* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the association of chloroplast dna with photosynthetic membrane
Nutriepigenomics wikipedia , lookup
DNA barcoding wikipedia , lookup
Mitochondrial DNA wikipedia , lookup
Comparative genomic hybridization wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Microevolution wikipedia , lookup
Genomic library wikipedia , lookup
Point mutation wikipedia , lookup
Primary transcript wikipedia , lookup
Nicotinic acid adenine dinucleotide phosphate wikipedia , lookup
SNP genotyping wikipedia , lookup
Cancer epigenetics wikipedia , lookup
DNA profiling wikipedia , lookup
DNA polymerase wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
DNA vaccination wikipedia , lookup
DNA damage theory of aging wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Genealogical DNA test wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
United Kingdom National DNA Database wikipedia , lookup
Molecular cloning wikipedia , lookup
Epigenomics wikipedia , lookup
Non-coding DNA wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Helitron (biology) wikipedia , lookup
Nucleic acid double helix wikipedia , lookup
DNA supercoil wikipedia , lookup
History of genetic engineering wikipedia , lookup
Deoxyribozyme wikipedia , lookup
J. Cell Sci. 36, 169-183 (1979)
Printed in Great Britain © Company of Biologists Limited
169
THE ASSOCIATION OF CHLOROPLAST DNA
WITH PHOTOSYNTHETIC MEMBRANE
VESICLES FROM SPINACH CHLOROPLASTS
R. J. ROSE
Department of Biological Sciences, The University of Newcastle,
Newcastle 2308, N.S.W., Australia
SUMMARY
To investigate the association between chloroplast DNA (cp DNA) and the photosynthetic
membranes of spinach chloroplasts, previously suggested by electron-microscope autoradiography, use has been made of vesicles formed by isolating chloroplasts directly in 3-5 mMMg3+.
These chloroplast vesicles consist of photosynthetic membranes, separate from chloroplast
envelope membranes. Light and electron microscopy confirm that the vesicles consist of swollen
stroma lamellar membranes with some peripheral grana lamellae that are much less swollen.
Vesicles labelled with [3H]thymidine were obtained from [3H]thymidine-labelled chloroplasts
from spinach disks in which chloroplast division and cp DNA synthesis and segregation were
occurring. The chloroplast vesicle fraction retains about 45 % of the cp DNA as determined by
liquid scintillation counting. The cp DNA-membrane associations do not appear to be dependent on the presence of Mg*+. The chloroplast vesicles can be autoradiographed for light
microscopy if they are fixed in formaldehyde and no centrifugation steps are used. Lightmicroscope autoradiography is consistent with a preferential labelling of grana as opposed to
stroma membranes, and long lengths of membrane are labelled. It appears that in spinach
chloroplasts cp DNA is associated with granal thylakoids at intervals along the length of a
continuous photosynthetic membrane system. Such an organization would facilitate cp DNA
segregation during chloroplast division.
INTRODUCTION
Chloroplasts contain DNA which codes for some of the proteins required for
chloroplast development and function (Ellis, 1977; Kung, 1977). In higher plants
there are many chloroplasts per cell, and chloroplast replication is associated with the
full development of the leaf mesophyll cell (Possingham & Saurer, 1969). Some
mechanism is required to ensure that chloroplast DNA (cp DNA) is transmitted to
chloroplast progeny.
The chloroplast replication process can be conveniently studied in cultured spinach
leaf disks, where, using autoradiography to detect [3H]thymidine incorporation into
chloroplasts, it has been shown that chloroplast division is associated with cp DNA
synthesis and an orderly transmission of the cp DNA to the daughter chloroplasts
(Rose, Cran & Possingham, 1974; Possingham & Rose, 1976). From electronmicroscope autoradiography of sectioned chloroplasts, and chloroplast fractionation
studies, it has been argued that it is the association of the cp DNA with the photosynthetic membranes that facilitates the cp DNA segregation process in spinach
chloroplasts (Rose & Possingham, 1976a). Furthermore, rare light-microscope
170
R. J. Rose
autoradiographs of whole chloroplasts showed continuous spiral labelling patterns
which suggested that the cp DNA molecules occurred at intervals along the lamellar
membrane system (Rose & Possingham, 1976 a). However, these latter autoradiographs could not be obtained routinely and a method using isolated photosynthetic
membranes to autoradiograph the photosynthetic membrane system was sought. In
this paper, chloroplast membrane vesicles have been used to study the association
of cp DNA with the photosynthetic membrane system.
MATERIALS AND METHODS
Incubation of cultured leaf disks with
^H]thymidine
Spinach (Spinacia oleracea L) leaf disks were obtained from the base of 2-cm-long leaves and
cultured on sterile nutrient agax using the methods of Possingham & Smith (1972). The leaf
disks were grown at 23 °C under fluorescent light of intensity 6 mW cm"1, with a 14-h day.
Twenty to twenty five disks per plate were incubated in 50 /tCi [6 — 'HJthymidine (20-30 Ci/mM,
Radiochemical Centre, Amersham, U.K.) added in 1 ml to the surface of the 20 ml of nutrient
agar medium in 9-cm Petri dishes. All manipulations to the cultured disks were made in a
sterile cabinet employing a laminar air-flow system. The incubation in [6 —3H]thymidine
('H — TdR) was continued for approximately 72 h (day o to day 3) which allows almost 3
cycles of chloroplast division, and ensures that all the cp DNA is extensively labelled (Possingham & Rose, 1976). In the experiment from which Figs. 16 and 17 were obtained, the incubation was from day 3 to day 6 where there would be about 2 cycles of division.
Isolation and cytology of chloroplasts and chloroplast vesicles
Intact chloroplasts were isolated in either 0-4 M sucrose + 3 5 mM MgClj (MgCL is subsequently referred to as Mg1+), or 0-35 M Mga+, while chloroplast vesicles were isolated in
3 5 mM Mg*+. The disks were razor chopped in the appropriate medium to release the
chloroplasts or vesicles, and then filtered through jo-fim steel mesh.
For light-microscope cytology the freshly isolated chloroplasts and chloroplast vesicles were
examined with phase-contrast or Nomarski interference-contrast optics using a Leitz Orthoplan
microscope.
For electron-microscope cytology a similar procedure to that of Fowke (1975) for isolated
plant protoplasts was used. The isolated chloroplasts or vesicles were fixed in centrifuge tubes
in their original isolation medium containing 1 % glutaraldehyde at pH 6-5 for 2 h at room
temperature. Following the initial fixation, the chloroplasts or vesicles were pelleted at 1000 g
and transferred to 3 % glutaraldehyde in isolation medium for a further 3 h. The isolated
chloroplasts and vesicles were washed in isolation medium, then washed with 0-05 M sodium
phosphate buffer at pH 6-8 at o °C. Postfixation in 1 % osmium tetroxide in 0-05 M sodium
phosphate buffer at pH 6-8 at o °C was carried out overnight. The chloroplasts and vesicles
were then washed with distilled water and dehydrated in an ethanol series at o °C, gradually
transferred to pure propylene oxide in glass vials at room temperature, and finally embedded
in Araldite in small alfoil trays. Thin sections were stained with uranyl acetate in 50 % ethanol
and lead citrate before viewing in an AEI EM 6 G electron microscope.
Autoradiography
Light-microscope autoradiography was carried out with isolated chloroplasts and chloroplast
vesicles following incubation of the spinach disks in [6 —'HJthymidine. Isolated chloroplasts
were fixed in 4 % formaldehyde in 01 M sodium phosphate buffer at pH 7-2 at o °C for 20 min
in test tubes, then transferred to 4% formaldehyde in 35 mM phosphate buffer at pH 7-2 for
at least 12 h at 5 °C. Isolated chloroplast vesicles were fixed directly in 4 % formaldehyde in
3-5 mM phosphate buffer at pH 72 at o °C for 20 min, then transferred to 5 °C for at least
12 h. The chloroplasts or chloroplast vesicles sedimented by gravity to the bottom of the tube
Association of chloroplast DNA with membranes
171
and the supernatant was removed and replaced with distilled water. The chloroplasts or chloroplast vesicles were pipetted on to a glass slide, air dried, and processed for autoradiography
using Ilford L4 emulsion. The autoradiographic techniques have been described, as has the
specificity of incorporation of 3H-TdR into DNA (Rose, Cran & Possingham, 1975). Following
the development of the slides they were dehydrated in an ethanol series and mounted in Euparal.
Silver grains over the chloroplast vesicles were scored as to whether they were associated
with grana-rich or stroma-rich membrane areas. Circular vesicles with a peripheral grana-rich
region were scored. This did not exclude vesicles which had grana across the stroma membrane
area or in the centre of the vesicle (Figs. 10-17). Vesicle diameter and stroma membrane
diameters were measured. The stroma membrane area was calculated directly, and the grana
membrane area by difference between the total vesicle area minus the central stroma area.
Grains per unit area over the different membranes were obtained from 100 vesicles scored
from slides from the same experiment as Figs. 10-15, but with an exposure period of 5-5 weeks.
Background values were obtained adjacent to each vesicle scored, and corrections made for the
counting of that particular vesicle.
[3H]thymidine incorporation into chloroplasts and chloroplast vesicles as determined by
liquid scintillation counting
Chloroplasts or 'chloroplast vesicles' were isolated in the appropriate isolation medium at
o °C as described earlier and immediately placed on a single step discontinuous sucrose gradient
of 20 % and 60 % sucrose and centrifuged at 27 500 g with a sorvall HB-4 rotor for 25 min. The
chloroplast fractions were collected from the 20 %/6o % interface and the sucrose diluted with
distilled water to resuspend the fraction and allow it to be placed at the top of the 20 %
sucrose. The fractions were then centrifuged and collected again using the same procedures as
for the first centrifugation. With this procedure nuclei go to the bottom of the tube and are
separated from the chloroplast fractions; it was routinely monitored with the light microscope.
After the double centrifugation procedure the chloroplasts and chloroplast vesicles were
used to determine chlorophyll concentrations and the incorporation of *H-TdR into trichloroacetic acid (TCA)-precipitable material.
Chlorophyll was determined by collecting an aliquot from the chloroplast fractions on a
Whatman GF/A glass fibre filter. The filter was washed thoroughly with 35 mM Mg2+, then
the chlorophyll extracted with 80 % acetone and estimated spectrophotometrically (Arnon,
1949)The incorporation of 'H-TdR into TCA-precipitable material was determined by precipitation with 5 % TCA containing 0-5 mg/ml unlabelled thymidine. The precipitate was washed
initially by centrifugation then collected on Whatman GF/A filters. The filters were washed
with s % TCA, ether-ethanol, and finally ether before air drying and counting in a toluenebased scintillation fluid.
Dialysis experiments
Chloroplast vesicles free of nuclei were collected as described above and 1 ml of known
chlorophyl concentration placed in dialysis tubing for dialysis against 1 1. of distilled water, or
in the case of controls for dialysis against 35 mM Mgs+. Dialysis was carried out for 24 h. The
dialysis solutions were changed twice. After dialysis the vesicles were pelleted at iooog and the
3
H-TdR present in TCA-precipitable material was determined, in both treatments.
RESULTS
When chloroplasts from cultured spinach disks are isolated in 3-5 mM
they form many structures similar to those in Figs. 1-3. It is well known that chloroplasts isolated in such hypotonic solutions lose their outer envelopes (Spencer &
Wildman, 1962; Douce, Holtz & Benson, 1973). The structures formed in Figs. 1-3
are large vesicles formed from the photosynthetic membranes. I have referred to
these structures as chloroplast vesicles.
172
I
R. J. Rose
5,
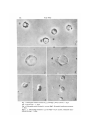
Fig. i. Chloroplast vesicles isolated in 35 mM Mg2+. phase-contrast, x 1450.
Fig. 2. As for Fig. 1. x 3450.
Fig. 3. Chloroplast vesicle isolated in 3-5 mM Mg*+. Nomarski interference-contrast,
x 3650.
Figs. 4, 5. Chloroplasts isolated in 3-5 mM Mg1+ + o'4M sucrose. Nomarski interference-contrast, x 2800.
Association of chloroplast DNA with membranes
173
The chloroplast vesicle structures are similar to those described by Spencer &
Wildman (1962) when using 20 raM sucrose and lower concentrations as osmoticum.
Subsequently, Spencer & Unt (1965) noted the ability of low concentrations of Mg2+
to form balloon-like structures and to stabilize grana and intergrana membranes
relative to the disruption that occurs in distilled water. Magnesium was preferred for
the study reported here as more and larger vesicles formed thanfrom a sucrose solution
of equal osmotic pressure and they preserved more effectively for subsequent autoradiography. The interpretation of Spencer & Wildman (1962) based on lightmicroscope cytology is that the vesicles arise by swelling of the stromal membranes,
and the grana which are more resistant to swelling, occur at the surface of these stromal
balloons. This latter interpretation is consistent with the cytological observations of
the current study. In Fig. 2 the grana appear as dark structures at the vesicle periphery,
while using Nomarski interference-contrast optics the grana appear as small raised
knobs (Fig. 3). Control chloroplasts isolated in 0-4 M sucrose + 3'5 mM Mg 2+ are
shown in Figs. 4 and 5. In the chloroplasts of Fig. 4 no grana can be visualized, while
they are just apparent in Fig. 5. Grana are visible in type II but not type I chloroplasts
(Spencer & Unt, 1965). Type I chloroplasts are morphologically intact while type
II chloroplasts have envelope breakage (Coombs & Greenwood, 1976) and were in
the minority (about 20%) in control preparations from which Figs. 4 and 5 were
obtained.
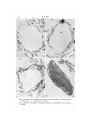
When the chloroplast vesicles are fixed in glutaraldehyde, processed, and examined
in the electron microscope they appear as in Figs. 6-8. They are bound by a membrane
to which adhere varying numbers of grana lamellae. The number of grana lamellae
seen in a single section will vary according to the section plane. The grana are partially
disrupted due to the isolation in the 3-5 mM Mg2+ and possibly due in part to the
difficulty in preserving the vesicles for electron microscopy. Nevertheless, grana
clearly are present at the vesicle periphery, and the vesicles form from stromal
membranes. It is clear from electron microscopy and light-microscope observations
of the vesicles that they are not uniformly covered with grana but there are a few
granal rich areas adhering to the surface of the vesicles. An isolated intact chloroplast
is shown in Fig. 9.
The chloroplast vesicles can be fixed and processed gently with minimal manipulation for light-microscope autoradiography. When the vesicles dry down on to the
slide only the grana rich regions can be readily visualized and the stroma appears
clear. Observations indicate that the grana rich regions adhere first to the slide and
then the remainder of the vesicle dries down. Many of these structures appear circular
surrounded by grana with central stroma regions (Figs. 15 and 16).
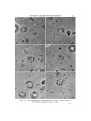
Autoradiographs of chloroplast vesicles are shown in Figs. 10-15, a n ^ show grains
following the vesicle periphery where there are granal rich regions. In Fig. 12 a line of
grains extends towards the centre of the dried-down vesicle, it is likely that this is
also a row of grana as at least some patterns like this will be seen, depending on how
many granal areas there are and their distribution around the vesicle. Uniform labelling of the vesicles would show grains right across the vesicle, and would indicate
stroma membrane labelling. Figs. 10-15 should be compared with Figs. 1-3.
12
CEL 36
Figs. 6-8. Electron micrographs of chloroplast vesicles isolated in 3-5 mM Mga+. Fig. 6,
x 11 800; Fig. 7, x 11 350; Fig. 8, x 15500.
Fig. 9. Electron micrograph of chloroplast isolated in 3-5 mM Mg1+ + 0'4 M sucrose,
x
26000.
Association of chloroplast DNA with membranes
Figs. 10-15. Autoradiographs of 3H-TdR-labelled chloroplast vesicles isolated in
3-5 mM Mg1+. Exposed 10 weeks, x 1900.
12-2
i76
R. J. Rose
•21
Association of chloroplast DNA with membranes
177
Further autoradiographs of chloroplast photosynthetic membranes are shown in
Figs. 16 and 17 from a separate experiment. The underlying membranes can be seen
more readily in these photographs. Fig. 16 is the type of pattern shown in Figs. 1015 where there are peripheral grana rich regions. Fig. 17 shows a partially swollen
chloroplast in which a number of swollen stromal regions are apparent, and the large
vesicles free of central grana have not formed from this structure. The silver grains
follow the relatively unswollen grana-rich regions, and not the swollen stromal regions.
Most vesicles observed show the reported labelling patterns. Such patterns are not
observed in the presence of the detergent Triton X-100 which solubilizes the
chloroplast membranes. If vesicles are located on the slide then checked for incorporation, 84% are clearly labelled at the level shown in Figs. 10-17. The remaining 16%
are virtually unlabelled.
To obtain a quantitative estimate of silver grain distribution in relation to grana
and stroma membrane areas, circular vesicles with a peripheral grana area were scored
as indicated in the Materials and methods section.
The grana membrane area is enriched in grana, but is not uniformly covered with
grana (Figs. 6, 7, 11, 15). The stroma membrane areas are enriched in stroma membranes but may contain grana (Figs. 12, 17). The peripheral grana pattern could arise
from a stroma membrane sheet interconnecting a row of grana on opposite sides of
the sheet. On swelling a vesicle with an encircling ring of grana could be formed (e.g.
Figs. 8, 9).
There are on average more than twice as many silver grains per unit grana-rich
membrane area, compared to per unit stroma-rich area, and two thirds of the vesicle
area is grana rich, giving four times as many grana grains (Table 1). A better indication
of a preferential grana membrane labelling pattern, can be seen from the frequency
distribution histograms of grains per unit area (Fig. 1). Thirty nine percent of the
vesicles show less than o-ioo grains//tm2 over stroma membranes compared with 1 %
of grana membranes having this labelling density. It is these vesicles with the low
stroma membrane labelling that have the clearest membrane separation pattern
(Figs. 10, 11, 13-16), while the more heavily labelled stroma areas would likely
contain grana (Figs. 12, 17). The range of values over the 2 membrane areas is fairly
similar, it is the distribution which is quite different.
While the grana membrane areas have more membrane material because of their
stacking, cp DNA would not be expected to occur between grana thylakoids. Electron
microscopy (Rose & Possingham, 1976a), suggests that the cp DNA is membrane
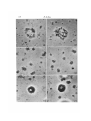
Figs. 16, 17. Autoradiographs of 3 H-TdR-labelled chloroplast vesicles isolated in
3-5 mM Mg 1 + . Separate experiment from that of Figs. 10-15. Exposed 6 weeks,
x 3800.
Figs. 18,19. Autoradiographs of 3 H-TdR-labelled chloroplasts isolated in 0-35 M Mg 2 + .
Same experiment as Figs. 10-15. Exposed 10 weeks, x 1900.
Fig. 20. Autoradiograph of 3 H-TdR-labelled nucleus, isolated in 3-5 mM Mg 1 + .
Same experiment as Figs. 10-15. Exposed 10 weeks, x 1900.
Fig. 21. Autoradiograph of 3 H-TdR-labelled nucleus, isolated in 0-35 M Mg 2 + . Same
experiment as Figs. 10-15. Exposed 10 weeks, x 1900.
178
R.J.Rose
attached, and extends into the stroma matrix. It seems more likely that the labelled
cp DNA is associated with the grana surface.
Autoradiographs of whole chloroplasts are shown in Figs. 18 and 19. These latter
chloroplasts were isolated in 0-35 M Mg 2+ and similar results were obtained from
Table 1. Silver grain distribution over chloroplast vesicles
Stromarich
Vesicle
diameter, diameter, Area,
flm
fim
/tm1
9/60
5-63
47'5i
Grana-rich
Stroma-rich
Grains/zim1
±S.E.
Total
grains
Area,
/tm1
Grains//tms
±S.E.
Total
grains
0432 ±0-023
2052
24-90
0188 ±0019
4-68
The terms ' grana' and ' stroma' in the Table refer to membranes of the grana and stroma
lamellae respectively.
1 .1 .
40 -
A
30 -
.
20 -
—
.
10 •
ZJ
40 -
•
•
r—1
.
0-8 0-9
1-0
E
30 ••
20
10
0
0-1
0-2
0-3 0-4
0-5
0-6 0-7
1-1
1-2
Grains//jm2
Fig. 22. Frequency distribution of silver grains/^ni1 for stroma-rich (A) and granarich (B) membrane areas of chloroplast vesicles. Vesicles were isolated from tissue
incubated 72 h (day o to day 3) in *H-TdR.
Association of chloroplast DNA zoith membranes
179
chloroplasts isolated in 0-4 M sucrose. When chloroplasts or chloroplast vesicles were
dried-down on to slides for autoradiography, nuclei were also present in the preparation.
Figs. 20 and 21 show that nuclei appear similar in autoradiographs of the 2 different
types of treatment. Nuclei are much more heavily labelled than chloroplasts because
of their much greater DNA content and are known to synthesize DNA in spinach leaf
disks (Rose et al. 1975). The unlabelled circular region is the nucleolar region. Labelled
nuclei can be clearly distinguished from chloroplasts.
Table 2. \?H]thymidine incorporation into chloroplasts and chloroplast vesicles
Treatment
3
H-Tdr
incorporation,
cpm/fig
chlorophyll
(mean)
Isolated in 35 mM Mg t + + O'4M sucrose
(chloroplast fraction)
Isolated in 3 5 mM Mg ! +
(chloroplast vesicle fraction)
% retention
of label, ±S.E.
2496
1149
45 ±15
Table 3. Dialysis of chloroplast vesicle fraction
3
Treatment
Isolated in 35 mM Mg*+,
dialysed against 3-5 mM Mg 1+
Isolated in 3-5 mM Mg a+ ,
dialysed against H2O
H-TdR
incorporation,
cpm//ig
chlorophyll
(mean)
538
364
0/
/o
retention
of label,
± S.F.
100
66 ±13
In order to gain some indication of how much cp DNA remained associated with
the autoradiographed chloroplast vesicles, the incorporated radioactivity in control
chloroplasts and chloroplast vesicles was determined by liquid scintillation counting.
Results are shown in Table 2. A variable but substantial amount of radioactivity was
retained by the vesicles. Considering the shearing of cp DNA that would occur in
vesicle formation the radioactive cp DNA must be tightly bound to the membrane.
Clearly, however, it is fragments and not whole molecules that are being autoradiographed.
The question arises that the autoradiographs could represent indiscriminate cation
binding of cp DNA. However the specific types of patterns obtained argue against
this. Nevertheless, the possibility of indiscriminate binding was investigated by
separating chloroplast vesicles from nuclei, and dialysing them against distilled water.
Results are shown in Table 3. Following dialysis two thirds of the radioactive cp DNA
still remained with the membrane fraction that could be pelleted at iooog1. The loss
of radioactivity that did occur is most likely due to further fragmentation of the
180
R. J. Rose
membrane system that occurs in distilled water (Spencer & Unt, 1965), which would
reduce membranes sedimenting at 1000 g. Indiscriminate Mg24" binding of cp DNA
to the membrane cannot account for the binding of most cp DNA to the chloroplast
vesicles.
DISCUSSION
By using chloroplast vesicles free of outer-envelope membranes, the photosynthetic
membrane system of spinach chloroplasts can be autoradiographed directly, after
having incorporated 3 H-TdR during the growth of cultured spinach disks. From the
data obtained it can be argued that what are being autoradiographed are fragments
of cp DNA attached to the photosynthetic membranes. When the chloroplast envelope
breaks, and is lost, after chloroplasts are isolated in 3-5 mM Mg2*, substantial cp DNA
is retained by the thylakoid membranes. The 45 % label retention obtained in this
study, compares with a 61 % value obtained (Rose & Possingham, 1976 a) using isolated
chloroplasts swollen in 4 mM Mg Cl2 but also containing 10 mM buffer, which causes
less swelling of thylakoid membranes (Douce et al. 1973). Furthermore, cp DNA
and membrane associations have been observed by electron microscopy in osmotically
shocked spinach chloroplasts (Woodcock & Fernandez-Moran, 1968).
The chloroplast vesicle cytology and autoradiography provides evidence that is
consistent with 2 points. First, that cp DNA occurs along the photosynthetic membrane system (e.g. in. Figs. 14 and 17 about 29 and 36/6m respectively of membrane
are labelled) and secondly, that cp DNA is preferentially associated with the grana
lamellae (Table 1, Fig. 1).
The significance of these results for spinach chloroplasts lies in providing an
explanation for the segregation of cp DNA in an equitable way in the dividing
chloroplast. That dividing spinach chloroplasts do segregate their DNA in a fairly
equal way to daughter progeny has been shown using pulse-chase experiments in
cultured spinach disks (Rose et al. 1974).
The electron-microscope autoradiography data obtained with thin sections (Rose &
Possingham, 1976a) and the current study using light-microscope autoradiography
of whole, large vesicles is most readily explained by the cp DNA occurring at intervals
along a continuous photosynthetic membrane system, attached to the grana lamellae.
That the photosynthetic membrane system is a continuum is the current understanding
of the chloroplast morphology of higher plants (Coombs & Greenwood, 1976;
Gunning & Steer, 1975). There are some recent indications from observations by
light microscopy of living chloroplasts (Jope, Atchison, Pringle & Wildman, 1977)
and from light microscopy using a fluorescent dye staining for DNA (Jope, James &
Wildman, 1977) that are also consistent with cp DNA occurring along the photosynthetic lamellar system of chloroplasts.
A preferential association of cp DNA with the grana, raises the question of how
many granal stacks have an associated cp DNA molecule. Overall the chloroplast
vesicle studies suggest that all grana could have associated cp DNA as most vesicles
are extensively labelled even though some label is lost during preparation. However,
Association of chloroplast DNA with membranes
181
unlabelled grana regions do occur, and 16% of the vesicles are unlabelled. It is of some
value to consider that higher plant chloroplasts usually have 20-30 cp DNA copies
(Kirk, 1972) and 40-60 grana (Gunning & Steer, 1975).
It should be emphasized that in the spinach disk system studied, there is a regular
growth and division cycle, coupled to cp DNA synthesis and segregation (Rose et al.
1974; Possingham & Rose, 1976). It is therefore possible that cp DNA replication
is coupled to membrane growth in a way similar to that postulated for bacteria
i.e. that chromosome duplication and separation is coupled to an extension of the
membrane surface (Ryter, 1968). Evidence has also recently been presented for the
association of mitochondrial DNA at or near its origin of replication, with the
inner mitochondrial membrane (Albring, Griffith & Attardi, 1977). A role in mitochondrial DNA replication and or segregation is suggested. Whether in higher plant
chloroplasts there is additional significance for cp DNA being associated with the
granal membranes (e.g. in granal development), rather than other membranes such as
the inner envelope is unclear. Bisalputra & Bisalputra (1969) have suggested that the
contact between the nucleoid and photosynthetic lamellae in the brown alga
Sphacelaria may be significant in the growth of the chloroplast membrane system.
Herrmann (1970) and Kowallik & Herrmann (1972) have studied the organization
of cp DNA in the chloroplasts of higher plants using Beta vulgaris L. They have
concluded that cp DNA in mature chloroplasts is distributed within several nucleoids
(depending on chloroplast size) with each nucleoid containing 4-8 genetic units. The
degree of clustering of cp DNA molecules might depend on the degree of coupling
between cp DNA replication and membrane growth, which would be influenced by
chloroplast development and physiology. In this regard it should be noted that cp
DNA synthesis and plastid division are readily separated experimentally i.e. cp DNA
synthesis without plastid division (Rose et al. 1975; Kass & Paolillo, 1977) and plastid
division without cp DNA synthesis (Boasson & Laetsch, 1969; Rose & Possingham,
19766).
The association of cp DNA with thylakoids is also important in cp DNA segregation in the brown alga, Sphacelaria (Bisalputra & Bisalputra, 1970). Sphacelaria has
a ring nucleoid (Bisalputra & Bisalputra, 1969) attached to the inner thylakoids of
the peripheral lamellae which loop around the rim of the chloroplast (Bisalputra &
Bisalputra, 1969; Bisalputra & Burton, 1970). A similar situation exists in Ochromonas
(Gibbs, Cheng & Slenkis, 1974) where it is known that there are a number of cp DNA
molecules, which are probably distributed along the nucleoid (Gibbs & Poole, 1973;
Gibbs et al. 1974).
Chloroplasts of higher plants (Rose & Possingham, 1976a) and many algae (see
literature cited by Gibbs et al. 1974) when studied by electron microscopy have
nucleoids apparently scattered throughout the chloroplast. It is possible that in such
chloroplasts the situation is similar to that in spinach, where there appears to be a
specific organization of cp DNA in relation to the photosynthetic membrane system.
182
R.J.Rose
I wish to thank Margaret Gibberd for her valuable assistance throughout the study, and
Margaret Brosnan for assistance with sectioning for electron microscopy. The project was
supported by ARGC grant No. D2-76/15280.
REFERENCES
M., GRIFFITH, J. & ATTARDI, G. (1977). Association of a protein structure of probable
membrane derivation with HeLa cell mitochondrial DNA near its origin of replication.
Proc. natn. Acad. Sci. U.S.A. 74, 1348-1352.
ARNON, D. J. (1949). Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta
vulgaris. PL Physiol., Lancaster 24, 1-15.
BISALPUTRA, T. & BISALPUTRA, A. A. (1969). The ultrastructure of chloroplast of a brown alga
Sphacelaria sp. I. Plastid DNA configuration - the chloroplast genophore. J. Ultrastruct.
Res. 29, 151-170.
BISALPUTRA, T. & BISALPUTRA, A. A. (1970). The ultrastructure of chloroplast of a brown alga
Sphacelaria sp. III. The replication and segregation of chloroplast genophore. J'. Ultrastruct.
Res. 32, 417-429BISALPUTRA, T. & BURTON, H. (1970). On the chloroplast DNA-membrane complex in
Sphacelaria sp. J. Microscopie 9, 661-666.
BOASSON, R. & LAETSCH, W. M. (1969). Chloroplast replication andgrowth in tobacco. Science,
N.Y. 166, 749-751COOMBS, J. & GREENWOOD, A. D. (1976). Compartmentation of the photosynthetic apparatus.
In The Intact Chloroplast (ed. J. Barber), pp. 1-51. Amsterdam: Elsevier/North-Holland.
DOUCE, R., HOLTZ, R. B. & BENSON, A. A. (1973). Isolation and properties of the envelope of
spinach chloroplasts. J. biol. Chem. 248, 7215-7222.
ELLIS, R. J. (1977). Protein synthesis by isolated chloroplasts. Biochim. biophys. Acta 463,
185-215.
FOWKE, L. C. (1975). Electron microscopy of protoplasts. In Plant Tissue Culture Methods
(ed. O. L. Gamborg & L. R. Wetter), pp. 55-59. Saskatoon: National Research Council
of Canada.
GIBBS, S. P., CHENG, D. & SLANKIS, T. (1974). The chloroplast nucleoid in Ochromonas
danica. I. Three-dimensional morphology in light- and dark-grown cells. J. Cell Sci. 16,
557-577GIBBS, S. P. & POOLE, R. J. (1973). Autoradiographic evidence for many segregating DNA
molecules in the chloroplast of Ochromonas danica. J. Cell Biol. 59, 318-328.
GUNNING, B. E. S. & STEER, M. W. (1975). Ultrastructure and the Biology of Plant Cells.
London: Edward Arnold.
HERRMANN, R. G. (1970). Multiple amounts of DNA related to the size of chloroplasts. I. An
autoradiographic study. Planta 90, 80—96.
JOPE, C. A., ATCHISON, B. A., PRINGLE, R. C. & WILDMAN, S. G. (1977). Spiral, string of grana,
chloroplast model tested by computer simulation. In Acides NucMiques et Synthese des
Protiins Chez les Vigitaux, pp. 153-157. Colloques internationaux C. N. R. S. No. 261.
JOPE, C , JAMES, T. W. & WILDMAN, S. G. (1977). Visualization of higher plant chloroplast
DNA by fluorescence microscopy with 4, 6-diamidino-2-phenylindole (DAPI). J. Cell Biol.
75, 3°4flKASS, L. B. & PAOLILLO, D. J. JR. (1977). Autoradiographic evidence for the effects of light
on RNA and DNA synthesis during replication in spores of Polytrichum. J. Cell Sci. 28,
ALBRING,
61-70.
KIRK, J. T. O. (1972). The genetic control of plastid formation: recent advances and strategies
for the future. Subcellular Biochem. 1, 333-361.
KOWALLIK, K. V. & HERRMANN, R. G. (1972). Variable amounts of DNA related to the size
of chloroplasts. IV. Three-dimensional arrangement of DNA in fully differentiated chloroplasts of Beta vulgaris L. J. Cell Sci. 11, 357-377.
KUNG, S. D. (1977). Expression of chloroplast genomes in higher plants. A. Rev. PI. Physiol.
28, 4QI-437POSSINGHAM, J. V. & ROSE, R. J. (1976). Chloroplast replication and chloroplast DNA synthesis
in spinach leaves. Proc. R. Soc. B 193, 295-305.
Association of chloroplast DNA with membranes
183
J. V. & SAURER, W. (1969). Changes in chloroplast number per cell during leaf
development in spinach. Planta 86, 186—194.
POSSINGHAM, J. V. & SMITH, J. W. (1972). Factors affecting chloroplast replication in spinach.
J. exp. Bot. 23, 1050-1059.
POSSINGHAM,
ROSE, R. J., CRAN, D. G. & POSSINGHAM, J. V. (1974). Distribution of DNA in dividing
spinach chloroplasts. Nature, Lond. 251, 641-642.
R. J., CRAN, D. G. & POSSINGHAM, J. V. (1975). Changes in DNA synthesis during cell
growth and chloroplast replication in greening spinach leaf disks. J. Cell Sci. 17, 27-41.
ROSE, R. J. & POSSINGHAM, J. V. (1976a). The localization of ['HJthymidine incorporation
in the DNA of replicating spinach chloroplasts by electron-microscope autoradiography.
J. Cell Sci. 20, 341-355ROSE, R. J. & POSSINCHAM, J. V. (19766). Chloroplast growth and replication in germinating
spinach cotyledons following massive gamma-irradiation of the seed. PL Phystol., Lancaster
57, 41-46.
RYTER, A. (1968). Association of the nucleus and the membrane of bacteria: a morphological
study. Bad. Rev. 32, 39-54.
SPENCER, D. & UNT, H. (1965). Biochemical and structural correlations in isolated spinach
chloroplasts under isotonic and hypotonic conditions. Aust.J. biol. Sci. 18, 197-210.
SPENCER, D. & WILDMAN, S. G. (1962). Observations on the structure of grana-containing
chloroplasts and a proposed model of chloroplast structure. Aust. J. biol. Sci. 15, 599-610.
WOODCOCK, C. L. F. & FERNANDEZ-MORAN, H. (1968). Electron microscopy of DNA conformations in spinach chloroplasts. J. tnolec. Biol. 31, 627-631.
ROSE,
{Received 20 July 1978 - Revised 11 October 1978)