* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Life after meiosis: patterning the angiosperm male gametophyte
Cell membrane wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell culture wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell growth wikipedia , lookup
Cellular differentiation wikipedia , lookup
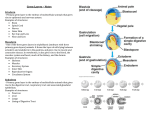
Cell–Cell Communication in Plant Reproduction Life after meiosis: patterning the angiosperm male gametophyte Michael Borg1 and David Twell Department of Biology, University of Leicester, Leicester LE1 7RH, U.K. Abstract Pollen grains represent the highly reduced haploid male gametophyte generation in angiosperms. They play an essential role in plant fertility by generating and delivering twin sperm cells to the embryo sac to undergo double fertilization. The functional specialization of the male gametophyte and double fertilization are considered to be key innovations in the evolutionary success of angiosperms. The haploid nature of the male gametophyte and its highly tractable ontogeny makes it an attractive system to study many fundamental biological processes, such as cell fate determination, cell-cycle progression and gene regulation. The present mini-review encompasses key advances in our understanding of the molecular mechanisms controlling male gametophyte patterning in angiosperms. A brief overview of male gametophyte development is presented, followed by a discussion of the genes required at landmark events of male gametogenesis. The value of the male gametophyte as an experimental system to study the interplay between cell fate determination and cell-cycle progression is also discussed and exemplified with an emerging model outlining the regulatory networks that distinguish the fate of the male germline from its sister vegetative cell. We conclude with a perspective of the impact emerging data will have on future research strategies and how they will develop further our understanding of male gametogenesis and plant development. Introduction Plant development is a continuous and highly plastic process that originates from populations of undifferentiated pluripotent stem cell niches called meristems. The interplay between cell proliferation and differentiation is critical for proper development and is tightly co-ordinated both temporally and spatially in response to intrinsic and extrinsic cues. This interrelationship dictates the patterning and growth of single cells right through to their organization at the multicellular level into tissues and organs [1]. Our understanding of how these processes are integrated has mostly come through observations at the individual cell level [2]. The male gametophyte represents an excellent system to exploit since its simple cell lineage and highly orchestrated development makes it ideal to dissect the molecular control of cellular patterning and its integration with the cell cycle. In angiosperms, the male gametophyte plays a vital role in plant fertility and crop production through the generation and delivery of the male gametes to the embryo sac for double fertilization. Major advances in genetic and genomic technologies have fuelled significant progress in our understanding of male gametophyte development at the molecular level [3]. Comprehensive transcriptomic Key words: Arabidopsis, asymmetric division, cell cycle, cell fate, germline, male gametophyte. Abbreviations used: APC, anaphase-promoting complex; CDK, cyclin-dependent kinase; CYCB1;1, cyclin B1;1; FBL17, F-box-like 17; GCS1, GENERATIVE CELL SPECIFIC 1; GEM1, GEMINI 1; GEX2, GAMETE-EXPRESSED2; MGH3, MALE GAMETE-SPECIFIC HISTONE H3; PAKRP1, phragmoplastassociated kinesin-related protein 1; Rb, retinoblastoma; RBR, Rb-related; TIO, two in one; TUBG, γ -tubulin. To whom correspondence should be addressed (email [email protected]). 1 Biochem. Soc. Trans. (2010) 38, 577–582; doi:10.1042/BST0380577 datasets from Arabidopsis are readily available [4], including those from the male gametophyte [5,6] and recently from isolated sperm cells [7]. Moreover, the identification of various gametophytic mutants has been pivotal in identifying genes required for cellular patterning of the male gametophyte. In the present paper, we review some of the key advances made in understanding male gametophyte development, with a focus on genetic studies of landmark events required to pattern the highly reduced male gametophyte. We refer the reader to other reviews that comprehensively discuss other aspects of male gametophyte patterning, including transcriptomic advances [3,8–11]. We also outline a model of the emerging regulatory network governing male germline specification and maintenance during male gametophyte development. Overview of male gametophyte development In animals, germline cells are determined early in embryogenesis and remain as a distinct population of stem cells throughout life [12,13]. In contrast, the male germline in angiosperms is only established after meiosis, when haploid microspores divide asymmetrically to form a small germ cell and large vegetative cell. Unlike the animal germline, the plant male germline is not regenerated through mitotic stem cell divisions, but instead undergoes a single round of mitosis to produce the functional pair of sperm cells required for double fertilization. Formation of the male gametophyte in angiosperms takes place within the stamens and the landmark events C The C 2010 Biochemical Society Authors Journal compilation 577 578 Biochemical Society Transactions (2010) Volume 38, part 2 Figure 1 Male gametophyte development in Arabidopsis Schematic diagram representing the distinct morphological stages of male gametophyte development in Arabidopsis. Diploid pollen mother cells undergo meiotic division to produce a tetrad of haploid microspores. The released microspores undergo a highly asymmetric division to produce a bicellular pollen grain with a small germ cell engulfed within the cytoplasm of a large vegetative cell. Whereas the vegetative cell exits the cell cycle, the germ cell undergoes a further mitotic division to produce twin sperm cells. The sperm cells then continue through the cell cycle to reach G2 -phase before karyogamy and double fertilization. A colour-coded timeline of the cell-cycle progression in each cell lineage is provided. Listed on the timeline are mutations that affect male gametophyte development at the point at which they are known to act. PMI, pollen mitosis I; PMII, pollen mitosis II. are outlined in Figure 1. Diploid pollen mother cells first undergo meiotic division to produce tetrads of haploid microspores. The microspores released then enlarge, and the microspore nucleus migrates to a peripheral position. The microspore then undergoes an asymmetric cell division, and the small germ cell subsequently becomes engulfed within the cytoplasm of the larger vegetative cell. The vegetative cell has dispersed nuclear chromatin and exits the cell cycle in G1 phase, whereas the germ cell has condensed nuclear chromatin and continues through a further round of mitosis to produce twin sperm cells. Male gametophyte patterning mutants Asymmetric division of the microspore is a vital process during male gametogenesis since it marks the stage at which the germline is set aside during pollen development. The resulting germ cell will then undergo a further mitotic division along with cell specification to produce twin sperm cells (Figure 1). The haploid nature of the gametophyte genome makes it a powerful model to test genetics-led hypotheses, and many studies have exploited gametophytic genetics to discover genes that are important for male gametophyte development (Figure 1). Genes regulating asymmetric division The asymmetry of microspore division is critical for the formation of the male germline, as induced symmetric C The C 2010 Biochemical Society Authors Journal compilation division results in two daughter cells that both follow a default vegetative cell fate programme [14]. Very few mutants and their corresponding genes have been isolated that affect asymmetric microspore division, and, perhaps unsurprisingly, most of these genes have important general cellular functions. Nevertheless, these are essential in establishing and maintaining the cell polarity required for asymmetric division and germ cell fate. MOR1 (MICROTUBULE ORGANIZATION 1)/ GEM1 (GEMINI 1) is a member of the MAP215 family of microtubule-associated proteins and plays a vital role in microspore polarity and cytokinesis by stimulating the growth and stability of interphase, spindle and phragmoplast microtubule arrays [15–17]. Similarly, the two functionally redundant γ -tubulin genes, TUBG1 and TUBG2, demonstrate further the importance of microtubules in establishing correct microspore division asymmetry [18]. In contrast with the above mutants, two-in-one (tio) microspores complete asymmetric nuclear division, but fail to complete cytokinesis, resulting in binucleate pollen grains. TIO is the plant homologue of the serine/threonine protein kinase FUSED [19] and localizes to the phragmoplast midline to play an essential role in centrifugal cell plate expansion. This could operate through the canonical NACK-PQR MAPK (mitogen-activated protein kinase) pathway [20] at the cell plate, since HINKEL (AtNACK1 in Arabidopsis) and TETRASPORE (AtNACK2 in Cell–Cell Communication in Plant Reproduction Figure 2 A model of the regulatory events that distinguish male germ cell and vegetative cell fate Following asymmetric division of the microspore, the cell-cycle inhibitors KRP6 and KRP7 are present in both the germ cell and vegetative cell. Transient expression of FBL17 specifically in the germ cell leads to the degradation of these KRPs, allowing CDKA to complex with cyclin D and subsequently phosphorylate RBR. Upon phosphorylation, RBR-mediated repression of the E2F/DP pathway is relieved and allows progression of the germ cell through S-phase. FBL17 is not expressed in the vegetative cell, and so CDKA inhibition by high levels of KRP6 and KRP7 is maintained, resulting in continued repression of the E2F/DP pathway by unphosphorylated RBR. Together, these two pathways serve to enforce vegetative cell fate by preventing entry into the cell cycle. Gamete specification begins shortly after germ cell division, where the expression of DUO1 and DUO3 in the germ cell leads to the activation of common and distinct germline differentiation genes. Once S-phase has been completed, the DUO1-dependent activation of the G2 /M-phase regulator CYCB1;1 promotes germ cell-cycle progression and entry into mitosis. In parallel, DUO3 also controls G2 /M-phase transition, independent of CYCB1;1, but involving unknown mechanisms. DUO1 and DUO3 therefore integrate germline differentiation with cell-cycle progression. Ultimately, the co-operation of these parallel pathways results in a pair of fully differentiated sperm cells equipped with a complement of essential germline factors such as GCS1 that are required for successful gamete fusion. Arabidopsis) double-mutant microspores show cell plate expansion and cytokinesis defects similar to tio microspores [21]. Two functionally redundant microtubule motor kinesins, PAKRP1 (phragmoplast-associated kinesin-related protein 1)/kinesin-12A and PAKRP1L (PAKRP1-like)/kinesin12B, also localize to the midline of the phragmoplast, and double-mutant microspores fail to form a well-organized antiparallel microtubule array between reforming nuclei [22]. It is evident that symmetrical cell division leads to failure of germline formation. These observations strengthen the hypothesis that correct differentiation of the germ cell lineage depends on persistent cell fate determinants being correctly segregated during asymmetric division [14]. Furthermore, the failure to clearly establish germ cell fate in gem1 [15] and tio [19] mutants, which arise from failure of cytokinesis, indicates that the confinement of such cell fate determinants by a new cell wall is critical to ‘seal in’ germ cell fate. Basic components of cellular machinery are thus important for patterning the male gametophyte and demonstrate how the orderly completion of cell-cycle events is intricately linked with cellular differentiation. The major outstanding challenge is not only to identify additional components involved in establishing microspore polarity, but also to discover the linked cell fate determinants responsible for specifying the differing fates of the vegetative and germ cells. Genes required for germ cell division The differential control of cell-cycle progression in the vegetative and germ cells is paramount in ensuring that twin C The C 2010 Biochemical Society Authors Journal compilation 579 580 Biochemical Society Transactions (2010) Volume 38, part 2 sperm cells are produced for double fertilization. A number of mutants have been described in Arabidopsis that produce bicellular pollen (a single germ cell within the vegetative cell) due to failure of germ cell division. Components of the canonical cell-cycle machinery are expected to play essential roles in germ cell division, and the analysis of CDKA;1 (A-type cyclin-dependent kinase) in Arabidopsis has revealed an essential role in germ cell division [23,24]. Germ cell division fails in cdka;1 mutants, whereas the DNA synthesis (S-) phase is delayed. A very similar phenotype to the cdka;1 mutant is observed when the FBL17 (F-box-like 17) gene is disrupted [25,25a]. Substrate-specific F-box proteins associate with Skp1 and CUL1 to form SCF (Skp1/Cullin/F-box) E3 ubiquitin protein ligase complexes [26,27]. FBL17 is transiently expressed only in the male germ cell after asymmetric microspore division and targets the CDK (cyclin-dependent kinase) inhibitors KRP6 and KRP7 for proteasome-dependent degradation, enabling the germ cell to progress through S-phase [25]. Conversely, the persistence of KRP6/KRP7 in the vegetative cell is proposed to maintain inhibition of CDKA activity and vegetative cell-cycle progression. Germline-specific expression of FBL17 thus enables differential control of the cell cycle in the germ and vegetative cells, effectively licensing germ cells for progression through S-phase (Figure 2). Similarly, proteasome-mediated degradation of KRP6 in microspores by two RING-finger E3 ligases, RHF1a and RHF2a, allows regular progression through microspore and subsequent germ cell mitotic cell cycles during male gametophyte development [28]. It is well established that the Rb (retinoblastoma)-E2F pathway controls cell-cycle progression in a variety of organisms [29], including plants [30,31]. Once phosphorylated by CDKs during the latter stages of G1 -phase, Rb repression of E2F/DP family transcription factors is alleviated, activating downstream cell-cycle genes that commit cells to S-phase [32]. A recent study has demonstrated that the plant homologue RBR (Rb-related) plays a pivotal role in male gametophyte patterning [33]. Loss of RBR does not have a major impact on microspore division, but does result in a complex array of phenotypes characterized by the limited hyperproliferation of the vegetative cell and, to a lesser extent, the germline [33]. Hyperproliferation in the absence of RBR is caused by CDKA;1 activity, since introduction of a cdka;1 mutant allele prevents cell proliferation in rbr pollen [33]. Cell fate is also incorrectly specified in a fraction of rbr vegetative cells. These rbr vegetative cells appear to retain the progenitorlike character of the microspore and attempt to imperfectly reiterate asymmetric division to produce an additional cell with a range of fate identities. These findings point to RBR primarily controlling cell-cycle progression, while having a secondary impact on cell fate commitment. This places RBR repression of the E2F/DP pathway downstream of KRPdependent CDKA;1 inhibition, thus both mechanisms cooperate to ensure commitment to vegetative cell fate by enforcing cell-cycle exit. C The C 2010 Biochemical Society Authors Journal compilation Integrating germ cell division with cell fate determination Single germ cell phenotypes are also characteristic of duo pollen (duo) mutants. Asymmetric microspore division is completed in these mutants; however, the resulting germ cell fails to undergo cell division [34]. In the duo1 mutant, germ cells complete S-phase, but fail to enter mitosis. DUO1 encodes a novel conserved R2R3 Myb protein specifically expressed in the male germline [35]. We have demonstrated recently that failure of mitotic entry in duo1 germ cells is caused by the lack of germline activation of the G2 /M-phase regulator cyclin B1;1 (CYCB1;1) [36]. Unlike fbl17 and cdka;1 mutant germ cells, which are able to exclusively fertilize the egg cell [24,25], duo1 pollen is unable to fertilize. This suggests that, in addition to cell-cycle defects, key features of gamete differentiation are incomplete in duo1. By monitoring the expression of genes associated with germ cell specification, we have demonstrated that, in addition to controlling germline expression of CYCB1;1, DUO1 also dictates correct differentiation of the male germline (Figure 2). The three molecular markers that are suppressed in duo1 pollen reported the expression of three germline-expressed genes: MGH3 (MALE GAMETE-SPECIFIC HISTONE H3), a male germline-specific histone H3.3 variant [37], GEX2 (GAMETE-EXPRESSED2), encoding a putative membraneassociated protein [38,39], and GCS1 (GENERATIVE CELL SPECIFIC 1) (HAP2), encoding a sperm cell-surface protein required for fertilization [40,41]. Thus the lack of GCS1 expression in the absence of DUO1 is sufficient to explain why duo1 pollen is infertile. In addition to the suppressed expression of these normally germline-specific markers in duo1 germ cells, ectopic expression of DUO1 is sufficient to activate their expression in a range of non-germline cells [36]. When DUO1 is ectopically expressed in seedlings, transcripts of MGH3, GEX2 and GCS1 are detected only in response to DUO1. Whereas these germline differentiation genes are activated in response to ectopic DUO1 expression, this is not sufficient to induce CYCB1;1 transcripts. Thus DUO1-dependent expression of CYCB1;1 in the germline may involve indirect transcriptional mechanisms or post-transcriptional controls. The recent discovery of DUO3 adds a further node to an emerging germline regulatory network (Figure 2). Like DUO1, DUO3 is a key regulatory protein that is required for germ cell division and correct gamete specification [42]. DUO3 is a conserved nuclear protein in land plants and contains domains related to GON-4, a cell lineage regulator of gonadogenesis in Caenorhabditis elegans and haemopoeisis in zebrafish [43,44]. duo3 germ cells either fail to divide or show a delay in division, and, unlike DUO1, DUO3 promotes entry into mitosis independently of CYCB1;1. A proportion of duo3 germ cells show persistent expression of CYCB1;1, indicating that failure of division in duo3 could be due to incomplete activation of the APC (anaphase-promoting Cell–Cell Communication in Plant Reproduction complex), which normally degrades CYCB1;1 to promote exit from mitosis [45]. Use of the same molecular markers that are suppressed in the duo1 mutant has identified distinct yet overlapping control of germline differentiation genes by DUO1 and DUO3. Complete expression of GCS1 and GEX2 requires both DUO1 and DUO3, whereas expression of MGH3 depends solely upon DUO1. The mechanism by which DUO1 and DUO3 activate the expression of common targets remains unknown, yet it has been proposed that they interact in a transcriptional complex or that they may act in tandem through a DUO3-dependent chromatin-remodelling route [42]. Whereas DUO1 expression is restricted to the male germline, DUO3 is widely expressed in sporophytic tissues, particularly at sites of cell division, as well as in both the vegetative cell and germ cell in developing pollen. Consistent with its wide expression pattern, analysis of duo3 homozygous plants revealed embryo developmental defects associated with a reduced rate and co-ordination of cell division [42]. Thus, like DUO1 in the male gametophyte, DUO3 has an essential developmental role in cell-cycle progression and cell specification, but DUO3 may function as a general regulator in both gametophytic and sporophytic tissues. Perspectives The wealth of genetic and genomic resources for various plant species now available makes this an exciting time for male gametophyte biology [8]. Comprehensive transcriptomic studies have massively increased our knowledge base of the complexity and dynamics of haploid gene expression in the developing gametophyte and sperm cells [5,7]. These transcriptomic datasets have already been exploited to discover a network level response of MIKC* MADS box transcription factors involved in pollen maturation [46,47]. Moreover, the recent application of novel proteomic technologies has further widened our perspective of pollen biochemistry [48,49]. Recent evidence has emerged for the expression and function of critical components of small RNA pathways in the developing male gametophyte [50,51]. These small RNA pathways add another layer to an already complex and diverse gametophytic gene expression programme [8]. The discovery of DUO1 and DUO3 provides compelling insights into how the processes of cell proliferation and cell differentiation are integrated during male germline development. Many challenges lay ahead if we are to fully understand the complexity and architecture of these overlapping regulatory networks. In addition to uncovering the plethora of downstream target genes and the nature of their activation, an unresolved issue is the regulation of these germline determinants and how their activation is linked with asymmetric division of the microspore. Analysis of the DUO1 promoter has provided encouraging evidence of upstream regulators, since its regulation is dependent on positive elements in its minimal promoter [36] and not on a proposed derepression mechanism involving the lily repressor protein GRSF (germline restrictive silencing factor) [36,52]. Furthermore, the pathways by which DUO1 and DUO3 co-ordinate germ cell division remain largely unknown. Analysing these mechanisms not only will provide further insights into male gametogenesis, but also will have an impact on our understanding of how cell-specific modules are recruited to co-ordinate cell-cycle progression using basic cell-cycle components. The future therefore promises to deliver some exciting and novel discoveries in male gametophyte biology. Exploitation of the male gametophyte as an experimental system has already had a major impact on our knowledge of fundamental cellular processes and the machinery that drives them. Much of this knowledge has been gained through transcriptomic analyses [8] and the genetic approaches discussed in the present review. The future challenge is to apply systematic approaches and to exploit new knowledge of gametophytic gene expression to formulate new hypotheses. Concerted efforts are thus expected to not only deliver a corroborated and system level perspective of the mechanisms controlling male gametogenesis and gamete function, but also provide insight into the complex interplay between cell proliferation and differentiation in plants. Funding Work in the Twell laboratory is supported by research grants from Biotechnology and the Biological Sciences Research Council (BBSRC). M.B. is supported by a BBSRC-funded studentship. References 1 2 3 4 5 6 7 8 9 10 11 12 Gutierrez, C. (2005) Coupling cell proliferation and development in plants. Nat. Cell Biol. 7, 535–541 Jakoby, M. and Schnittger, A. (2004) Cell cycle and differentiation. Curr. Opin. Plant Biol. 7, 661–669 Twell, D., Oh, S.-A. and Honys, D. (2006) Pollen development, a genetic and transcriptomic view. Plant Cell Monogr. 3, 15–45 Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L. and Gruissem, W. (2004) Genevestigator: Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136, 2621–2632 Honys, D. and Twell, D. (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol. 5, R85 Pina, C., Pinto, F., Feijo, J.A. and Becker, J.D. (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol. 138, 744–756 Borges, F., Gomes, G., Gardner, R., Moreno, N., McCormick, S., Feijo, J.A. and Becker, J.D. (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol. 148, 1168 Borg, M., Brownfield, L. and Twell, D. (2009) Male gametophyte development: a molecular perspective. J. Exp. Bot. 60, 1465 Becker, J.D. and Feijo, J.A. (2007) How many genes are needed to make a pollen tube? Lessons from transcriptomics. Ann. Bot. 100, 1117–1123 Honys, D., Renák, D. and Twell, D. (2006) Male gametophyte development and function. in Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st edn (da Silva, J.A.T., ed.), pp. 76–87, Global Science Books, London Singh, M.B. and Bhalla, P.L. (2007) Control of male germ-cell development in flowering plants. BioEssays 29, 1124–1132 Hayashi, K., de Sousa Lopes, S.M.C. and Surani, M.A. (2007) Germ cell specification in mice. Science 316, 394–396 C The C 2010 Biochemical Society Authors Journal compilation 581 582 Biochemical Society Transactions (2010) Volume 38, part 2 13 14 15 16 17 18 19 20 21 22 23 24 25 25a 26 27 28 29 30 31 32 C The Strome, S. and Lehmann, R. (2007) Germ versus soma decisions: lessons from flies and worms. Science 316, 392–393 Eady, C., Lindsey, K. and Twell, D. (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell 7, 65–74 Park, S.K., Howden, R. and Twell, D. (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125, 3789–3799 Whittington, A.T., Vugrek, O., Wei, K.J., Hasenbein, N.G., Sugimoto, K., Rashbrooke, M.C. and Wasteneys, G.O. (2001) MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613 Twell, D., Park, S.K., Hawkins, T.J., Schubert, D., Schmidt, R., Smertenko, A. and Hussey, P.J. (2002) MOR1/GEM1 plays an essential role in the plant-specific cytokinetic phragmoplast. Nat. Cell Biol. 4, 711 Pastuglia, M., Azimzadeh, J., Goussot, M., Camilleri, C., Belcram, K., Evrard, J.L., Schmit, A.C., Guerche, P. and Bouchez, D. (2006) γ -Tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18, 1412–1425 Oh, S.-A., Johnson, A., Smertenko, A., Rahman, D., Park, S.K., Hussey, P.J. and Twell, D. (2005) A divergent cellular role for the FUSED kinase family in the plant-specific cytokinetic phragmoplast. Curr. Biol. 15, 2107–2111 Sasabe, M., Takahashi, Y., Soyano, T., Tanaka, H., Kousetsu, K., Suzuki, T. and Machida, Y. (2006) The NACK-PQR MAP kinase cascade controls plant cytokinesis. in Biotechnology in Agriculture and Forestry 58. Tobacco BY-2 Cells: from Cellular Dynamics to Omics (Nagata. T., Matsuoka, K., Inzé and D., eds, eds), pp. 79–94, Springer, Berlin Oh, S.-A., Bourdon, V., Das ‘Pal, M., Dickinson, H. and Twell, D. (2008) Arabidopsis kinesins HINKEL and TETRASPORE act redundantly to control cell plate expansion during cytokinesis in the male gametophyte. Mol. Plant. 1, 794–799 Lee, Y.R.J., Li, Y. and Liu, B. (2007) Two Arabidopsis phragmoplastassociated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell 19, 2595–2605 Iwakawa, H., Shinmyo, A. and Sekine, M. (2006) Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 45, 819–831 Nowack, M.K., Grini, P.E., Jakoby, M.J., Lafos, M., Koncz, C. and Schnittger, A. (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat. Genet. 38, 63–67 Kim, H.J., Oh, S.-A., Brownfield, L., Hong, S.H., Ryu, H., Hwang, I., Twell, D. and Nam, H.G. (2008) Control of plant germline proliferation by SCFFBL17 degradation of cell cycle inhibitors. Nature 455, 1134–1137 Gusti, A., Baumberger, N., Nowack, M., Pusch, S., Eisler, H., Potuschak, T., De Veylder, L., Schnittger, A. and Genschik, P. (2009) The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS ONE 4, e4780 Petroski, M.D. and Deshaies, R.J. (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6, 9–20 Smalle, J. and Vierstra, R.D. (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55, 555–590 Liu, J., Zhang, Y., Qin, G., Tsuge, T., Sakaguchi, N., Luo, G., Sun, K., Shi, D., Aki, S., Zheng, N. et al. (2008) Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell 20, 1538–1554 Harbour, J.W. and Dean, D.C. (2000) The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14, 2393–2409 Wildwater, M., Campilho, A., Perez-Perez, J.M., Heidstra, R., Blilou, I., Korthout, H., Chatterjee, J., Mariconti, L., Gruissem, W. and Scheres, B. (2005) The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 123, 1337–1349 Ebel, C., Mariconti, L. and Gruissem, W. (2004) Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 429, 776–780 Weinberg, R.A. (1995) The retinoblastoma protein and cell cycle control. Cell 81, 323–330 C 2010 Biochemical Society Authors Journal compilation 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 Chen, Z., Hafidh, S., Poh, S.H., Twell, D. and Berger, F. (2009) Proliferation and cell fate establishment during Arabidopsis male gametogenesis depends on the retinoblastoma protein. Proc. Natl. Acad. Sci. U.S.A. 106, 7257–7262 Durbarry, A., Vizir, I. and Twell, D. (2005) Male germ line development in Arabidopsis: duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol. 137, 297–307 Rotman, N., Durbarry, A., Wardle, A., Yang, W.C., Chaboud, A., Faure, J.E., Berger, F. and Twell, D. (2005) A novel class of MYB factors controls sperm-cell formation in plants. Curr. Biol. 15, 244–248 Brownfield, L., Hafidh, S., Borg, M., Sidorova, A., Mori, T. and Twell, D. (2009) A plant germline-specific integrator of sperm specification and cell cycle progression. PLoS Genet. 5, 3 Okada, T., Endo, M., Singh, M.B. and Bhalla, P.L. (2005) Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J. 44, 557–568 Engel, M.L., Holmes-Davis, R. and McCormick, S. (2005) Green sperm: identification of male gamete promoters in Arabidopsis. Plant Physiol. 138, 2124–2133 Alandete-Saez, M., Ron, M. and McCormick, S. (2008) GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol. Plant 1, 586–598 Mori, T., Kuroiwa, H., Higashiyama, T. and Kuroiwa, T. (2006) Generative cell specific 1 is essential for angiosperm fertilization. Nat. Cell Biol. 8, 64–71 von Besser, K., Frank, A.C., Johnson, M.A. and Preuss, D. (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133, 4761–4769 Brownfield, L., Hafidh, S., Durbarry, A., Khatab, H., Sidorova, A., Doerner, P. and Twell, D. (2009) Arabidopsis DUO POLLEN3 is a key regulator of male germline development and embryogenesis. Plant Cell 21, 1940–1956 Friedman, L., Anna-Arriola, S.S., Hodgkin, J. and Kimble, J. (2000) Gon-4, a cell lineage regulator required for gonadogenesis in Caenorhabditis elegans. Dev. Biol. 228, 350–362 Liu, Y., Du, L., Osato, M., Teo, E.H., Qian, F., Jin, H., Zhen, F., Xu, J., Guo, L., Huang, H. et al. (2007) The zebrafish udu gene encodes a novel nuclear factor and is essential for primitive erythroid cell development. Blood 110, 99–106 Peters, J.M. (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7, 644–656 Verelst, W., Twell, D., de Folter, S., Immink, R., Saedler, H. and Munster, T. (2007) MADS-complexes regulate transcriptome dynamics during pollen maturation. Genome Biol. 8, R249 Verelst, W., Saedler, H. and Münster, T. (2007) MIKC* MADS–protein complexes bind motifs enriched in the proximal region of late pollen-specific Arabidopsis promoters. Plant Physiol. 143, 447–460 Grobei, M.A., Qeli, E., Brunner, E., Rehrauer, H., Zhang, R., Roschitzki, B., Basler, K., Ahrens, C.H. and Grossniklaus, U. (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res. 19, 1786–1800 Zou, J., Song, L., Zhang, W., Wang, Y., Ruan, S. and Wu, W.H. (2009) Comparative proteomic analysis of Arabidopsis mature pollen and germinated pollen. J. Integr. Plant Biol. 51, 438–455 Grant-Downton, R., Hafidh, S., Twell, D. and Dickinson, H.G. (2009) Small RNA pathways are present and functional in the angiosperm male gametophyte. Mol. Plant 2, 500–512 Chambers, C. and Shuai, B. (2009) Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol. 9, 87 Haerizadeh, F., Singh, M.B. and Bhalla, P.L. (2006) Transcriptional repression distinguishes somatic from germ cell lineages in a plant. Science 313, 496–499 Received 21 August 2009 doi:10.1042/BST0380577