal plane, dehydrated in a series of graded alcohols, and embedded in celloidin. The LGNs then were sectioned serially at 25 m in the coronal plane, and every fifth section was stained with thionine, mounted on a glass slide, and coverslipped for histologic analysis. Care was taken to standardize the dehydration and celloidin processing times for each brain. Linear tissue shrinkage, assumed to be uniform, was determined by inserting a pair of fine tungsten wires into each block of LGN tissue and carefully measuring the distance between the wires, at the level of the tissue, before and after the dehydration and celloidin embedding processes. The percentage linear shrinkage for each block of tissue then was derived from the formula SL ⫽ 100 ⫺ (AP/BP ⫻ 100), where BP and AP represent the before and after processing distances between the tungsten wires, respectively. On the basis of linear shrinkage (SL), it then was possible to derive correction factors for both areal and volumetric shrinkage. The correction factor for areal shrinkage (ACF ⫽ 1/(1 ⫺ SL)2, when multiplied by the measured area of each neuron or LGN region (M or P), then yielded the true area of that structure. The correction factor for volumetric shrinkage was derived from the measurement of linear shrinkage (SL) using the formula: VCF ⫽ 1/(1 ⫺ SL)3. Cell Size The effects of elevated IOP on cell size were studied by measuring at ⫻1000 the cross-sectional areas of approximately 2400 geniculate neurons in each animal. Because glaucomatous visual field loss is most common within the central 30°,21all neurons were sampled from a rostral– caudal location of the nucleus that corresponded to a visual field representation of approximately 10 to 15° azimuth.22–24 In addition, because retinal ganglion cell loss in glaucoma can be diffuse, LGN regions representing retinal input from superior retina (nasal LGN), inferior retina (temporal LGN), nasal retina (contralateral LGN), and temporal retina (ipsilateral LGN) were examined (Fig. 1). Cell samples (approximately 100 neurons/ layer) were taken from the full width of each lamina and included only those neurons with clearly visible nucleoli. Measurements of soma area were obtained from the cell drawings 1372 Weber et al. IOVS, May 2000, Vol. 41, No. 6 (model C5985 chilled CCD; Hamamatsu, Bridgewater, NJ) and image analysis software (Image-Pro Plus; Media Cybernetics, Silver Spring, MD). The laminar area of the M and P region included in each section was obtained by measuring the total area of each region and subtracting that portion occupied by interlaminar space. Total laminar volumes for the M and P regions of each animal were derived by summing the measured areas, multiplying by section thickness (25 m), and correcting for sample size (every fifth section) and volumetric tissue shrinkage. Statistical Comparisons FIGURE 1. Primate retinogeniculate pathway showing the regions of the LGN examined and the approximate locations of their retinal inputs. Layers 1 and 2 are the M-layers and layers 3 through 6 are the P-layers. Ganglion cells in nasal retina project to the contralateral LGN, whereas those in temporal retina project ipsilaterally. The nasal region of the LGN receives its afferent input from ganglion cells in superior retina (B, C), and the temporal region of the LGN is innervated by ganglion cells located in inferior retina (A, D). using a digitizing tablet and commercial software (SigmaScan; Jandel Scientific, Corte Madera, CA). All cell area measurements were corrected for tissue shrinkage. Cell Density and Number Cell density in the M- and P-layers was estimated by counting with a grid eyepiece reticle and ⫻50 oil immersion objective all neurons displaying clear nucleoli that were located within a sample volume that was 130 ⫻ 130 ⫻ 25 m (section thickness). Cell counts were derived from three regions per layer in each of three adjacent LGN sample sections (every fifth section), for nine sample regions per LGN layer. The rostral– caudal and medial–lateral location of each sample region corresponded with the approximate LGN location from which cell size measurements were obtained. All cell counts were corrected according to the method of Abercrombie,25 using the diameter of the nucleolus (approximately 3 m) as the object thickness. All sample areas were corrected for volumetric shrinkage as described. Estimates of total cell number for the M and P regions of normal and glaucomatous animals were derived by multiplying the corrected sample densities (neurons/ cubic millimeter) by their respective LGN volume measurements (in cubic millimeters). It is important to note that our measurements of LGN cell density and number for normal animals agree closely with similar estimates made using comparable histologic techniques and both the Abercrombie25 and optical dissector stereologic methods.23 LGN Volume LGN volume was determined by capturing digital images of each LGN sample section using a high-resolution video camera All data are presented as means ⫾ 1 SD. Differences in mean soma size and cell density were compared using two-way analysis of variance (ANOVA) statistical methods (SPSS, Chicago, IL) combined with the Bonferroni adjustment for multiple comparisons (NCSS 2000, Kaysville, UT). Paired comparisons of LGN cell size distributions were made using the Kolmogorov–Smirnov test for two independent samples (SPSS). Differences in LGN volume and cell number were compared using one-way ANOVA, followed by the Dunnett test for multiple comparisons (SPSS). Correlation and partial correlation analyses (SPSS) were used to compare the relations between mean, peak, and duration of elevated IOP; cell size, density, and number; and LGN volume. In all cases, P ⫽ 0.05 was used as the level of significance. RESULTS Qualitative Observations Figure 2 shows examples of the cellular organization of the normal primate LGN (Fig. 2A) and after different periods of elevated IOP (Figs, 2B, 2C, 2D). The sections are presented in the coronal plane, with the nasal region of the nucleus to the left. The two ventral layers (Fig. 2A, layers 1, 2) are the Mlayers, and the four dorsal layers (Fig. 2A, layers 3 through 6) are the P-layers. The LGN shown in Figure 2B is from animal M81102 (2.5 weeks, 46 mm Hg). Although at the time of death the optic nerve head was deeply cupped and the temporal region of the disc was pale, the LGN did not appear abnormal (cf., Fig. 2A). By contrast, the LGN in Figure 2C (animal M85128: 8 weeks, 52 mm Hg), displays clear cellular differences between those layers receiving input from the glaucomatous eye (affected layers: 2, 3, and 5) and those receiving input from the normal eye (unaffected layers: 1, 4, and 6). Neurons in the affected layers appear to be smaller and less well stained than those in the unaffected layers, and this cellular difference becomes more pronounced after 6 months of relatively high mean IOP (Fig. 2D). At the time of death, the glaucomatous eye of this animal (G-1: 49 mm Hg) was deeply cupped and uniformly pale, and intracellular analysis of the retina14 indicated that few ganglion cells remained. For animals with 4 weeks of elevated IOP, the LGN of the animal with the lower mean IOP (M86005: 46 mm Hg) resembled that of the 2.5-week animals (Fig. 2B), whereas the LGN from the animal with the higher mean IOP (AA02: 62 mm Hg) more closely resembled the LGN of the 8-week animal shown in Figure 2C. The LGNs from animals that had 16 and 27 weeks of elevated IOP, but lower mean levels (37 and 28 mm Hg, respectively), most closely resembled the LGNs of the 2.5- and 8-week animals shown in Figures 2B and 2C. IOVS, May 2000, Vol. 41, No. 6 Glaucoma and the Primate LGN 1373 FIGURE 2. Photomicrographs of cresyl violet–stained coronal sections from the right LGN of a normal animal (A) and animals that had the pressure in one eye elevated for 2.5 (B), 8 (C), and 24 weeks (D). In all cases, the nasal region of the nucleus is to the left, and the temporal region is to the right. Layers 1, 4, and 6 receive retinal input from the normal contralateral eye, whereas layers 2, 3, and 5 are innervated by the glaucomatous ipsilateral eye. Prolonged elevation of IOP resulted in a decrease in the size and Nissl substance within neurons receiving input from the glaucomatous eye, resulting in their pale appearance. Scale bar, 500 m. Quantitative Observations Cell Size. Because chronic elevation of IOP may not affect ganglion cells in all areas of the retina equally, we examined LGN regions receiving input from the superior, inferior, nasal, and temporal hemiretinas of each eye (Fig. 1). The results of these regional comparisons, in animals with mean IOPs more than 40 mm Hg, are shown in Figure 3. For neurons receiving input from superior versus inferior retina (Fig. 3A), no selective difference was seen within either the M- or P-layers of the LGN. Neurons receiving input from both hemiretinas showed approximately an 8% decrease in soma size after 2.5 weeks of elevated pressure. After 4 to 8 weeks, the mean soma size for neurons in both the M and P regions was only 75% of normal. After 6 months of elevated IOP, neurons in the M-layers had decreased in size approximately 58% (from 490 to 208 m2), FIGURE 3. Comparison of the change in mean soma size versus duration of elevated IOP for M- and P-cells receiving input from ganglion cells located in either the superior versus inferior (A) or nasal versus temporal (B) regions of the retina. Comparable decreases in mean soma size were measured in all retinal target regions of the LGN (*P ⬍ 0.05 versus normal; 2-way ANOVA with Bonferroni adjustment). whereas neurons in the parvocellular layers had decreased approximately 59% (from 289 to 118 m2). The 16-week animals (mean IOP: 37 mm Hg) showed approximately a 16% decrease in LGN cell size in both the M- and P-layers, whereas those with 27 weeks of elevated IOP (mean IOP: 28 mm Hg) showed a decrease of approximately 32%. Cell size comparisons for LGN neurons receiving input from nasal versus temporal retina also showed no differential effect (Fig. 3B). After 2.5 weeks of elevated IOP, neurons in both the M- and P-layers were approximately 8% smaller than normal. By 4 weeks the mean soma size of M-cells receiving input from either hemiretina had decreased from 492 to 340 m2 (31%). Over this same period, parvocellular neurons showed a decrease in area of approximately 26% (from 289 to 215 m2). Although little additional change in soma size oc- 1374 Weber et al. curred for LGN neurons located in either region between 4 and 8 weeks, cells in both the M- and P-layers underwent an additional 10% to 15% decrease in soma size between 8 and 24 weeks, reaching final mean values of 230 and 130 m2, respectively. Nasal–temporal differences in soma size for M- and P-cells from animals with 16 and 27 weeks of elevated IOP were comparable with those described for neurons receiving input from superior versus inferior retina: approximately a 16% decrease in both M- and P-cells after 16 weeks of elevated IOP and approximately a 28% decrease in the size of M-cells and 34% decrease in the size of P-cells with 27 weeks of elevated IOP. Chronic elevation of IOP also resulted in a decrease in the mean soma sizes of LGN neurons receiving input from the normal eye (Fig. 4). The percentage difference in soma size for both M- and P-cells within the affected versus unaffected layers was small after 2.5 to 4 weeks of elevated IOP (3.6 –5.3%). This difference increased to approximately 14% by 8 weeks, and to 48% after 24 weeks. Animals with 16 weeks of elevated IOP also showed decreased mean soma sizes in both the affected and unaffected layers. Neurons in the unaffected M- and Players showed an approximately 12% decrease in mean soma size, and those in the affected layers were 16% smaller than normal. Despite mean IOPs of only approximately 28 mm Hg, and their showing little change in cup-to-disc ratio (Table 1), the animals with 27 weeks of elevated IOP showed approximately a 26% decrease in M-cell size and a 34% decrease in P-cell size for both affected and unaffected layers. Figure 5 compares the size distributions of neurons in the magnocellular (Figs. 5A, 5B) and parvocellular (Figs. 5C, 5D) regions of the normal and glaucomatous LGN. The upper curve in each plot represents the size distribution of neurons located in the right temporal region of the normal LGN, whereas the lower curves represent the size distributions of neurons mea- FIGURE 4. Comparison of pressure-induced changes in mean soma size for neurons located in the affected and unaffected layers of the LGN. Neurons in the affected layers receive direct synaptic input from axons of ganglion cells located in the glaucomatous eye, whereas those in the unaffected layers receive their input from ganglion cells in the normal eye. Neurons in both regions showed changes in soma size as a result of chronic elevation of IOP (*P ⬍ 0.05 versus normal; 2-way ANOVA with Bonferroni adjustment). IOVS, May 2000, Vol. 41, No. 6 sured in matched regions of animals with 2.5, 4, 8, and 24 weeks of elevated IOP. In the normal LGN, M-cells (Figs. 5A, 5B) ranged in size from approximately 121 to 799 m2, with a mean soma area of 474 m2. As expected, normal P-cells (Figs. 5C, 5D) were smaller, ranging in size from approximately 108 to 525 m2, with a mean soma area of 290 m2. After 2.5 to 24 weeks of elevated IOP, neurons in the unaffected M-layers were smaller than normal, averaging only approximately 400 m2. Although this decrease resulted in a small but significant leftward shift in the cell size distributions for the 4-, 8-, and 24-week animals, the overall range of cell sizes for neurons in the normal and unaffected M-layers was similar (Fig. 5A: normal, 121 to 799 m2; unaffected, 119 to 802 m2). The shift toward smaller soma sizes was more pronounced in the affected M-layers, where both the overall mean and range of soma sizes were significantly different from normal (Fig. 5B: mean, 324 versus 400 m2; range, 130 – 680 m2 versus 119 – 802 m2). The unaffected P-layers (Fig. 5C) also showed a modest but significant shift toward smaller soma sizes in both mean cell area (229 versus 280 m2) and the overall range of cell sizes (108 – 472 m2 versus 126 –525 m2). Similarly, the size distributions for neurons in the affected P-layers (Fig. 5D) were significantly different from those for neurons in the matched layers of normal animals. Neurons in the affected layers ranged in size from 48 to 460 m2 (mean, 196 m2), compared with a range of 108 to 472 m2 (mean, 280 m2) for neurons in the normal layers. In all cases, LGN neurons from animals with high mean pressures (approximately 49 mm Hg) and long durations (24 weeks) of elevated IOP were affected most severely. Cell Density. Neuronal densities were derived from cell counts made in approximately the same regions of the LGN used to compare differences in cell size. In all animals, cell densities for both the affected and unaffected regions of the Mand P-layers were obtained from measurements made in the right LGN (Fig. 6). To reduce intralaminar variability, cell density measurements for normal animals were obtained only from LGN layers that corresponded with the layers examined in the experimental animals (e.g., animals with glaucomatous right eyes had the cell densities in the affected layers of the right LGN [layers 2, 3, and 5] compared only with the density of neurons measured in layers 2, 3 and 5 of normal right LGNs). Figure 6 shows that chronic elevation of IOP, regardless of the magnitude, resulted in an increase in the density of neurons in both the M- and P-layers. The percentage change in cell density was greatest in the unaffected layers (M, 38%; P, 70%). The increase in cell density for the unaffected layers of both regions was significantly different from normal in animals with 8 weeks or more of elevated IOP. No significant difference was found in changes in cell density in the affected layers, despite a 45% increase in the P-layers. Changes in cell density in the M-layers was more modest, showing a peak increase of 26% after 8 weeks but increases of only 22% and 9% after 16 and 27 weeks, respectively. In comparing the affected and unaffected layers, only the density of neurons in the affected M-layers of animals with 27 weeks of elevated pressure was found to be significantly less than the density of neurons in the unaffected layers. LGN Volume. Figure 7 shows the effects that different levels and durations of elevated IOP have on LGN volume. As indicated in the Materials and Methods section, these data represent the total laminar volume (affected and unaffected IOVS, May 2000, Vol. 41, No. 6 Glaucoma and the Primate LGN 1375 FIGURE 5. Three-point smoothed histograms showing the size distributions of M- (A, B) and P-cells (C, D) in the normal (upper traces) and glaucomatous (lower traces) LGNs of animals after different durations of elevated IOP. Left: normal versus unaffected layers; right: normal versus affected layers. For all comparisons versus normal (Kolmogorov–Smirnov test; P ⬍ 0.001, except 2.5-week unaffected magnocellular, P ⫽ 0.376). layers) for each region. This served to minimize errors in identifying affected versus unaffected layers in regions of the LGN containing laminar discontinuities (e.g., Fig. 2C). As with cell density, all volumetric data were derived from the right LGN of each animal. However, comparable measurements from the left LGN of four animals (normal, 8, 16, and 27 weeks) indicated no left–right bias. For normal animals, total laminar volume was found to be approximately 68.2 mm3 (M, 56.4 mm3; P, 11.8 mm3). Both regions of the nucleus showed significant decreases in laminar volume after as little as 2.5 weeks of elevated IOP. The volume of the P region decreased approximately 23%, to 43.4 mm3, whereas the volume of the M region decreased 35%, to 7.7 mm3. Although 4 weeks yielded little additional change in volume, both regions decreased to approximately 55% of normal by 8 weeks. A similar magnitude of change was seen in both the M- and P-layers of the animals with 16 and 27 weeks of elevated IOP, despite their lower mean pressures. Neuronal Number. Estimates of total cell number in the LGNs of normal and glaucomatous animals were determined from the cell density and LGN volume measurements, after corrections for tissue shrinkage (Fig. 8). For normal animals, the total number of neurons in the LGN was estimated to be approximately 1.27 million, with 10.3% located in the M-layers and 89.7% in the P-layers. Compared with matched normal layers, the affected M-layers of glaucomatous animals showed a significant decrease (38%) in the number of surviving neurons for each period studied, whereas the P-layers did not (10% decrease). Relation of IOP to Changes in LGN Morphology. Correlation and partial correlation analyses were used to analyze the relations between mean, peak, and duration of elevated IOP, and glaucoma-related changes in LGN cell size, density, and number, as well as LGN volume (Table 2; ⫹ indicates P ⬍ 0.05). In the M-layers, correlations were found between mean and peak IOP and decreases in cell size, number, and LGN volume. No correlation was seen for duration of IOP versus cell size, or cell density versus any component of IOP. In the P-layers, correlations were found between mean, peak, and duration of elevated IOP and the decrease in cell size and LGN 1376 Weber et al. IOVS, May 2000, Vol. 41, No. 6 FIGURE 8. Histograms showing estimates of the mean number of neurons in the normal and affected M (left) and P (right) regions of the glaucomatous LGN after different durations of elevated IOP (*P ⬍ 0.05 versus normal; 1-way ANOVA with Dunnett test). FIGURE 6. Comparison of the change in cell density in the affected and unaffected M- and P-layers of the LGN after different durations and mean levels of elevated IOP (*P ⬍ 0.05 versus normal; 2-way ANOVA with Bonferroni adjustment). volume, as well as the increase in cell density within this region. No correlation was seen between any component of IOP and the decrease in P-cell number. DISCUSSION We used the monkey model of glaucoma and standard histologic techniques to examine the degenerative effects that chronic elevation of IOP has on the cytoarchitecture of the primate LGN. The results show that elevation of IOP had a profound effect on the size, density, and number of neurons in the LGN, as well as the laminar volume of the nucleus itself. In FIGURE 7. Histograms showing the change in laminar volume for the M- and P-layers of the LGN as a result of different levels and durations of elevated IOP. For each region, total laminar volume was derived by combining the volumes of the affected and unaffected layers, and excluding intralaminar volume (*P ⬍ 0.05 versus normal; 1-way ANOVA with Dunnett test). addition, they show that these cellular changes were not restricted to LGN layers receiving input from the glaucomatous eye, but also were present, albeit to a lesser degree, in laminae innervated by the normal eye. The cell size data reveal two important relationships between elevation of IOP and LGN degeneration. First, they show that longer durations of elevated IOP, even at low to moderate levels, resulted in larger reductions in the soma sizes of both Mand P-cells. Second, they indicate that higher mean levels of IOP shortened the time during which the cellular changes occur. Animals with 2.5 weeks of elevated pressure at mean levels of approximately 45 mm Hg showed decreases in mean soma size that were comparable with animals with 16 weeks of elevated IOP and mean levels of approximately 36 mm Hg. Similarly, animals with 8 weeks of elevated IOP (approximately 46 mm Hg) displayed reductions in mean soma size that were comparable with those with 27 weeks of elevated IOP and mean IOPs of approximately 28 mm Hg. At each of the different periods studied, similar percentage decreases in mean cell area were measured in both the Mand P-layers. Although this suggests that IOP affects the sizes of M- and P-cells equally, it is important to note that these data reflect changes in the population mean and not necessarily those of single neurons. This distinction is important, because different populations of neurons may reach their final mean sizes through completely different mechanisms (e.g., cell loss versus shrinkage). The histograms in Figure 5 suggest that both cell loss and shrinkage contribute to the decrease in mean soma size within the M- and P-layers in glaucoma. They also indicate that in both regions the largest neurons may be affected most severely. Both areas show an absence of very large neurons, and both display modest increases in the percentage of small to medium sized neurons, as would be expected if larger neurons also were shrinking (cf., Figs. 5B, 5D). That cell size, and not cell class, may be the main factor governing glaucomatous changes in the LGN is consistent with similar results in the primate retina.12,13 The leftward shift of the lower ends of the parvocellular, but not magnocellular, distributions for animals with 4, 8, and 24 weeks of elevated IOP suggests that general cell shrinkage might play a greater role in determining final mean soma size in the P-layers, whereas cell loss is the primary mechanism in the M-layers. This would be Glaucoma and the Primate LGN IOVS, May 2000, Vol. 41, No. 6 1377 TABLE 2. Correlation/Partial Correlation Analysis: IOP Versus Cell Morphology Magnocellular Mean IOP Peak IOP Duration Parvocellular Size Density Number Volume Size Density Number Volume ⫹ ⫹ ⫺ ⫺ ⫺ ⫺ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫹ ⫺ ⫺ ⫺ ⫹ ⫹ ⫹ ⫹ P ⬍ 0.05. consistent with our cell number estimates: We found approximately a four times greater loss of M-cells than P-cells in the glaucomatous animals. To date, only one study has examined cellular changes in the primate LGN with glaucoma. Chaturvedi et al.15 used human autopsy material to compare the density of neurons in the magno- and parvocellular regions of normal patients and patients with documented glaucoma. Based on a small (approximately 1 neuron/per square millimeter) but significant decrease in cell density in the M- but not P-layers, the authors concluded that glaucoma has a preferential effect on the Mregion of the LGN. Our data also indicate that glaucoma has a differential effect on M- versus P-cell density. However, they show that chronic elevation of IOP results in an increase in LGN cell density and that this increase in cell density is approximately two times greater in the P-layers than in the M-layers (59% versus 31%). The large increase in P-cell density most likely resulted from a significant decrease in laminar volume, but not cell number, in this region of the nucleus, whereas the modest increase in M-cell density probably reflected a complex balance between decreasing laminar volume and cell loss (Figs. 7, 8). As expected, the largest increases in cell density were measured in the unaffected M- and P-layers, where cell loss was minimal. The apparent contradiction between our cell density results and those of Chaturvedi et al.15 could be explained by animal– human variation or differences in the duration of glaucoma for their patients (years) versus our animals (weeks). If during the initial stages of the disease process, cell density is influenced primarily by a rapid reduction in LGN volume due to early axonal degeneration (described later), then the increases in cell density reported here would be expected. As the disease progresses and cell loss becomes more prevalent, then either no change or a decrease in cell density might be expected, depending on the relation between LGN volume and cell number. This most closely reflects the results of Chaturvedi et al.15 In agreement with Ahmad and Spear,23 we found the total laminar volume of the normal rhesus LGN to be approximately 68 mm3. After only 2.5 weeks of elevated IOP, the volume of the P-layers decreased approximately 23%, whereas the Mlayers underwent a 35% reduction in size. Despite its smaller percentage decrease, the actual reduction in P volume is approximately threefold greater than the reduction in M volume (13 mm3 versus 4.2 mm3). Because P-cells undergo little change in size or number during this period (Figs. 4, 8), this initial decrease in P volume most likely reflects an early loss of axonal material.26 The more pronounced change within the P region might be due to several factors. First, because the P region contains approximately eight times as many neurons, there simply are many more retinal axons and terminal arbors within this region. Second, because the axons of M-ganglion cells enter the dorsal surface of the nucleus and traverse the P-layers on their way to the ventral M region,27 atrophy of these fibers also may contribute to a decrease in parvocellular volume. Within the M-layers, the decrease in laminar volume most likely involves a complex combination of axon degeneration, cell shrinkage, and neuronal cell loss (Figs. 4, 5, and 8). One unexpected result of this study was the relatively constant level of cell loss across animals with different durations of elevated IOP (Fig. 8). After 2.5 weeks of elevated pressure, the affected M-layers show approximately a 38% loss of neurons, and little change thereafter. A similar pattern was seen for the P-layers; however, the onset of cell loss was delayed approximately 1.5 weeks relative to the M-layers. One possible explanation for this step-like pattern in LGN cell loss is that each region contains subpopulations of neurons with different sensitivities to optic nerve injury. Although the data in Figure 8 may represent an initial loss of the most sensitive neurons, longer durations of elevated IOP may be needed to show the loss of more resistant neurons. That the M-layers of the primate LGN may contain neurons with different sensitivities to axonal injury has been suggested previously,28 and Golgi studies have described at least two types of relay cells in this region of the nucleus.29 An obvious question is, why do LGN neurons degenerate after damage to the optic nerve? In the case of retinal ganglion cell death and glaucoma, pressure-induced damage to the optic nerve has been shown to disrupt axonal transport,9,30,31 which then results in a decrease in the amount of trophic material ganglion cells obtain from their target neurons in the LGN. Because elevation of IOP does not damage LGN neurons or their connections with target neurons in visual cortex directly, it seems likely that neurons in the glaucomatous LGN degenerate primarily because of a decrease in neuronal activity within the retino-geniculo-cortical pathway. LGN neurons may require a minimum level of activity to maintain connections with visual cortex and obtain sufficient amounts of trophic material for their survival. A reduction in neuronal activity may also explain the cellular changes seen within the unaffected layers, a phenomenon not restricted to glaucoma.8,32 Because LGN neurons representing each eye innervate common targets in visual cortex, a reduction in cortical activity that produces a general decrease in cortical trophic levels would have an effect on cells in all layers of the nucleus. At present, there is good evidence that retinal ganglion cells in the glaucomatous eye die by apoptosis.33,34 Although similar studies have not been conducted in the LGN, transynaptic apoptotic cell death has been described in other systems.35,36 This does not preclude concomitant involvement of 1378 Weber et al. necrotic cell death, however, because both necrosis and apoptosis have been demonstrated in the LGN after visual cortex damage37 and in the retina after pressure-induced ischemia38,39 and optic nerve crush.40 Interestingly, neurons undergoing each type of cell death appear to follow a different time course: Neurons that die soon after the insult do so mainly by necrosis, whereas those that die later undergo apoptosis. Although we cannot address the functional significance of our cellular changes, it is of interest to note that Smith et al.41 reported few abnormalities concerning the functional integrity of LGN neurons or their retinal afferents in monkeys with experimental glaucoma. This result is somewhat surprising, considering the structural abnormalities of ganglion cells in the glaucomatous eye14 and the transneuronal changes described in this study. One possible explanation is that retinal ganglion cells and LGN neurons are capable of maintaining much of their functional integrity, despite significant changes in morphology. Current retinal studies are examining this possibility. Although many questions remain, these data demonstrate clearly that chronic elevation of IOP has a profound degenerative effect on the primate LGN, a major structure involved in the integration and transfer of visual information. Although our estimates of pressure-induced cell loss suggest that glaucoma has a more pronounced effect on neurons in the M- than in the P-pathway, perhaps the more important message is that in strategies intended to mitigate glaucomatous neuropathy, treatment of the entire central visual pathway, and not the eye alone should be considered. Acknowledgments The authors thank Todd Perkins and Michael Bueche for assisting with clinical evaluations of the eyes; Elaine Bostad, Jane Walsh, Judy McMillan, and Charles Greenfeld for technical assistance; and Dan Houser and Shelly Zimbric of the Wisconsin Regional Primate Research Center for assistance with the animals. References 1. Lennie P. Parallel visual pathways: a review. Vision Res. 1978;20: 561–594. 2. Kaplan E, Lee BB, Shapley RM. New views of primate retinal function. In: Osborne N, Chader G, eds. Progress in Retinal Research. Oxford, UK: Pergamon; 1990:273–336. 3. Dacey D, Lee BB. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. 4. DeMonasterio FM, Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol (Lond). 1975;251:167– 195. 5. Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form, color, movement and depth. J Neurosci. 1987;7:3416 –3468. 6. Cowan WM. Anterograde and retrograde transneuronal degeneration in the central and peripheral nervous system. In: Ebbesson S, Nauta WJH, eds. Contemporary Research Methods in Neuroanatomy. New York: Springer–Verlag, 1970:217–251. 7. Pearson HE, Stoffler DJ. Retinal ganglion cell degeneration following loss of postsynaptic target neurons in the dorsal lateral geniculate nucleus of the adult cat. Exp Neurol. 1992;116:163–171. 8. Headon MP, Sloper JJ, Powell TPS. Initial hypertrophy of cells in undeprived laminae of the lateral geniculate nucleus of the monkey following early monocular visual deprivation. Brain Res. 1982; 238:439 – 444. 9. Quigley HA, Anderson DR. The dynamics and location of axonal transport blockade by acute intraocular pressure elevation in primate optic nerve. Invest Ophthalmol. 1976;15:606 – 616. IOVS, May 2000, Vol. 41, No. 6 10. Quigley HA. Pathophysiology of the optic nerve in glaucoma. In: McAllister JA, Wilson RP, eds. Glaucoma. London: Butterworths; 1986:30 –53. 11. Quigley HA, Dunkelberger GR, Green WR. Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology. 1988;95:357–363. 12. Glovinsky Y, Quigley HA, Pease ME. Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1993;34:395– 400. 13. Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991;32:484 – 490. 14. Weber AJ, Kaufman PL, Hubbard WC. Morphology of single ganglion cells in the glaucomatous primate retina. Invest Ophthalmol Vis Sci. 1998;39:2304 –2320. 15. Chaturvedi N, Hedley–Whyte E, Dreyer EB. Lateral geniculate nucleus in glaucoma. Am J Ophthalmol. 1993;116:182–188. 16. Vickers JC, Schumer RA, Podos SM, Wang RF, Riederer BM, Morrison JH. Differential vulnerability of neurochemically identified subpopulations of retinal ganglion cell neurons in monkey model of glaucoma. Brain Res. 1995;680:23–35. 17. Vickers JC, Hof PR, Schumer RA, Wang RF, Podos SM, Morrison JH. Magnocellular and parvocellular visual pathways are both affected in a macaque monkey model of glaucoma. Aust NZ J Ophthalmol. 1997;25:239 –243. 18. Kaufman PL, Davis GE. Minified Goldmann applanating prism for tonometry in monkeys and humans. Arch Ophthalmol. 1980;98: 542–546. 19. Gaasterland DE, Kupfer C. Experimental glaucoma in the rhesus monkey. Invest Ophthalmol. 1974;13:455– 457. 20. Quigley HA, Hohman RM. Laser energy levels for trabecular meshwork damage in the primate eye. Invest Ophthalmol Vis Sci. 1983;24:1305–1307. 21. Heijl A. Visual field loss and perimetry in glaucoma. In: Podos SM, Yanoff M, eds. Textbook of Ophthalmology: Glaucoma. Vol. 7. London: Mosby; 1994;7:6 –7.27. 22. Malpeli JG, Lee D, Baker FH. Laminar and retinotopic organization of the macaque lateral geniculate nucleus: magnocellular and parvocellular magnification functions. J Comp Neurol. 1996;375:363– 377. 23. Ahmad A, Spear PD. Effects of aging on the size, density and number of rhesus monkey lateral geniculate neurons. J Comp Neurol. 1993;334:631– 643. 24. Connolly M, Van Essen D. The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. J Comp Neurol. 1984;226:544 – 564. 25. Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239 –247. 26. Glees P, Le Gros Clark WE. The termination of optic fibers in the lateral geniculate body of the monkey. J Anat. 1941;75:295–308. 27. Le Gros Clark WE, Penman GG. The projection of the retina in the lateral geniculate body. Proc R Soc Lond B Biol Sci. 1934;114:291– 313. 28. Goldby F. A note on transneuronal atrophy in the human lateral geniculate body. J Neurol Neurosurg Psychiatry. 1957;20:202– 207. 29. Saini KD, Garey LJ. Morphology of neurons in the lateral geniculate nucleus of the monkey. A Golgi study. Exp Brain Res. 1981;42: 235–248. 30. Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axonal transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. 31. Dandona L, Hendrickson A, Quigley, HA. Selective effects of experimental glaucoma on axonal transport by retinal ganglion cells to the dorsal lateral geniculate nucleus. Invest Ophthalmol and Vis Sci. 1991;32:1593–1599. 32. Headon MP, Sloper JJ, Hiorns RW, Powell TPS. Shrinkage of cells in undeprived laminae of the monkey lateral geniculate nucleus following late closure of one eye. Brain Res. 1981;229:187–192. 33. Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and IOVS, May 2000, Vol. 41, No. 6 34. 35. 36. 37. after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774 –786. Garcia–Valenzuela E, Shareef S, Walsh J, Sharma SC. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;61:33– 44. DeGiorgio LA, Dibinis C, Milner TA, Saji M, Volpe BT. Histological and temporal characteristics of nigral transneuronal degeneration after stiatal injury. Brain Res. 1998;795:1–9. Loez–Mascaraque L, Price JL. Protein synthesis inhibitors delay transneuronal death in the piriform cortex of young adult rats. Neuroscience. 1997;79:463– 475. Kalil, RE, Fedynyshyn, JP. Axotomy induces two cytologically distinct types of cell death in the dorsal lateral geniculate nucleus (LGN) of the adult rat. Soc Neurosci Abstr. 1998;24:1303. Glaucoma and the Primate LGN 1379 38. Joo C-K, Choi J-S, Ko HW, et. al. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40:713–720. 39. Buchi ER. Cell death in the rat retina after a pressure-induced ischemia-reperfusion insult: an electron microscopic study, I: ganglion cell layer and inner nuclear layer. Exp Eye Res. 1992;55:605– 613. 40. Bien A, Seidenbecher CI, Böckers TM, Sabel BA, Kreutz MR. Apoptotic versus necrotic characteristics of retinal ganglion cell (RGC) death after partial optic nerve injury. J Neurotrauma. 1999;16: 153–163. 41. Smith EL, Chino YM, Harwerth RS Ridder WH, Crawford MLJ, DeSantis L. Retinal inputs to the monkey lateral geniculate nucleus in experimental glaucoma. Clin Vis Sci. 1993;8:113–139.