* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download organometallic reagents
Fischer–Tropsch process wikipedia , lookup
Aromaticity wikipedia , lookup
George S. Hammond wikipedia , lookup
Bottromycin wikipedia , lookup
Kinetic resolution wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Elias James Corey wikipedia , lookup
Stille reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
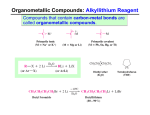
8-7 Organometallic Reagents: Sources of Nucleophilic Carbon for Alcohol Synthesis If the carbonyl carbon of an aldehyde or ketone could be attacked by a nucleophilic carbon atom, R:-, instead of a hydride ion, both an alcohol and a new carbon-carbon bond would be formed. The class of compounds called organometallic reagents are strong bases and good nucleophiles and are useful in this kind of synthesis. 28 Alkyllithium and alkylmagnesium reagents are prepared from haloalkanes. Alkyllithium and alkylmagnesium compounds can be prepared by reaction of alkyl halides with lithium or magnesium in ethoxyethane (diethylether) or oxacyclopentane (THF). The order of reactivity of the haloalkane is: Cl < Br < I (CH3CH2 )2 O, 0o 10o C CH3Br + 2 Li CH3Li + LiBr Methyl-lithium Grignard reagents, RMgX, can be formed from primary, secondary, and tertiary haloalkane, as well as from haloalkenes and halobenzenes. Grignard reagents are very sensitive to moisture and air and are formed in solution and used immediately. The metal atoms in a Grignard reagent are electron-deficient and become coordinated to two solvent molecules: 30 The alkylmetal bond is strongly polar. The carbon-lithium bond in CH3Li has about 40% ionic character, and the carbon-magnesium bond in CH3MgCl has about 35% ionic character. The metal atom is strongly electropositive and is at the positive end of the dipole. The formation of a Grignard reagent is an example of reverse polarization. In the haloalkane, the carbon atom attached to the halogen was electrophilic. In the Grignard reagent, the carbon atom has become nucleophilic. The alkyl group in alkylmetals is strongly basic. Carbanions are the conjugate bases of alkanes (estimated pKa’s of about 50), and as a result are extremely basic, much more so than amines or alkoxides. Because of their basicity, carbanions are extremely sensitive to moisture or other acidic functional groups. This reaction is one method which can be used to convert alkylhalides into alkanes. 32 A more direct way of producing an alkane from a haloalkane is by an SN2 displacement of the halide by a hydride ion from LiAlH4. NaBH4 is not reactive enough to carry out this displacement. A deuterium atom can be introduced into an alkane by the reaction of D2O with an organometallic reagent: 8-8 Organometallic Reagents in the Synthesis of Alcohols Useful reactions of organometallic reagents are to react aldehydes and ketones giving an alcohol containing a new C-C bond. Reaction with formaldehyde produces a primary alcohol. 34 Aldehydes other than formaldehyde form secondary alcohols. Ketones react to form tertiary alcohols. Alkyllithium and Grignard reagents cannot be used to displace halide ions from haloalkanes as the reaction is too slow. 8-9 Complex Alcohols: An Introduction to Synthetic Strategy Mechanisms help in predicting the outcome of a reaction. Bromide is a better leaving group than fluoride. 36 Example 2: How does a Grignard reagent add to a carbonyl group? The negatively polarized alkyl group in the organometallic reagent attacks the positively polarized carbonyl carbon in the carbonyl group. Example 3: What is the product of the radical halogenation of methylcyclohexane? The tertiary bond is weaker than a primary or secondary C-H bond. Br2 is very selective in radical halogenations. 38 New reactions lead to new synthetic methods. We now have several synthetic methods at our disposal: Each of the products formed by these reactions can be altered by further chemical reactions leading to more and more complicated molecules. Finding suitable starting materials and an efficient synthetic path to a desired target molecule is a problem called total synthesis. A successful synthesis is characterized by: • Brevity • High overall yield • Readily available starting materials (commercially available and inexpensive) • Reagents should be relatively nontoxic and easy to handle. 40 Retrosynthetic analysis simplifies synthesis problems. The most frequent synthetic task is building up larger, more complicated molecules from smaller simple fragments. The best approach in designing a synthetic route to a desired product is to work the synthesis backwards on paper. This approach is called retrosynthetic analysis. In this approach, strategic C-C bonds in the target molecule are broken at points where bond formation seems possible. The reason that retrosynthetic analysis is useful is that fewer possible reactions need to be considered compared to an analysis starting with large numbers of possible starting materials and chemical reactions. An analogy would be to start at the tip of a branch of a tree and work backwards to the main trunk. If you started at the trunk and tried to find a particular branch, you would encounter many dead end paths and would have to constantly backtrack. Consider the retrosynthetic analysis of 3-hexanol: The double-shafted arrow indicates a strategic disconnection. Two inferior retrosynthetic analyses are: These strategies are inferior to the first because they do not simplify the target structure: no C-C bonds are broken. 42 Retrosynthetic analysis aids in alcohol construction. Consider the retrosynthetic analysis of the preparation of 4-ethyl-4-nonanol. The strategic bonds are around the functional group. Of the three paths, a,b, and c, is best: The building blocks are almost equal in size (5 and 6 carbon fragments), providing the greatest simplification in structure. The 3-hexanone can also be subjected to retrosynthetic analysis: The 3-hexanol was subjected to an earlier retrosynthetic analysis. The complete synthetic scheme will be: 44 Watch out for pitfalls in planning syntheses. Try to minimize the total number of transformations required to convert the initial starting material into the desired product. A seven-step synthesis with a yield of 85% at each step gives an overall efficiency of conversion of 32%. A four-step synthesis with three yields at 95% and one at 45% gives an overall efficiency of conversion of 39%. A convergent synthesis of the same number of steps is preferable to a linear synthesis. Do not use reagents having functional groups that would interfere with the desired reaction. This problem could be overcome by using two equivalents of a Grignard reagent, or by protecting the hydroxy functionality in the form of an ether. Do not try to make a Grignard reagent from a bromoketone. It will react with its own or another molecule’s ketone group. It is possible to protect the ketone group in this case. 46 Take into account any mechanistic and structural constraints affecting the reactions under consideration: • Radical brominations are more selective than chlorinations. • Remember the structural limitations on nucleophilic reactions. • Remember the lack of reactivity of the 2,2-dimethyl-1halopropanes. • These hindered systems form organometallic reagents and may be modified in this manner. • Tertiary haloalkanes do not undergo SN2 reactions, but eliminate in the presence of bases: 8 Important Concepts 1. Alcohols are alkanols (IUPAC) – Names derived from stem prefixed by alkyl and halo substituents Alcohols Have Polar and Short O-H Bond – 2. • • • • Hydroxy group is hydrophilic (hydrogen bonding) Unusually high boiling points Appreciable water solubility Alkyl part is hydrophobic 3. Alcohols Are Amphoteric – • • • • Deprotonation by bases whose conjugate acids are weaker than the alcohol Protonation yields alkyloxonium ions Acidity: primary > secondary > tertiary alcohol Electron-withdrawing substituents increase acidity 8 Important Concepts 4. Reverse Polarization – i.e., conversion of the alkyl group in a haloalkane, Cδ+-Xδ-, into its nucleophilic analog in an organometallic compound, Cδ--Mδ+. 5. Aldehyde and Ketone Carbonyl Carbons are Electrophilic – C=O carbon is subject to attack by hydride hydrogens or organometallic alkyl groups. Aqueous workup yields alcohols. 6. Oxidation of Alcohols – Yields aldehydes and 7. ketones (Chromium IV reagents). Retrosynthetic Analysis – Identifies efficient sequence of reactions by identifying strategic bonds.