* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Centrosome Dynamics during the Meiotic Progression in the Mouse
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cell growth wikipedia , lookup
Kinetochore wikipedia , lookup
List of types of proteins wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Spindle checkpoint wikipedia , lookup
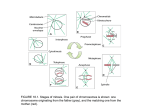
Eur J Clin Chem Clin Biochem 1996; 34:659-660 © 1996 by Walter de Gruyter · Berlin · New York LETTER TO THE EDITOR Centrosome Dynamics during the Meiotic Progression in the Mouse Oocyte Sarah Baatout. Paul Jacqitet, Louis de Saint-Georges and Lucile Baugnet-Mahieu Laboratory of Radiobiology, Belgian Nuclear Energy Study Centre, CEN-SCK, Mol, Belgium Sir, The centrosome is the most important microtubule organizing centre and a major point for microtubule growth within the cell. Because of their microtubule nucleating capacity, centrosomes are responsible for many functions, such as the organization of the interphase cytoskeleton and cytoplasm and the formation of the mitotic spindle. Centrosomes are known to participate in the location of the cleavage furrow during cytokinesis (1). In this context, it is useful to note that several types of proteins including motor molecules such as kinesin (2) and dynein (3), microtubule-associated proteins such as the microtubule associated protein MAPI (4), Ca/calmodulin kinase II (5), centractin and centrin (6) are associated with centrosomes. In animals, almost all mitotic cells possess two centrosomes, one at each spindle pole, consisting of a pair of centrioles surrounded by osmiophilic material called pericentriolar material and which contains the microtubule nucleating capacity of the centrosome. However, plant cells, yeast and fungi, and most notably, mammalian oocytes are known to divide without centrioles (7). In the mouse, centrioles are still present in oogonia and early oocytes. With respect to cytoplasmic centrosomes, their consistent reduction in number from the germinal vesicle stage to early diakinesis, coupled with their increase in size at this stage, suggest that centrosomal material aggregates at this early step of maturation. This is followed in prometaphase by increased numbers of smaller centrosomes that are no longer detectable in metaphase of meiosis-I but reappear at anaphase. Coupled with the total absence of immunodetectable centrosomes during telophase and their re-emergence at metaphase-II, cycles of aggregation, dispersion and reassembly seem to occur at specific cell cycle transitions. Unlike mitotic cells, metaphase-II-arrested mouse oocytes contain, in addition to spindle pole-associated centrosomes, a population of nonspindle-associated microtubule organizing centres (8). After fertilization, those organizing centres nucleate microtubules and participate in pronuclear movement and in the formation of subsequent mitotic spindles (8). In addition, it is not until the late morula and early blastocyst that centrioles reappear during preimplantation development in the mouse. In the oocyte, the ability to form asters is temporally correlated with an increase in maturation promoting factor activity which occurs upon meiotic resumption (9). The cell cycle of eukaryotes is regulated in part by the maturation promoting factor, which is a complex formed by the cdc2 (a serine/threonine kinase) and cyclin B (a protein which is synthesized and degraded in a cell cycle dependent manner). The maturation promoting factor activity increases greatly from the beginning of mitosis, is maximal at metaphase, and thereafter abruptly decreases thus stimulating mitotic events. In the mouse, it has been shown that maturation promoting factor level peaks at metaphase of meiosis-I and -II, with low levels detectable between these stages (10). When exogenous cdc2 kinase is added to isolated centrosomes, both phosphorylation and Mphase microtubule growth dynamics result, suggesting that cdc2 can regulate changes in centrosome-directed microtubule dynamics (11). Furthermore, investigations of Gotoh and colleagues have shown that maturation promoting factor acts locally to regulate spindle formation and that regulation of cdc2 itself is dependent on microtubules (12). Immunodetectable centrosomes are always phosphorylated at cell cycle steps characterized by high levels of maturation promoting factor; conversely, at telophase, when centrosomes are not immunodetectable, phosphorylated foci are absent when maturation promoting factor level is known to be low. This suggests that the phosphorylation status of centrosomal material would determine the apparent assembly and disassembly dynamics of centrosomes correlated with specific cell cycle stage during meiotic maturation. The suppression of centrosome-nucleated microtubule assembly observed at metaphase-I and -II occurs when both maturation promoting factor and cytostatic factor levels are thought to be elevated. Purified centrosomes injected into unactivated metaphaseII-arrested Xenopus oocytes do not support microtubule growth. However, when these eggs are artificially activated, microtubule growth occurs. Since the increase in nucleation capacity appears in conjunction with the loss of cytostatic factor, it is possible that cytostatic factor is involved in the differential regulation of microtubule dynamics of the centrosome during both metaphase-ί and -II. Cytostatic factor has been identified as the c-mos protooncogene product and its destruction at fertilization is dependent on the calcium-activated enzyme, calpain (9). In addition, the interaction between c-mos protein and tubulin suggests that c-mos protein may bind to tubulin subunits and prevent assembly or it may act directly on the centrosome to alter microtubule dynamics (13). Vertebrate oocytes are also unique in exhibiting asymmetric cleavage at the time of polar body formation. The polarity exhibited by the mammalian oocyte at the lime of polar body formation has been thought to reflect the effect of the meiotic spindle on the oocyte plasma membrane and subcortical cytoplasm. Documented examples of this polarity include changes in the cortical organization of the oocyte membrane during maturation, with the establishment of an acttn-rich microvillus-free domain overlying the spindle, that exhibit differences in lipid and protein mobility and the exclusion of cortical granules (14). Whether a functional relationship exists between cytoplasmic centrosomes and polarity determination in the oocyte remains to be established. Since the mouse oocyte appears to segregate its centrosomes into spindle organizing and cytoplasmic components, it is possible that this structural segregation is used to coordinate the unique demands of karyokinesis and cytokinesis for asymmetric cleavage during meiotic progression. Further studies will be necessary in order to characterize the functional roles of centrosome populations during the meiotic cell cycle. Letter to the editor 660 References 1. Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol 1986; 105:245-81. 2. Neighbors BW, Williams RC, Mclntosh JR. Localization of kinesin in cultured cells. J Cell Biol 1988; 106:1193-204. 3. Pfarr CM, Cove M, Grisson PM, Hays TS, Porter ME, Mclntosh JR. Cytoplasmic dynein localizes to kinetochores during mitosis. Nature 1990; 345:263-5. 4. Sherline P, Mascaro RN. Epidermal growth factor induces rapid centrosomal separation in HeLa and 3T3 cells. J Cell Biol 1982; 93:507-11. 5. Ohta Y, Ohta T, Miyamoto E. Ca/calmodulin-dependent protein kinase II: localisation in the interphase nucleus and the mitotic apparatus of mammalian cells. Proc Natl Acad Sei USA 1990; 87:5341-5. 6. Rout M, Kilmartin J. Components of the yeast spindle and spindle pole body. J Cell Biol 1990; 111:1913-27. 7. Maro B, Karsenti E. Centrosomes and the spatial distribution of microtubules in animal cells. Trends Biochem Sei 1986; 11:460-3. 8. Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sei USA 1986; 83:105-9. 9. Sagata N, Wanatabe N, Vande Woude GF, Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 1989; 342:512-8. 10. Hashimoto N, Kishimoto T. Regulation of meiotic metaphase by a cytoplasmic maturation-promoting factor during mouse oocyte maturation. Dev Biol 1988; 126:242-52. 11. Verde F, Berrez J, Antony C, Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xeriopus eggs: requirement for phosphorylated factors and cytoplasmic dynein. J Cell Biol 1991; 112:1177-87. 12. Gotoh Y, Nisihia E, Matsuda S, Shiina N, Kosako H, Shiokawa K, et al. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature 1991; 349:251-4. -r 13. Zhou R, Oskarsson M, Paules PS, Schulz N, Cleveland D, Vande Woude GF. Ability of the c-mos product to associate with and phosphorylate tubulin. Science 1991; 251:671-5. 14. Ducibella T, Anderson E, Albertini DF, Aalberg J, Rangarajan S. Quantitative studies of changes in cortical granule number and distribution in the mouse oocyte during meiotic maturation. Dev Biol 1988; 130:184-97. Received April 4/May 27, 1996 Corresponding author: Sarah Baatout, Laboratory of Radiobiology, Belgian Nuclear Study Energy Centre, CEN-SCK, Boeretang 200, B-2400 Mol, Belgium Eur J Clin Chem Clin Biochem 1996; 34:661 © 1996 by Waiter de Gruyter · Berlin · New York LETTER TO THE EDITOR Searching for an International Name for an Old Discipline Xavier Fuentes-Arderiu Servei de Bioquimica Clinica, Ciutat Sanitaria i Universitäria de Bellvitge, UHospitalet de Llobregat, Barcelona, Spain Sir, The advancements in the European Union will it make necessary to adapt and harmonize many areas, including the professional fields of the scientific disciplines which have considerable differences among the European countries (1). This also applies, of course, to the discipline corresponding to the activity carried out in a general clinical laboratory. This discipline may be defined as the branch of health sciences devoted to the in vitro observation of biological properties useful in prevention, diagnostics, prognostics and monitoring of diseases, by means of the techniques of the basic sciences; in other words, it is the set of knowledge, considered as a whole, belonging to the individual disciplines Clinical Biochemistry, Clinical Immunology, Clinical Microbiology, Clinical Parasitology and Laboratory Haematology (though in some countries Anatomical Pathology and Clinical Toxicology are also included). But what is the name of this discipline? In the European Union there are different names depending on the country. The most usual names are: Clinical Analyses (e. g. in Spain), Clinical Biology (e. g. in Belgium), Clinical Pathology (e.g. in Portugal), Laboratory Diagnostic Pathology (e. g. in Italy), Laboratory Medicine (e. g. in Germany), and Medical Biology (e. g. in France). In my opinion, within the European Union — and ideally within the world — a unique name to refer to this branch of scientific knowledge should exist. This unique name should have two essential characteristics: (i) to avoid any preponderance, it should be a name that is not traditionally used in any country of the European Union, and (ii) it should be a name not in conflict with the diversity of the university background (graduates in biology, chemistry, medicine, pharmacy, etc.) of the professionals of such activity. A possible solution may be the name Clinical Laboratory Sciences. I strongly uphold this term because it has the above characteristics and is the most useful to describe this plural discipline. This opinion is probably supported by many clinical laboratory professionals, as suggested from the public-opinion poll about the preference between the slogans "Serving clinical laboratory science worldwide" and "Serving laboratory medicine worldwide", proposed by the Journal of the International Federation of Clinical Chemistry as a reaction to a Letter to the Editor (2). The votes collected by H. J. Lin, chair of the Editorial Board of the journal, were: 49 votes for "Serving clinical laboratory science worldwide", and 10 votes for "Serving laboratory medicine worldwide" (Lin HJ, personal communication, 1993). Dealing with scientific matters, reason should always prevail over sentiment! References 1. Büttner J. Clinical chemistry: a professional field for physicians and natural scientists in Europe. Eur J Clin Chem Clin Biochem 1991; 29:3-12. 2. Fuentes-Arderiu X, Castineiras-Lacambra MJ. Laboratory medicine or clinical laboratory sciences? J Int Fed Clin Chem 1992; 4:136. Received October JO, 1995 Corresponding author: Dr. X. Fuentes-Arderiu, Servei de Bioquimica Clinica, Ciutat Sanitaria i Universitaria de Bellvitge, E-08907 L'Hospitalet de Llobregat, Barcelona, Spain