* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture 19 Membranes 2: Membrane Proteins
P-type ATPase wikipedia , lookup
Biochemistry wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Protein moonlighting wikipedia , lookup
Membrane potential wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Lipid bilayer wikipedia , lookup
Protein adsorption wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Proteolysis wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
SNARE (protein) wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
List of types of proteins wikipedia , lookup
Trimeric autotransporter adhesin wikipedia , lookup
Cell membrane wikipedia , lookup
BIOC 460, Spring 2008
Lecture 19
Membranes 2:
Membrane Proteins
Next 2 lectures: Membrane Transport
Reading: Berg, Tymoczko & Stryer, 6th ed., Chapter 12, pp. 336-348
Bacteriorhodopsin Jmol structure:
http://www.biochem.arizona.edu/classes/bioc462/462a/jmol/rhodopsin/rhodop1.htm
PGH2 Synthase (COX 2) Jmol structure:
http://www.biochem.arizona.edu/classes/bioc462/462a/jmol/cox12/cox121.htm
Bacterial porin Jmol structure:
http://www.biochem.arizona.edu/classes/bioc462/462a/jmol/porin/newporin.html
Key Concepts
•
•
•
•
•
•
Membrane functions (review): selective permeability barriers,
information processing, organization of reaction sequences, energy
conversion
Lipids (lipid bilayer) responsible for permeability barrier
Proteins perform essentially all other membrane functions, including
modulation of permeability barrier by allowing or assisting some solutes to
cross membrane (transport processes)
Fluid mosaic model of membrane structure: 2-dimensional "fluid"
composed of lipids and proteins (both often with attached carbohydrates
on outer side of membrane)
Proteins: peripheral, lipid-anchored, or integral
Mobility of components within the membrane:
–
Lateral diffusion: rapid for both proteins and lipids within the plane
of the membrane (except for proteins anchored, for example to
cytoskeleton)
–
Transverse diffusion ("flip-flop") of both proteins and lipids is
extremely slow, unless mediated by protein "flippases".
–
Lipid composition in the 2 leaflets of bilayer is asymmetric, as is
protein distribution.
LEC 19, Membranes 2 - Membrane
Proteins
1
BIOC 460, Spring 2008
Key Concepts, continued
•
Membrane fluidity essential to function, regulated by fatty acid
composition of lipids and (eukaryotes) by cholesterol content
Integral membrane proteins typically assume one of two secondary
structures to get polar groups of polypeptide backbone across
hydrophobic core of lipid bilayer: membrane-spanning α helices (about 20
residues long) or antiparallel β sheets wrapped into β barrels.
Examples:
–
Glycophorin: single hydrophobic transmembrane α helix
–
Bacteriorhodopsin: 7 transmembrane helices
–
Prostaglandin H2 Synthase: on surface of ER membrane but
anchored in membrane by a set of α-helices with hydrophobic R
groups that extend into membrane core
–
Porins: large β barrel with aqueous channel down the center
•
•
Learning Objectives
•
•
•
•
•
Terminology (as applied to membrane proteins): peripheral, integral,
lipid-anchored (operational definition -- how can they be extracted
from membrane?); trans-membrane helix; antiparallel β barrel
Briefly explain what “lipid rafts” are in membranes.
Explain in structural terms how an integral membrane protein can deal
with its polar backbone groups in spanning the hydrophobic core of a
lipid bilayer.
Name 2 types of secondary structural elements used by integral
membrane proteins to cross membranes. Describe where the R
groups are located in these secondary structural elements relative to
the hydrophobic lipid core.
Discuss the structural properties of the following examples of
membrane proteins: glycophorin A, bacteriorhodopsin, prostaglandin
H2 synthase, and a porin. Include in your discussion the type(s) of
secondary structure and types of R groups found in the
transmembrane (membrane-spanning) structural components of these
membrane proteins.
LEC 19, Membranes 2 - Membrane
Proteins
2
BIOC 460, Spring 2008
Lipid Rafts
• lateral diffusion (sideways in plane of membrane) rapid for lipids and for
many proteins (but NOT for proteins "anchored" by attachment to other
proteins, e.g., in cytoskeleton on inner side of membrane, or extracellular
matrix, or both -- e.g., fibronectin)
• Timescale of msec to sec, lipids DO diffuse freely in plane of membrane.
• On much shorter time scale, 25 µsec intervals, lipids zoom around in a small
confined area of membrane, then seem to "hop" to another small confined
area, as if there were "fences" they had to hop over (networks of interacting
membrane proteins?)
• Very recent research: different types of membrane lipids are NOT randomly
located all through the membrane -- some phosphosphingolipids and
cholesterol cluster together in membrane ”rafts" (patches of lipid about 50
nm wide), thicker and more "ordered" structurally than the more disordered
neighboring microdomains rich in glycerophospholipids.
• Lipid rafts have a few thousand lipid molecules and about 10-50 protein
molecules, and are particularly enriched in 2 classes of lipid-anchored
integral membrane proteins.
• Rafts may "organize" some membrane receptors and signaling proteins
whose functions require protein-protein interactions (collision probability
much greater in "corral" of local raft).
• Proteins and lipids move in and out of rafts, but on slower time scale (sec
rather than µsec), but biochemical processes work on the faster time scale
in which rafts are stable clusters.
Membrane Proteins
• mediate nearly all membrane functions except establishment of
permeability barrier
• membrane protein functions:
– "pumps" (active transport)
– "gates" ("passive" transport, facilitated diffusion)
– receptors
– signal transduction
– enzymes
– energy transduction
• Membrane protein distribution:
– Both amount of protein in general and which specific proteins are
present varies with function of membrane, i.e., with type of
membrane and with cell type.
– Examples:
• myelin (membrane around myelinated nerve fibers,
function=electrical insulation) mostly lipid (only ~18% protein)
• plasma membrane: enzymes, receptors, etc. (~50% protein)
• mitochondrial inner membrane and chloroplast thylakoid
membrane: electron transport, energy transduction (ATP
synthesis) (~75% protein)
LEC 19, Membranes 2 - Membrane
Proteins
3
BIOC 460, Spring 2008
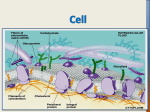
Schematic Diagram of Membrane Structure
Nelson & Cox, Lehninger Principles of Biochemistry, 4th ed., Fig. 11-3
Fluid Mosaic Model of Membrane Structure
(Singer and Nicholson, 1972)
• model of biological membranes as 2-dimensional "solutions" of
globular proteins embedded in fluid lipid bilayer, structurally and
functionally asymmetric with respect to the 2 sides of bilayer
• Fatty acyl chains in interior of membrane form a fluid, hydrophobic
region; surface is hydrophilic.
• Proteins and lipids can diffuse laterally (in plane of membrane) unless
they're "anchored" to something,
but transverse diffusion ("flip-flop") is very
slow unless there's a catalyst for it.
• Carbohydrates
on both proteins
(glycoproteins)
and lipids
(glycolipids)
are exposed on
extracellular
surface of
plasma
membrane.
Nelson & Cox, Lehninger
Principles of Biochemistry,
4th ed., Fig. 11-3
LEC 19, Membranes 2 - Membrane
Proteins
4
BIOC 460, Spring 2008
Membrane Protein Asymmetry
• All biological membranes are asymmetric.
• Membrane protein orientation: Proteins definitely oriented: inner side,
or outer side, or spanning membrane, but in a specific orientation
• Example: asymmetry of the Na+-K+ transport system in plasma
membranes.
– Transport enzyme pumps Na+ out of cell and K+ into cell, against
a concentration gradient (unfavorable, so requires input of free
energy, coupling to processes resulting in net hydrolysis of ATP on
inside of cell)
Berg et al.,Fig. 12-34
Membrane Proteins
•
3 types based on association with membrane:
1. Peripheral
2. Integral
3. Lipid-anchored
1.
•
•
Peripheral membrane proteins
weakly associated with membrane at surface
bind to polar lipid heads and/or to integral membrane proteins
–
electrostatic interactions predominate (ionic bonds and/or
hydrogen bonds)
–
easily extractable from membranes by high salt concentrations
(disrupting electrostatic interactions), or by EDTA (chelates Ca2+
and Mg2+)
usually water-soluble (globular)
There can also be fibrous proteins attached to membrane surface
(cytoskeletal proteins).
Some peripheral "membrane" proteins come and go from membrane,
e.g., using reversibly attached lipid anchor.
–
Covalently attached C14 fatty acyl chain (myristoyl group) slips
into lipid bilayer, holding protein to surface of membrane.
•
•
•
LEC 19, Membranes 2 - Membrane
Proteins
5
BIOC 460, Spring 2008
2. Integral membrane proteins
• tightly bound to membrane - interact with interior (membrane core, lipid
tails) (hydrophobic interactions)
• require detergents (or organic solvents) for extraction from membranes
• water-insoluble
• Some proteins have both peripheral and integral domains.
• Some completely span membrane ("transmembrane proteins").
• Glycoproteins always have carbohydrates on extracellular side.
– carbohydrates attached by O-glycosidic
(acetal) bonds to OH groups of specific
Ser or Thr residues, or
– N-glycosidic
bonds to Asn
residues)
Nelson & Cox, Lehninger
Principles of Biochemistry,
4th ed., Fig. 11-3
3. Lipid-anchored membrane proteins
• Proteins that are covalently linked to lipids, a requirement for their
association with the membrane (lipid inserts into membrane, associating
protein with membrane)
• Some lipid anchors can be reversibly attached to/detached from
proteins.
– "switching device" to alter affinity of protein for membrane
• role in signal transduction pathways in eukaryotic cells
– e.g., N-myristoylation: C14 fatty acid attached
enzymatically in amide linkage to α-amino of
N-terminal Gly of membrane protein
– examples of reversibly
N-myristoylated
proteins:
– PKA (cAMPdependent
protein
kinase)
– α subunit of
G proteins
Nelson & Cox, Lehninger
Principles of Biochemistry,
4th ed., Fig. 11-3
LEC 19, Membranes 2 - Membrane
Proteins
6
BIOC 460, Spring 2008
Transmembrane Proteins
How does an integral membrane protein accommodate its polar
backbone (peptide N-H and C=O groups) in a stable way across
hydrophobic core of lipid bilayer?
• By hydrogen-bonding as many as possible of polar backbone groups
(really ALL of them), i.e., by forming secondary structures, either
α-helical or β barrel motifs for membrane-spanning parts of
transmembrane protein.
Examples of Transmembrane Proteins demonstrating
transmembrane secondary structural motifs:
1. glycophorin A (erythrocyte membranes)
2. bacteriorhodopsin (purple membrane of Halobacterium halobium, a saltloving bacterium)
3. prostaglandin H2 synthase (COX, enzyme involved in biosynthesis of
prostaglandins/inflammatory response)
4. porins (channel-forming proteins -- outer membranes of gram-negative
bacteria and outer membranes of mitochondria and chloroplasts)
1. Glycophorin A:
•
•
•
•
has a single transmembrane α-helix
integral membrane protein in erythrocytes
total MW ~31,000 but only 131 aa residues
Remember, average "residue mass" of an amino acid residue in a protein
is about 110, so glycophorin A would have MW about 14,400.
• Where does additional mass come from?
• Glycophorin A is a glycoprotein
by mass ~60% carbohydrate, ~40% protein.
• Most of protein (N-terminal portion) on outside of cell, exposed to water;
mainly hydrophilic residues, heavily glycosylated (lots of carbohydrates in
glycosidic bonds to specific Ser, Thr, and Asn residues)
• Carbohydrates include ABO and MN blood group antigen-determining
structures.
• Extracellular part of protein also receptor for influenza virus binding to cells
• C-terminal portion on cytosolic side of membrane, interacts with
cytoskeletal proteins
• One 19-AA residue hydrophobic segment is exactly right length to span
membrane if it’s coiled into an α-helix -- with hydrophobic R groups
oriented outward, toward "solvent" (hydrophobic core of lipid bilayer).
LEC 19, Membranes 2 - Membrane
Proteins
7
BIOC 460, Spring 2008
• Hydrophobic 19-20 amino acid sequences are a very common way for
proteins to span biological membranes, in α-helical conformation.
• Polar peptide backbone groups (carbonyl oxygens and amide N-H groups)
fully hydrogen-bonded
– Hydrogen-bonding "neutralizes" these polar groups, and
– "screens" them from contact with lipid core by R groups on outside of
helix.
• hydrophobic core of membrane (2X length of tails) is ~30 Å across, so 20residue α-helix (20 residues x 1.5 Å "rise" per residue) is right length to
reach across hydrophobic core.
Berg et al., Fig. 12-27a
2. Bacteriorhodopsin
• 7 transmembrane α-helices (common motif for integral membrane
proteins involved in signaling processes, "7-TM helix receptors")
• from purple membranes of bacteria of the genus Halobacterium, salt-loving
archaebacteria
• 247 amino acid residues (26,000 MW)
• When oxygen is scarce, uses light energy to pump protons across
membrane, out of cell, against a concentration gradient (example of
primary active transport)
• generates and maintains [H+] gradient (pH gradient) across cell membrane
• Resulting transmembrane proton gradient = "stored" potential energy,
used by a different protein (ATP synthase) to drive ATP synthesis in a
photosynthetic mode
• globular shape,
most of protein
embedded in
membrane
•Jmol structure of
bacteriorhodopsin)
Berg et al., Fig. 12-18
LEC 19, Membranes 2 - Membrane
Proteins
8
BIOC 460, Spring 2008
2. Bacteriorhodopsin
• Most of protein structure in 7 closely packed transmembrane helices
arranged in a bundle almost perpendicular to plane of membrane
• outer sides of helices (interact with hydrophobic interior of membrane):
many hydrophobic R groups
• "inner" sides of helices, facing interior of bundle, have some charged
residues (some charged/polar residues interact with cofactor (retinal),
some involved in proton translocation)
• Arrangement sort of opposite of water-soluble globular proteins: in an
integral membrane protein, not only are most R groups on interior of
protein hydrophobic, but R groups on outside of protein are hydrophobic,
in contact with
membrane lipid
core.
(Can have
hydrophilic groups
in interior if needed
to interact with
prosthetic group
like retinal, or with
water in channels,
as in porin
structures)
Berg et al., Fig. 12-18
3. Prostaglandin H2 synthase
• COX, enzyme involved in biosynthesis of prostaglandins/inflammatory
response
• cyclooxygenase (COX) activity inhibited by aspirin and other nonsteroidal
anti-inflammatory drugs (NSAIDs) like ibuprofen, naproxen, Celebrex, Vioxx,
etc.)
• integral membrane protein, homodimeric, with subunit structures primarily
α-helical
• protein does NOT span membrane -- it's on surface of endoplasmic
reticulum (ER) membrane, extending into lumen of ER, but firmly anchored
in membrane by a set of α-helices with hydrophobic R groups
extending into membrane core.
Berg et al., Fig. 12-23
LEC 19, Membranes 2 - Membrane
Proteins
Berg et al.,
Fig. 12-24
9
BIOC 460, Spring 2008
3. PGH2 synthase
• REVIEW the notes on enzyme inhibition and examples of irreversible and
reversible inhibitors, and lipids notes on phospholipases.
• substrate, arachidonic acid, is a 20-C fatty acid (VERY hydrophobic)
released from membrane lipids by PLA2.
• Substrate can enter enzyme active site via hydrophobic channel in the
enzyme without entering aqueous environment.
• NSAIDs block that channel, inhibiting the enzyme, preventing prostaglandin
synthesis, and thus reducing inflammation. (What type of inhibitor would
naproxen be -- competitive, noncompetitive, or uncompetitive?)
• Jmol structure of COX (cyclooxygenase) with inhibitor in substrate entry
channel (Aspirin acetylates Ser 530 residue in channel.)
Berg et al., Fig. 12-23
Berg et al.,
Fig. 12-24
4. Porins
• (This is a review from Lecture 6, so we will not go over it again in class, but
it's part of the Lecture 19 material.)
• channel-forming proteins found in outer membranes of gram-negative
bacteria and also in outer membranes of mitochondria and chloroplasts
trimers of 3 identical subunits in the membrane (homotrimers)
• each subunit a large (16 or 18-stranded) antiparallel β sheet rolled up into
a large β barrel
• Remember, R groups in β sheets alternate "sticking out" on opposite sides
of the sheet.
• Polar R groups line aqueous
central channel across membrane
and also for residues interacting with
H2O at surfaces of membrane).
• Hydrophobic R groups face outside
of barrel (in contact either with other
subunits or with hydrophobic core of
membrane).
• Polar peptide backbone groups
(carbonyl O and amide N-H groups)
fully hydrogen-bonded in β barrel
secondary structure.
Berg et al., Fig. 2-50
LEC 19, Membranes 2 - Membrane
Proteins
10
BIOC 460, Spring 2008
Structure of one subunit of a bacterial porin
• Jmol structure of a porin subunit
• left: side view, in plane of membrane; right: view from periplasmic space
(from inside, looking out through pore in outer membrane)
Berg et al., Fig. 12-20
On a single strand in β conformation,
where are the side chains of the amino acid
residues? (all on one side? Alternating sides?
3 residues’ R groups on one side, 1 on the other side?)
Amino acid sequence of a porin
• β strands are indicated, with diagonal lines indicating direction of
hydrogen bonding along the β sheet
• hydrophobic residues (F, I, L, M, V, W and Y) shown in yellow
Berg et al., Fig. 12-21
•Note the more or less alternating hydrophobic and hydrophilic residues
in the β strands (adjacent R groups project out from sheet on opposite sides).
LEC 19, Membranes 2 - Membrane
Proteins
11