* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download and Rhizobiales-Like PPP-Family Protein Phosphatases from
Secreted frizzled-related protein 1 wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Gene nomenclature wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Lipid signaling wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Signal transduction wikipedia , lookup
Gene regulatory network wikipedia , lookup
Metalloprotein wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Point mutation wikipedia , lookup
Homology modeling wikipedia , lookup
Phosphorylation wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Gene expression wikipedia , lookup
Paracrine signalling wikipedia , lookup
Magnesium transporter wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Interactome wikipedia , lookup
Protein structure prediction wikipedia , lookup
Expression vector wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
UNIVERSITY OF CALGARY
Characterization of Novel Shewanella- and Rhizobiales-Like PPP-Family Protein
Phosphatases from Arabidopsis thaliana
by
Richard Glen Uhrig
A THESIS
SUBMITTED TO THE FACULTY OF GRADUATE STUDIES
IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE
DEGREE OF DOCTOR OF PHILOSOPHY
DEPARTMENT OF BIOLOGICAL SCIENCES
CALGARY, ALBERTA
July, 2013
© Richard Glen Uhrig 2013
Abstract
Reversible protein phosphorylation catalyzed by protein kinases and phosphatases represents the
most prolific and currently best-characterized post-translational modification. Over the last
decade advancements in genome sequencing technologies has massively increased genomic
databases, resulting in the identification of previously unannotated protein kinases and
phosphatases from multiple organisms. The primary goal of the research presented here was to
elucidate the evolutionary, biochemical, cellular and biological characteristics of two recently
identified PPP-family protein phosphatase subclasses from the model photosynthetic Eukaryote
Arabidopsis thaliana. These two subclasses included the Shewanella-like (SLP1 and 2) and
Rhizobiales-like (RLPH2) phosphatases, which were named after their relatedness to
phosphatase orthologs from Shewanella and Rhizobiales bacteria. Heterologous protein
phosphatase expression in, and purification from, Escherichia coli revealed unique biochemical
characteristics including a complete insensitivity to PPP-family protein phosphatase inhibitors
okadaic acid and microcystin-LR, as well as diversity in their phosphorylated substrate
specificities. Bioinformatics complemented cell biology also uncovered unique subcellular
localizations for each enzyme, with AtSLP1, 2 and AtRLPH2 being chloroplastic, mitochondrial
and nuclear / cytosolic, respectively. Further identification of the AtSLP1 and AtSLP2 protein
interactome from both plant tissues and cell culture was accomplished by employing a tandem
affinity purification protein isolation strategy, with specific protein interactor complements
indicating independent regulatory roles within plant cells involving chloroplast energy
biosynthesis and mitochondrial intermembrane space processes, respectively. Lastly, phenotypic
analysis of atslp2 insertional mutant and 35S::AtSLP2 over-expression plants revealed biological
involvement in regulating gibberellic acid-related processes during seed germination as well as
ii
influencing fatty acid and amino acid contents during seed maturation. Research presented here
has made significant progress in our understanding of the previously uncharacterized SLP and
RLPH PPP-family phosphatase subclasses from Arabidopsis thaliana, by resolving their
molecular evolution, in addition to their subcellular, cellular and biochemical properties. A
functional understanding of AtSLP1 and AtSLP2 was also achieved through the identification of
their protein interaction partners, which, in conjunction with insertional knockout and protein
over-expression plant lines, lays a solid foundation for future research endeavors looking to
examine bacterial-like phosphatases from photosynthetic and non-photosynthetic Eukaryotes
alike.
iii
Acknowledgements
I would like to thank past and present members of the Moorhead lab for fruitful conversations
throughout the duration of my degree. I would also like to thank undergraduate students Rolaine
Bu, Keaton Colville and Arshpreet Deol for their contributions. Thanks also to Dr. Howard Ceri
for use of his confocal microscope and Dr. Gordon Chua for use of his microplate reader. I
would also like to extend my sincerest thanks to Dr. Greg Moorhead for giving me the
opportunity to work in his lab and for being a supportive and encouraging mentor over these past
years.
Research was supported by National Science and Engineering Research Council of Canada
(NSERC) Alexander Graham Bell CGS-D, Alberta Ingenuity Technology Futures (AITF)
Graduate Student Scholarship, The Izaak Walton Killam Doctoral Scholarship and The Dean's
Doctoral Scholarship.
Collaborations with: Dr. Anne-Claude Gingras, Dr. Marcus Samuel, Dr. Edward Yeung and Dr.
Alisdair Fernie
iv
Dedication
To my Family for a lifetime of encouragement and support.
v
Table of Contents
Abstract ............................................................................................................................... ii Acknowledgements ............................................................................................................ iv Dedication ............................................................................................................................v Table of Contents ............................................................................................................... vi List of Tables .......................................................................................................................x List of Figures and Illustrations ......................................................................................... xi List of Manuscripts and Contributions ..............................................................................xv List of Symbols, Abbreviations and Nomenclature ........................................................ xvii Epigraph .............................................................................................................................xx CHAPTER ONE: INTRODUCTION ..................................................................................1 1.1 Protein phosphorylation .............................................................................................1 1.2 Protein phosphatase families .....................................................................................3 1.3 Plant PPP protein phosphatases .................................................................................7 1.3.1 Type 1 (PP1) protein phosphatases ...................................................................9 1.3.2 Type 2A (PP2A) protein phosphatases............................................................14 1.3.3 Type 4 & 6 (PP4 & PP6) protein phosphatases ...............................................18 1.3.4 Type 5 (PP5) protein phosphatases .................................................................20 1.3.5 Type 7 (PP7) protein phosphatases .................................................................22 1.3.6 Novel plant bacterial-like PPP protein phosphatases ......................................23 1.3.7 Prokaryotic protein phosphorylation and PPP protein phosphatases ..............24 1.4 Research Objectives .................................................................................................26 CHAPTER TWO: EVOLUTION OF BACTERIAL-LIKE PPP PROTEIN
PHOSPHATASES IN PHOTOSYNTHETIC EUKARYOTES FEATURES
ANCESTRAL MITOCHONDRIAL ORIGIN OVERLAID BY HORIZONTAL GENE
TRANSFER ..............................................................................................................28 2.1 Introduction ..............................................................................................................28 2.2 Materials and Methods.............................................................................................30 2.2.1 Multiple sequence alignments .........................................................................30 2.2.2 Candidate sequence search, retrieval and validation .......................................30 2.2.3 Phylogenetic tree inference .............................................................................32 2.2.4 Subcellular localization prediction ..................................................................33 2.2.5 Analysis of sequence motifs and gene architecture .........................................34 2.3 Results ......................................................................................................................34 2.3.1 Eukaryotic bacterial-like SLP and RLPH protein phosphatases are PPP protein
phosphatases ....................................................................................................34 2.3.2 Distribution and interrelationships of bacterial-like PPP phosphatases ..........39 2.3.2.1 SLP phosphatases ..................................................................................39 2.3.2.2 RLPH phosphatases ...............................................................................44 2.3.3 Sequence motif identification ..........................................................................46 2.3.4 In silico gene structure analysis of bacterial-like PPP phosphatases from
photosynthetic Eukaryotes ...............................................................................49 2.4 Discussion ................................................................................................................49 2.5 Conclusion ...............................................................................................................59 vi
CHAPTER THREE: BACTERIAL-LIKE SLP PROTEIN PHOSPHATASES FROM
ARABIDOPSIS THALIANA ARE HIGHLY CONSERVED PLANT PROTEINS THAT
POSSESS UNIQUE PROPERTIES .........................................................................61 3.1 Introduction ..............................................................................................................61 3.2 Materials and Methods.............................................................................................62 3.2.1 Bioinformatics .................................................................................................62 3.2.2 Molecular cloning ............................................................................................62 3.2.3 Cell culture transfection and protoplast creation .............................................63 3.2.4 Transient AtSLP expression in Vicia faba epidermal leaf cells ......................64 3.2.5 Microscopy ......................................................................................................64 3.2.6 Heterologous protein expression and antibody production .............................65 3.2.7 Enzymatic analysis ..........................................................................................67 3.3 Results ......................................................................................................................68 3.3.1 AtSLP1 and AtSLP2 localize to different cellular compartments...................68 3.3.2 Temporal and spatial expression of AtSLP1 and AtSLP2 ..............................73 3.3.3 AtSLP phosphatase primary sequence and enzymatic properties ...................80 3.3.4 Characterization of purified untagged AtSLP1 ...............................................85 3.4 Discussion ................................................................................................................88 3.4.1 Subcellular targeting of the AtSLP phosphatases ...........................................88 3.4.2 Temporal and spatial differences in AtSLP phosphatase expression ..............92 3.4.3 Conservation of essential PPP protein phosphatase motifs in AtSLP1 and AtSLP2
..........................................................................................................................94 3.4.4 Metal cation preferences of AtSLP phosphatases ...........................................95 3.4.5 Inhibition by classic PPP protein phosphatase inhibitors ................................96 3.5 Conclusion ...............................................................................................................98 CHAPTER FOUR: PROTEIN INTERACTOME ANALYSIS OF SLP PHOSPHATASES 1
AND 2 FROM A. THALIANA ..................................................................................99 4.1 Introduction ..............................................................................................................99 4.2 Materials and Methods...........................................................................................102 4.2.1 Plant growth conditions .................................................................................102 4.2.2 Molecular cloning and expression .................................................................102 4.2.3 A. thaliana cell culture transfection and TAP pull-downs ............................103 4.2.4 Mass spectrometry .........................................................................................104 4.2.5 Transient tobacco BY2 cell expression and imaging ....................................106 4.2.6 Isolation of A. thaliana cell culture mitochondria .........................................107 4.2.7 Enzymatic analysis ........................................................................................108 4.2.8 Antibody production ......................................................................................109 4.2.9 Western immunoblotting ...............................................................................109 4.2.10 Native polyacrylamide gel electrophoresis (PAGE) ...................................110 4.3 Results ....................................................................................................................110 4.3.1 AtSLP1 specifically interacts with chloroplast F1 ATP synthase subunits β and γ
........................................................................................................................110 4.3.2 AtSLP2 interacts with mitochondrial redox relay protein AtMIA40 ............113 4.3.3 Reciprocal interaction analysis supports a specific AtSLP2-AtMIA40 interaction
........................................................................................................................116 4.3.4 AtSLP2 and AtMIA40 co-localize to the mitochondria ................................117 vii
4.3.5 AtMIA40 activates the Ser/Thr phosphatase activity of AtSLP2 .................120 4.4 Discussion ..............................................................................................................127 4.4.1 AtSLP1 phosphatase interacts with chloroplast F1 ATP synthase subunits β and γ.
........................................................................................................................127 4.4.2 AtSLP2 phosphatase is a novel client of redox relay protein AtMIA40 .......130 4.4.3 Co-localization of both AtSLP2 and AtMIA40 to the mitochondria ............136 4.5 Conclusion .............................................................................................................137 CHAPTER FIVE: EXPLORING THE BIOLOGICAL SIGNIFICANCE OF ATSLP1 AND
ATSLP2: A REVERSE GENETIC APPROACH ..................................................139 5.1 Introduction ............................................................................................................139 5.2 Methods .................................................................................................................142 5.2.1 Plant growth conditions and seed weight ......................................................142 5.2.2 Molecular cloning and expression .................................................................143 5.2.3 Seed extraction ..............................................................................................143 5.2.4 Plant DNA isolation and PCR genotyping ....................................................143 5.2.5 Immunoblot analysis .....................................................................................144 5.2.6 Quantitative PCR analysis .............................................................................144 5.2.7 Metabolite analysis ........................................................................................147 5.3 Results ....................................................................................................................147 5.3.1 Generation of homozygous insertional mutant and protein over-expression plant
lines ................................................................................................................147 5.3.2 AtSLP2 and AtMIA40 function to negatively regulate seed germination ....148 5.3.3 An AtSLP2-AtMIA40 protein complex negatively regulates GA biosynthesis150 5.3.4 AtSLP2 influences seed fatty acid and amino acid content ..........................159 5.4 Discussion ..............................................................................................................162 5.4.1 Negative regulation of seed germination through GA metabolism ...............162 5.4.2 The AtSLP2-AtMIA40 protein complex functions to negatively regulate GA
biosynthesis ....................................................................................................164 5.4.3 Influence of AtSLP on seed FA and AA composition ..................................166 5.5 Conclusion .............................................................................................................168 CHAPTER SIX: RHIZOBIALES-LIKE PHOSPHATASE 2 FROM ARABIDOPSIS
THALIANA IS A NOVEL PHOSPHOTYROSINE-SPECIFIC PPP PROTEIN
PHOSPHATASE ....................................................................................................169 6.1 Introduction ............................................................................................................169 6.2 Materials and Methods...........................................................................................171 6.2.1 Bioinformatics ...............................................................................................171 6.2.2 Molecular cloning of AtRLPH2 ....................................................................171 6.2.3 Transient expression of fluorescent AtRLPH2 constructs and microscopy ..172 6.2.4 Heterologous protein expression and purification .........................................173 6.2.5 Tandem affinity purification (TAP) isolation of AtSLP1 and AtRLPH2 .....174 6.2.6 Enzymatic analysis ........................................................................................174 6.3 Results ....................................................................................................................176 6.3.1 AtRLPH2 is a dual-localized nuclear and cytosolic protein .........................176 6.3.2 Purification of recombinant HIS6-tagged AtRLPH2 ....................................176 6.3.3 Enzymatic characterization of AtRLPH2 ......................................................180 viii
6.3.4 AtRLPH2 is a phosphotyrosine-specific PPP protein phosphatase ...............186 6.4 Discussion ..............................................................................................................192 6.4.1 Bridging the gap: AtRLPH2 is a phosphotyrosine-specific PPP protein
phosphatase ....................................................................................................192 6.4.2 Function of AtRLPH2 protein phosphatase ..................................................195 6.4.3 AtRLPH2 maintains an ancient insensitivity to naturally occurring protein
phosphatase inhibitors....................................................................................197 6.4.4 AtRLPH2 catalysis: Cysteine-mediated or metal-dependent catalytic mechanism
........................................................................................................................198 6.5 Conclusion .............................................................................................................199 CHAPTER SEVEN: PERSPECTIVES AND FUTURE DIRECTIONS ........................200 7.1 Summary ................................................................................................................200 7.2 Novel PPP phosphatase of the chloroplast: SLP1 phosphatases ...........................202 7.3 Connecting AtSLP2 to GA biosynthesis ...............................................................203 7.4 The SLP phosphatase of Schizosaccharomyces pombe .........................................204 7.5 Bridging the gap: the AtRLPH2 is a phosphotyrosine-specific PPP protein .........205 LITERATURE CITED: ...................................................................................................206 APPENDIX A.1. SLP PHYLOGENETIC TREE AND ALIGNMENT SEQUENCE
INFORMATION.....................................................................................................225 APPENDIX A.2. RLPH PHYLOGENETIC TREE AND ALIGNMENT SEQUENCE
INFORMATION.....................................................................................................230 APPENDIX B. PHYLOGENETIC TREE NODE SCORES ..........................................233 APPENDIX C.1. CLONING AND GENOTYPING PRIMERS ....................................234 APPENDIX C.2. PLANT GENOTYPING .....................................................................235 APPENDIX C.3. PRIMERS FOR QPCR OF GA RELATED GENES ..........................236 ix
List of Tables
Table 1.1: PPP protein phosphatase complement of A. thaliana and humans. ............................... 9 Table 2.1: In silico subcellular prediction data for eukaryotic SLP phosphatases used in
phylogenetic tree and alignment construction. ..................................................................... 42 Table 2.2: In silico subcellular prediction data for eukaryotic RLPH phosphatases used in
phylogenetic tree and alignment construction. ..................................................................... 46 Table 2.3: Intron quantity for photosynthetic Eukaryote SLP phosphatases. ............................... 51 Table 3.1: MALDI-TOF mass spectrometry identification of lower molecular weight
polypeptides in the Superdex 200 pool post-digest. ............................................................. 88 Table 4.1: AtSLP1- and AtSLP2-specific protein interactors. ................................................... 114 Table 4.2: Phosphorylated peptides used in AtSLP2 enzyme assays. ........................................ 129 Table 6.1: Malachite green dephosphorylation assay examining AtRLPH2 phosphatase
activity towards phosphorylated peptide substrates. ........................................................... 190 x
List of Figures and Illustrations
Figure 1.1: Reversible protein phosphorylation of serine, threonine and tyrosine. ........................ 2 Figure 1.2: PPP protein phosphatase inhibitors. ............................................................................. 6 Figure 1.3: Phylogeny and domain architecture of plant PPPs. ...................................................... 8 Figure 1.4: Tissue-specific transcript expression of the A. thaliana PPP protein
phosphatases. ........................................................................................................................ 11 Figure 1.5: A. thaliana PP1: from non-specific catalytic subunits to specialized cellular
functions. ............................................................................................................................... 13 Figure 1.6: Cellular events regulated by PP2A in plants. ............................................................. 16 Figure 2.1: Alignment of SLP phosphatases from both Prokaryotes and Eukaryotes. ................. 37 Figure 2.2: Alignment of RLPH phosphatases from both Prokaryotes and Eukaryotes. ............. 38 Figure 2.3: Orthogonal phylogenetic tree depicting SLP phosphatase distribution and
interrelationships across both Eukaryotes and Prokaryotes. ................................................. 41 Figure 2.4: Orthogonal phylogenetic tree depicting RLPH phosphatase distribution and
interrelationships across both Eukaryotes and Prokaryotes. ................................................. 45 Figure 2.5: Compiled bacterial-like phosphatase Motif 2 from SLP and RLPH phosphatases.... 47 Figure 2.6: Compiled bacterial-like phosphatase Motif 1 from SLP and RLPH phosphatases.... 48 Figure 2.7: Gene structure of eukaryotic SLP and RLPH phosphatases. ..................................... 50 Figure 2.8: Proposed model of RLPH phosphatase molecular evolution. .................................... 53 Figure 2.9: Proposed model of SLP phosphatase molecular evolution. ....................................... 54 Figure 3.1: Development of AtSLP1 and 2 stably-transfected A. thaliana cell culture. .............. 69 Figure 3.2: In vivo subcellular localization of AtSLP1 and 2 using stably-transfected A.
thaliana cell culture. ............................................................................................................. 70 Figure 3.3: Co-localization of AtSLP1 with fluorescent marker constructs in Vicia faba
epidermal leaf cells. .............................................................................................................. 71 Figure 3.4: Co-localization of AtSLP2 with fluorescent marker constructs in Vicia faba
epidermal leaf cells. .............................................................................................................. 72 Figure 3.5: AtSLP transcript expression data. ............................................................................... 74 xi
Figure 3.6: NCBI Gene Expression Omnibus (GEO) profile of diurnal AtSLP1 and AtSLP2
transcript cycling (www.ncbi.nlm.nih.gov/geoprofiles). ...................................................... 76 Figure 3.7: Colloidal blue stained 12 % SDS-PAGE of the HIS6-AtSLP1 and HIS6-AtSLP2
NiNTA eluates employed in producing anti-AtSLP1 and anti-AtSLP2 polyclonal
antibodies. ............................................................................................................................. 77 Figure 3.8: Western blot assessment of affinity-purified (AP) anti-AtSLP1 and anti-AtSLP2
IgG detection limits and specificity. ..................................................................................... 78 Figure 3.9: Spatial and temporal western blot analysis of AtSLP1 and AtSLP2 protein
expression. ............................................................................................................................ 79 Figure 3.10: Alignment of AtSLP1 and AtSLP2 full-length protein sequences with
representative PP1 sequences from Arabidopsis thaliana (TOPP2; At5g59160),
AtPP2A-1 (At1g59830) and Homo sapiens (HsPP1γ; NP_002701). ................................... 81 Figure 3.11: 12 % SDS-PAGE of NiNTA-purified HIS6-AtSLP1, HIS6-AtSLP2 and
uninduced bacteria cell line control eluates stained with Colloidal blue. ............................. 83 Figure 3.12: Enzymatic analysis of HIS6-AtSLP1 and HIS6-AtSLP2......................................... 84 Figure 3.13: Size exclusion and anion exchange chromatography steps of AtSLP1
purification. ........................................................................................................................... 86 Figure 3.14: Analysis of the major end-point purification steps employed to completely
purify AtSLP1. ...................................................................................................................... 87 Figure 3.15: Colloidal blue stained 12 % SDS-PAGE of purified HsPP1γ and HIS6-TOPP2. ... 89 Figure 3.16: Assessment of purified, untagged AtSLP1 sensitivity to small molecule
inhibitors. .............................................................................................................................. 90 Figure 4.1: Schematic depiction of tandem affinity purification (TAP) protein isolation
methodology. ...................................................................................................................... 105 Figure 4.2: Western blot analysis of dark-grown, wild-type A. thaliana cell culture................. 111 Figure 4.3: AtSLP-TAP pull-downs isolating AtSLP1- and AtSLP2-specific protein
interactors. ........................................................................................................................... 112 Figure 4.4: Transcript and protein expression of AtSLP1 and AtCF1 ATP synthase subunits. . 115 Figure 4.5: Reciprocal TAP pull-downs verify specific interaction between AtSLP2 and
AtMIA40. ............................................................................................................................ 118 Figure 4.6: Reciprocal non-denaturing PAGE verifies specific interaction between AtSLP2
and AtMIA40. ..................................................................................................................... 119 xii
Figure 4.7: AtSLP2 and AtMIA40 are mitochondrial-targeted proteins. ................................... 121 Figure 4.8: Anti-AtMIA40 IgG production and testing. ............................................................. 122 Figure 4.9: AtMIA40 activates AtSLP2 under reducing conditions........................................... 124 Figure 4.10: Effect of reductant only on AtSLP1 and AtSLP2 activity. .................................... 125 Figure 4.11: Amino acid sequence alignment of photosynthetic Eukaryote SLP2
phosphatases against select SLP1 phosphatases. ................................................................ 126 Figure 4.12: Substrate specificity of AtSLP2-AtMIA40 complex. ............................................ 128 Figure 4.13: Model depicting how AtSLP1 may operate in vivo. .............................................. 131 Figure 4.14: Model depicting how AtSLP2 and AtMIA40 may operate in vivo. ....................... 133 Figure 5.1: AtSLP and AtMIA40 gene models depicting insertional mutant plant lines. ............ 145 Figure 5.2: PCR and immunoblot analysis of AtSLP1 and AtSLP2 knockout and overexpression plant lines. ......................................................................................................... 146 Figure 5.3: Silique size and seed weight..................................................................................... 149 Figure 5.4: Depiction of atslp2-2, wild-type NÖ and 35S::AtSLP2 seeds germinated on 0.5 x
MS-Agar plates containing ABA or Uniconazole. ............................................................. 151 Figure 5.5: Depiction of atmia40 and wild-type Col-O seeds germinated on 0.5 x MS-Agar
plates containing ABA or Uniconazole. ............................................................................. 152 Figure 5.6: Quantitative analysis of seed testa cracking on 0.5 x MS-Agar plates containing
ABA or Uniconazole........................................................................................................... 153 Figure 5.7: Quantitative analysis of seed radicle emergence on 0.5 x MS-Agar plates
containing ABA or Uniconazole. ........................................................................................ 154 Figure 5.8: Immunoblot analysis of AtSLP2 and AtMIA40 protein expression in imbibed and
germinating seeds................................................................................................................ 155 Figure 5.9: Relative transcript expression of GA-related biosynthetic and signaling proteins
from Biological Arabidopsis Resource (BAR). .................................................................. 157 Figure 5.10: Quantitative PCR analysis of GA biosynthetic and signaling genes from 0 h, 6 h
and 12 h imbibed atslp2-2, wild-type and 35S::AtSLP2 seeds. .......................................... 158 Figure 5.11: Comparative analysis of atslp2-2 and 35S::AtSLP2 seed FA content and
composition. ........................................................................................................................ 160 Figure 5.12: Relative levels of AAs in atslp2-2, WT NÖ and 35S::AtSLP2 dry seeds. ............. 161 xiii
Figure 5.13: Model of AtSLP2-AtMIA40 protein complex function during seed germination. 163 Figure 6.1: AtRLPH1 and 2 transcriptional tissue expression profile. ....................................... 177 Figure 6.2: Subcellular localization of AtRLPH2....................................................................... 178 Figure 6.3: Purification of AtRLPH2-HIS6 from E. coli............................................................ 179 Figure 6.4: Calibrated Superdex 200 size exclusion chromatography of AtRLPH2-HIS6. ....... 181 Figure 6.5: AtRLPH2 pH activity profile, metal cation dependency, and sensitivity to metal
chelators EDTA and EGTA. ............................................................................................... 182 Figure 6.6: ClustalX alignment of representative RLPH phosphatases from across
photosynthetic Eukaryotes. ................................................................................................. 183 Figure 6.7: Cartoon depiction of PPP, PPM and PTP protein phosphatase motifs relative to
AtRLPH2. ........................................................................................................................... 184 Figure 6.8: Effect of hydrogen peroxide (H2O2) and N-ethylmaleimide (NEM) on AtRLPH2
phosphatase activity. ........................................................................................................... 185 Figure 6.9: AtRLPH2 sensitivity to inhibition by phosphate-containing small molecules. ....... 187 Figure 6.10: AtRLPH2 sensitivity to classic PPP and PTP protein phosphatase small
molecule inhibitors.............................................................................................................. 188 Figure 6.11: Phosphorylated substrate preferences of purified A. thaliana bacterial-like PPP
protein phosphatases. .......................................................................................................... 191 Figure 6.12: Phosphorylated substrate preferences of TAP-purified A. thaliana bacterial-like
PPP-family protein phosphatases. ....................................................................................... 193 xiv
List of Manuscripts and Contributions
Chapter 1
Uhrig, R.G., Labandera, A.M., and Moorhead, G.B. Arabidopsis PPP serine / threonine protein
phosphatases: many targets but few engines. Trends in Plant Science. Accepted Manuscript
PLANTS-D-13-00047R1.
I was primary author on the manuscript
Chapter 2
Uhrig, R.G*., Kerk, D*., and Moorhead, G.B. Evolution of bacterial-like PPP protein
phosphatases in photosynthetic eukaryotes features ancestral mitochondrial or archaeal origin
overlaid by lateral gene transfer. Manuscript in progress.
I will be co-primary author on the manuscript
I was responsible for all in silico subcellular localization, motif identification and gene structure
work. HMM work was split between myself and collaborator Dr. David Kerk (University of
Calgary). I initially formulated alignments and phylogenetic trees to trace the heritage of each
protein subclass, which was further resolved by collaborator Dr. David Kerk.
Chapter 3
Uhrig, R.G., and Moorhead, G.B. Two ancient bacterial-like PPP family phosphatases from
Arabidopsis thaliana are highly conserved plant proteins that possess unique properties. Plant
Physiology. 2011. 157(4): 1778-92.
I was primary author on the manuscript
Chapters 4 & 5
Uhrig, R.G., Liang, S., Goudreault, M., Yeung, E., Colville, K., Bu, R., Gingras, A.C., Samuel,
M.A., and Moorhead, G.B. Activation of Arabidopsis mitochondrial serine/threonine protein
phosphatase SLP2 by the oxidoreductase MIA40. Manuscript in progress.
I will be primary author on the manuscript
I was responsible for all work except the following. Mass spectrometry was performed by
collaborators Dr. Anne-Claude Gingras and Marilyn Goudreault (University of Toronto).
Quantitative PCR and atmia40 genotyping was performed by Siyu Liang (University of
Calgary). Pictures of germinating siliques were taken by Dr. Edward Yeung (University of
Calgary). Seed fatty acid and amino acid analysis was performed by the collaborating laboratory
of Dr. Alisdair Fernie (Max Planck Institute of Molecular Plant Physiology).
xv
Chapter 6
Uhrig, R.G., Labandera, A.M., Colville, K., and Moorhead, G.B. Rhizobiales-like phosphatase 2
(RLPH2) from Arabidopsis thaliana is a novel phosphotyrosine-specific PPP-family protein
phosphatase. Manuscript in progress.
I will be primary author on the manuscript
I was responsible for all work except the following. AtRLPH2- and AtSLP1-TAP
phosphorylated peptide assays were performed in conjunction with A.M. Labandera.
Other notable contributions during Ph.D. thesis
Uhrig, R.G., and Moorhead, G.B. Plant Proteomics: current status and future prospects. Journal
of Proteomics. doi: 10.1016/j.jprot.2013.01.018.
I was primary author on the manuscript
Skene-Arnold, T.D., Luu, A.H., Uhrig, R.G., De Wever, V., Nimick, M., Maynes, J., Fong, A.,
James, M., Trinkle-Mulcahy, L., Moorhead, G.B., and Holmes, C.F.B. Molecular mechanisms
underlying the interaction of Protein Phosphatase-1c with ASPP proteins. Biochemical Journal.
2013. 449: 649-59.
I was a contributing author on the manuscript
Tran, H.T., Uhrig, R.G., Nimick, M., and Moorhead, G.B. Interfacing protein lysine acetylation
and protein phosphorylation: Ancient modifications meet ancient proteins. Plant Signaling and
Behavior. 2012. 7(8).
I was a contributing author on the manuscript
Tran, H.T*., Nimick, M*., Uhrig, R.G*., Templeton, G., Morrice, N., Gourlay, R., Delong, A.,
and Moorhead, G.B. Arabidopsis thaliana Histone Deacetylase 14 (Hda14) is an α-Tubulin
Deacetylase that associates with Pp2a and enriches in the microtubule fraction with the putative
Histone Acetyltransferase Elp3. The Plant Journal. 2012. 71(2): 263-72.
I was co-primary author on the manuscript
Uhrig, R.G., and Moorhead, G.B. Okadaic acid and microcystin insensitive PPP-family
phosphatases may represent novel biotechnology targets. Plant Signaling and Behavior. 2011.
6(12).
I was primary author on the manuscript
Uhrig, R.G., Ng, K.K.S., and Moorhead, G.B. PII in higher plants: a modern role for an ancient
protein. Trends in Plant Science. 14(9): 505-11.
I was primary author on the manuscript
xvi
List of Symbols, Abbreviations and Nomenclature
Symbol
ABA
ABI5
AGO1
ATP
ADP
AMP
Asp
BLASTP
TBLASTN
BIN2
BR
BRI1
BSL1-3
BSU1
Ch
CDG1
cTP
cv
Cyto
DSP
DTT
EDTA
EGTA
ELP3
ER
GA
GEO
GEM
GFP
Gly-3P
Glu-6P
GSH
GST
H2O2
HAD
HDA14
HEAT
Definition
abscisic acid
abscisic acid insensitive 5
argonaute protein 1
adenosine 5'-triphosphate
adenosine 5'-diphosphate
adenosine 5'-monophosphate
Aspartate
basic local alignment search tool - search protein database
with a protein query
basic local alignment search tool - search translated
nucleotide database with a protein query
Bri1 insensitive kinase 2
brassinosteroid
brassinosteroid insensitive 1
Bri1 supressor-like 1-3
Bri1 suppressor 1
Chloroplast
constitutive differential growth 1
chloroplast transit peptide
column volume
Cytosol
dual specificity phosphatase
Dithiothreitol
ethylenedinitrilotetraacetic acid
ethylene glycol tetraacetic acid
elongator protein 3
endoplasmic reticulum
gibberellic acid
gene expression omnibus
GL2 expression modulator
green fluorescent protein
glycerol-3-phosphate
glucose-6-phosphate
reduced glutathione
glutathione S-transferase
hydrogen peroxide
haloacid dehalogenase
histone deacetylase 14
Huntingtin, elongation factor 3, protein phosphatase 2A,
and the yeast kinase target of rapamycin 1
xvii
HEPES
HGT
HIS6
HMCR
HMM
Hs
Hsp90
HTGS
IC50
I-2
MCLR
MIA40
mM
mRNA
MS
Mt
mTP
MWCO
NAA
NaF
NaOV
NCBI
NEM
NIPP1
NiNTA
nM
nm
NSCP
Nuc
OA
PAGE
PCR
PDC (E1α mitochondrial)
pAsp
pHis
pSer
pThr
pTyr
Perox
PHOT2
Pi
PP1-7
PPi
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
horizontal gene transfer
hexa-histidine
3-hydroxy-3-methylglutaryl coenzyme A reductase
hidden Markov model
homo sapiens (human)
heat shock protein 90
high throughput genomic sequences
half-maximal inhibitory concentration
inhibitor-2 protein
microcystin-LR
mitochondrial import and assembly protein 40
Millimolar
messenger ribonucleic acid
Murashige Skoog
Mitochondria
mitochondrial targeting peptide
molecular weight cutoff
1-naphthaleneacetic acid
sodium fluoride
sodium orthovanadate
National Center for Biotechnology Information
N-ethylmaleimide
nuclear inhibitor of protein phosphatase-1
nickel nitrilotriacetic acid
nanomolar
nanometers
non-specific co-purifying proteins
nucleus
okadaic acid
polyacrylamide gel electrophoresis
polymerase chain reaction
pyruvate dehydrogenase complex (mitochondrial E1α)
phosphorylated aspartate
phosphorylated histidine
phosphorylated serine
phosphorylated threonine
phosphorylated tyrosine
peroxisome
phototropin 2
inorganic phosphate
protein phosphatase type 1 - 7
pyrophosphate
xviii
PMSF
pNPP
PP2C
PPKL
PPM
PPP
PRSL
PTEN
PTP
PTPC
PTS1
RCN1
RISC
RFP
RLPH
RSS1
SBI1
SDS22
SDS
SEX4
SHLP1
SLP
Sp
SPS
SuSy
TCEP
TOPP2
TOR
TPR
µM
WGS
phenylmethylsulfonyl fluoride
para-nitrophenyl phosphate
phosphoprotein phosphatase 2C
protein phosphatase kelch-like
Mg2+-dependent protein phosphatase
phosphoprotein phosphatase
PP1 regulatory subunit 2-like protein
phosphatase and tensin homolog
protein tyrosine phosphatase
plant-type phosphoenolpyruvate carboxylase
peroxisomal targeting signal 1
roots curl in naphthylphthalamic acid 1
RNA induced silencing complex
red fluorescent protein
Rhizobiales-like phosphatase
rice salt sensitive 1
suppressor of Bri1
suppressor of DIS2
sodium dodecyl sulfate
starch excess 4
Shewanella-like phosphatase-1
Shewanella-like phosphatase
signal peptide
sucrose phosphate synthase
sucrose synthase
tris(2-carboxyethyl)phosphine
Arabidopsis thaliana PP1 isoform 2
target of rapamycin
tetratricopeptide
micromolar
whole-genome shotgun contigs
xix
Epigraph
"Think off-center."
- George Carlin
xx
Chapter One: Introduction
1.1 Protein phosphorylation
Reversible protein phosphorylation is an ancient regulatory mechanism which has
evolved to become one of the dominant means to control protein function, regulating essentially
every cellular process across the domains of life (Figure 1.1A; (Hunter and Pawson, 2012)).
Mass spectrometry-based phosphoproteomics studies have revealed that upwards of 70% of all
human proteins are phosphorylated, and most on multiple sites within a protein (Olsen et al.,
2006), while ongoing genome sequencing efforts have consistently found protein kinases and
phosphatases to constitute approximately 2-4% of the protein-encoding genes of most
Eukaryotes (Kerk et al., 2008; Hunter and Pawson, 2012). Given the expansive nature of protein
kinase and phosphatase families across Eukaryotes, there is no reason to believe protein
phosphorylation would be any less common in the Kingdom Plantae.
Phosphorylated proteins have been found in abundance throughout mitochondria (Ito et
al., 2009), chloroplasts (Reiland et al., 2009), nuclei (Olsen et al., 2006), cytosol (Olsen et al.,
2006) and even extracellularly (Tagliabracci et al., 2012), with phosphoproteomics documenting
plant proteomes to have a much higher abundance of phosphotyrosine than originally thought
despite the absence of classic protein tyrosine kinases and phosphatases. This led to the
discovery of phosphoserine (pSer), threonine (pThr) and tyrosine (pTyr) ratios much like other
higher Eukaryotes (~84-86%, 10-12% and 2-4%, respectively) (Figure 1.1B; (Sugiyama et al.,
2008; Nakagami et al., 2010; Nguyen et al., 2012)).
1
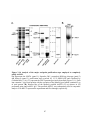
Figure 1.1: Reversible protein phosphorylation of serine, threonine and tyrosine.
(A) Reversible protein phosphorylation is catalyzed by the opposing actions of protein kinases
and protein phosphatases, which add a phosphoryl group and remove phosphate from a target
protein, respectively. Protein kinases convert adenosine 5'-triphosphate (ATP) to adenosine 5'diphosphate (ADP), transferring the phosphoryl group to a protein, while phosphatases use
water (H2O) to catalyze the removal of phosphate (Pi). (B) Structures of phosphorylated and
dephosphorylated serine, threonine and tyrosine. These amino acids comprise the bulk of the
phosphorylation events occurring in Eukaryotes. The estimated abundance of each
phosphorylated amino acid species in humans and plants is listed above. Highlighted in pink is
the attached phosphate molecule.
2
For historical and sometimes technical reasons, protein kinases are generally more well
characterized than protein phosphatases (Brautigan, 2013). In vitro, protein kinases display
substrate specificity based on protein primary sequence, while protein phosphatase catalytic
subunits are typically non-discriminate in the absence of additional protein binding partners
(Heroes et al., 2013). This has led to the notion that protein phosphatases lack specific regulation
and simply maintain a ‘housekeeping’ function. However, studies across a range of model
Eukaryotes has confirmed that in fact the phosphatases are not passive players in the
(de)phosphorylation balance, but rather dynamic and as highly regulated as their partner kinases.
Since the mid 1990s, biochemical and genetic studies have continued to uncover key roles for the
plant protein phosphatases in a wide range of biological contexts.
1.2 Protein phosphatase families
Protein kinases consist of one superfamily (Manning et al., 2002; Lehti-Shiu and Shiu,
2012), while the protein phosphatases are divided into four distinct families (Moorhead et al.,
2007; Genoud et al., 2008; Kerk et al., 2008; Shi, 2009; Bollen et al., 2010; De Munter et al.,
2013; Heroes et al., 2013) represented by the phosphoprotein phosphatases (PPP), Mg2+/Mn2+dependent phosphoprotein metallophosphatases (PPM/PP2C), phosphotyrosine phosphatases
(PTP) and aspartic acid (Asp)-dependent family enzymes. These four families are categorized
based on a combination of their catalytic mechanism, metal cation requirements, inhibitor
sensitivities and phosphorylated target substrates (Shi 2009). All four protein phosphatase
families possess at least one member which is capable of dephosphorylating pSer and pThr
residues, while the PTP phosphatases, which largely dephosphorylate pTyr residues, have
members which also dephosphorylate non-proteinaceous phosphorylated substrates (Tonks,
3
2006; Kerk et al., 2008; Silver et al., 2013). It is thought that the PPP protein phosphatases
catalyze upwards of 90% of the overall protein dephosphorylation reactions in eukaryotic cells
(Heroes et al., 2013); however, this number may be slightly lower in plants due to the immense
proliferation of Ser/Thr-specific PP2C enzymes (Xue et al., 2008; Fuchs et al., 2013).
Both the PPP and PPM protein phosphatases have been characterized to dephosphorylate
pSer and pThr residues (Kerk et al., 2008; Shi, 2009; Fuchs et al., 2013), and both have been
found to maintain a metal cation-based catalytic mechanism involving either Mn2+/Fe3+ (PPPs)
or Mg2+/Mn2+ (PPM/PP2Cs) (Shi, 2009; Fuchs et al., 2013). These similarities, however, are a
product of convergent evolution, as PPP and PPM/PP2C phosphatases possess distinct catalytic
pockets comprised of GDxHG, GDxVDRG, GNHE, HGG and RxxxD, DGxxG, DG, GxxDN
motifs, respectively (Shi, 2009; Fuchs et al., 2013). Furthermore, most PPM/PP2C protein
phosphatases possess additional motifs that assist in defining their subcellular localization and
substrate specificity in a similar manner to protein kinases (Ubersax and Ferrell, 2007; Xue et al.,
2008; Shi, 2009), while PPP protein phosphatases are expressed as bare catalytic subunits that
require interaction(s) with targeting subunits to define their intracellular specificity (Moorhead et
al., 2009).
Unlike PPM protein phosphatases, PPP protein phosphatases are sensitive to a number of
small molecule inhibitors, such as microcystin and okadaic acid, which bind a surface loop near
the active site (Figure 1.2; (Goldberg et al., 1995; Maynes et al., 2001)). These inhibitors are
pharmacological tools for experimentation and were instrumental in the initial categorization of
aforementioned PPP protein phosphatase subclasses based on differences in inhibitory sensitivity
(MacKintosh et al., 1990; Sheppeck et al., 1997). Microcystin-LR (MCLR) and okadaic acid
(OA) are naturally occurring compounds that were purified from the cyanobacterium Microcystis
4
aeruginosa (MacKintosh et al., 1990) and marine sponge Halichondria okadai, respectively
(Bialojan and Takai, 1988). Microcystis aeruginosa can have severe ecological ramifications
through its exogenous production of microcystins during large cyanobacterial blooms, which
result in fish kills and water toxification (Cohen et al., 1990; MacKintosh and MacKintosh,
1994; Dawson, 1998). These two compounds are responsible for inhibiting both PP1 and PP2Alike (PP2A, PP4 and PP6) protein phosphatases, but at different concentrations, aiding in the
differentiation between isolated phosphatases prior to the availability of sequenced genomes for
phylogenetic comparisons.
PTP phosphatases on the other hand, possess a completely different, but highly
conserved, metal cation-independent dephosphorylation mechanism (Jia et al., 1995; Kerk et al.,
2008; Tonks, 2013). Unlike either the PPP or PPM protein phosphatases, which maintain a
tertiary folded catalytic cleft capable of coordinating metal co-factors, PTP phosphatases possess
a structurally deeper, more compact HCx5R catalytic motif, which employs a deprotonated
cysteine to facilitate dephosphorylation of target pTyr residues (Jia et al., 1995; Pannifer et al.,
1998; Tonks, 2013). PTP phosphatases are divided into 2 main subclasses: tyrosine phosphatases
and dual specificity phosphatases (DSPs) (Kerk et al., 2008). The DSP phosphatases were
initially named based upon the ability of the first mitogen-activated protein kinase (MAPK)
phosphatase to dephosphorylate both residues of the pTxpY motif in the MAPK activation loop
(Caunt and Keyse, 2013); however, a number of DSP phosphatases possess the capability of
dephosphorylating non-proteinaceous species such as phosphatidylinositol phosphates (PTEN),
glucans (Laforin, SEX4) and mRNA (RNA-capping enzymes) (Kerk et al., 2008; Shi, 2009;
Kotting et al., 2010; Gentry et al., 2013; Silver et al., 2013).
5
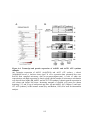
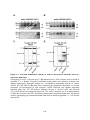
Figure 1.2: PPP protein phosphatase inhibitors.
Depicted are the molecular structures of (A) Microcystin-LR (MCLR) and (B) Okadaic acid
(OA). (C) Atomic structure of PP1-MCLR complex (1FJM; Goldberg et al., 1995). PP1 (green)
has four key amino acid stretches (blue) comprising the active site. The C-terminal SAPNYC
motif (red) contains the cysteine responsible for the covalent linkage of MCLR (yellow). Purple
spheres depict two Mn2+ cations that position in the active site to assist in PP1 enzymatic
activity. The upper panel depicts the PP1 atomic structure without microcystin, while the lower
panels depict the positioning of MCLR in the active site from two different angles.
6
The fourth family of protein phosphatases are the Asp-based catalytic phosphatases (Kerk
et al., 2008). These phosphatases are defined by an unique catalytic motif: the DxDxTV motif
(Kerk et al., 2008; Seifried et al., 2013). The upstream Asp (D) residues function to coordinate
the metal cation co-factor Mg2+ and are central to Asp-based phosphatase catalytic activity
(Seifried et al., 2013). This family is so far defined by a small group of RNA polymerase II
associating (FCP) and haloacid dehalogenase (HAD) protein phosphatases (Seifried et al., 2013).
1.3 Plant PPP protein phosphatases
The PPP protein phosphatase family of plants is comprised of type one (PP1) through
type 7 (PP7) phosphatases, which largely maintain both sequence and structural relatedness
(Figure 1.3). This is highlighted by their conserved catalytic mechanism involving the same four
canonical catalytic motifs found in other eukaryotic PPP protein phosphatases (Figure 1.3;
(Moorhead et al., 2007; Shi, 2009; Heroes et al., 2013)). Plants, however, lack the PP3 (or PP2B)
enzymes and, along with several other Eukaryotes, have additional unique PPP protein
phosphatases; the Kelch-repeat domain-containing (PPKL) protein phosphatases, the
Shewanella-like phosphatases (or SLPs) and the Rhizobiales-like phosphatases (or RLPHs),
which are all absent in mammals (Table 1.1; (MacKintosh and MacKintosh, 1994; Andreeva and
Kutuzov, 2004; Kerk et al., 2008)). When examining the tissue expression profile of A. thaliana
PPP protein phosphatases, it becomes evident that PPP protein phosphatases are likely involved
in regulating both cellular processes common across diverse tissue types as well as processes
central to highly specialized tissues (Figure 1.4). This is potentially highlighted by the abundant
AtPP1 phosphatases, which show wide-ranging transcriptional expression profiles outside of
7
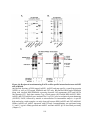
Figure 1.3: Phylogeny and domain architecture of plant PPPs.
The highly conserved core catalytic domain of each PPP subfamily is depicted in gray with
signature aspects of each motif highlighted. Green and blue amino acids represent those
involved in metal ion coordination and phosphate binding, respectively. Also described is the
microcystin inhibition docking motif SAPNYC (purple), which includes the reactive cysteine
(C) to which microcystin covalently attaches. PP7 maintains this motif, but lacks the reactive C,
whereas SLP and RLPH phosphatases completely lack this motif. Within these motifs ‘x’
represents any amino acid. Unique features of each subfamily are also depicted: TPR
(tetratricopeptide repeat), NLS (nuclear localization signal) and cTP (chloroplast transit
peptide). The Arabidopsis sequences used to compile the phylogenetic tree are: PP1 (TOPP1;
At2g29400), PP2A-1 (At1g59830), PP4-1 (At4g26720), PP5 (At2g42810), PP6-1 (At1g50370),
PP7 (At5g58500), SLP1 (At1g07010), SLP2 (At1g18480), RLPH1 (At3g09960), RLPH2
(At3g09970) and PPKL (BSU1; At1g03445). No canonical PP2B (calcineurin-A) is encoded in
plants. Neighbor-joining (NJ) phylogenetic tree was obtained using ClustalX 2.0.12 and was
visualized using FigTree v1.3.1. Tree topology is consistent with other studies examining PPP
protein phosphatases. The total number of amino acids for each enzyme is shown on the right
and, for presentation purposes only, two (×3) of the six Kelch-repeat motifs of the Kelch-like
domain are shown for BSU1. The number of genes encoding each subfamily of the PPP family
in Arabidopsis are shown in square brackets. Although the architecture of a specific gene
product is depicted (e.g. TOPP1), each additional protein maintains the same motifs and
domains.
8
silique and seed tissues, contrary to AtPP4 phosphatases, which are almost entirely expressed in
silique and seed tissues (Figure 1.4).
Table 1.1: PPP protein phosphatase complement of A. thaliana and humans.
PPP protein phosphatase subclasses found in only A. thaliana (light grey) and the total PPP
protein phosphatases encoded by each respective organism (dark grey) are highlighted. Table
was modified from Kerk et al., 2008., with labeled phosphatases (*) absent from this previous
study.
1.3.1 Type 1 (PP1) protein phosphatases
PP1 protein phosphatases are ~37 kDa monomeric proteins that are remarkably
conserved and ubiquitously expressed across Eukaryotes (Kerk et al., 2008). In A. thaliana there
are nine PP1 phosphatase isoforms which maintain 80 - 85 % identity amongst themselves and
76-90% identity to PP1 phosphatases from humans and fungi (Moorhead et al., 2009; Templeton
et al., 2011). With PP1 substrate specificity determined through interactions with a variety of
regulatory subunits, identification and characterization of these regulatory subunits has been of
paramount importance. Of the 200 known PP1 binding partners in humans, the vast majority
dock PP1 through a conserved binding site defined as 'RVxF', which maintains the consensus
9
(R/K)(R/K)(V/I)x(F/W) (Moorhead et al., 2007, 2007; Heroes et al., 2013). Numerous human
PP1 interactors have orthologs in plants that also possess conserved RVxF motifs and thus likely
associate with PP1 (Takemiya et al., 2009; Ogawa et al., 2011; Templeton et al., 2011; Takemiya
et al., 2013). The amino acids of PP1 responsible for coordinating the RVxF motif are also
conserved across Eukaryotes (Egloff et al., 1997), supporting this motif as an ancient proteindocking site. The prevalence of this motif contributes to PP1 involvement in a diversity of
cellular processes ranging from mitosis to metabolism in non-photosynthetic Eukaryotes (Cohen,
2002).
Recent biochemical evidence has demonstrated that plant PP1 regulatory proteins interact
with PP1 catalytic subunits through the RVxF motif as predicted, similarly implicating plant PP1
in the regulation of a number of cellular processes (Ogawa et al., 2011; Takemiya et al., 2013).
These binding partners function to either abolish PP1 activity by blocking access to the active
site (e.g. Inhibitor-2 protein), or by recruiting substrates and/or controlling active site access (Peti
et al., 2013). From a cellular localization perspective, the nine A. thaliana PP1 (AtPP1)
phosphatases, like the three human PP1 isoforms, localize to the nucleus and cytosol (Templeton
et al., 2011; Takemiya et al., 2013), and in plants are excluded from the plastid (MacKintosh et
al., 1991). Transcriptional expression analysis shows that they are present in a wide range of
tissues, but are largely excluded from siliques and seeds (Figure 1.4). As well, all AtPP1
phosphatases were found to be active phosphatases and to be differentially regulated by A.
thaliana inhibitor-2 (AtI2) protein (Templeton et al., 2011). Through a combination of MCLRor PP1-Sepharose affinity chromatography, several PP1 interactors have been uncovered in A.
thaliana, including NIPP1 (NUCLEAR INHIBITOR OF PROTEIN PHOSPHATASE 1), SDS22
(SUPPRESSOR OF DIS2), GEM (GL2-EXPRESSION MODULATOR) and I2 (Figure 1.5;
10
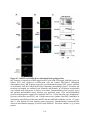
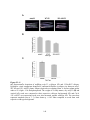
Figure 1.4: Tissue-specific transcript expression of the A. thaliana PPP protein
phosphatases.
Using the online database Genevestigator, the transcriptional expression profile of each PPP
protein phosphatase from A. thaliana was obtained (www.genevestigator.com) across a range of
tissues. These data were derived from compiled microarray expression analysis using the
Affymetrix 22k A. thaliana chip. At2g29400 (AtPP1-1; TOPP1) At5g59160 (AtPP1-2; TOPP2),
At1g64040 (AtPP1-3; TOPP3); At2g39840 (AtPP1-4; TOPP4), At3g46820 (AtPP1-5; TOPP5),
At5g43380 (AtPP1-6; TOPP6), At4g11240 (AtPP1-7; TOPP7), At5g27840 (AtPP1-8; TOPP8),
At3g05580 (AtPP1-9; TOPP9), At1g10430 (AtPP2A-1), At3g58500 (AtPP2A-2), At2g42500
(AtPP2A-4), At1g69960 (AtPP2A-5), At4g26720 (AtPP4-1), At5g55260 (AtPP4-2),
At2g42810 (AtPP5), At1g50370 (AtPP6-1), At3g19980 (AtPP6-2), At5g63870 (AtPP7),
At1g03445 (AtBSU1), At4g03080 (AtBSL1), At2g27210 (AtBSL2), At1g07010 (AtSLP1),
At1g18480 (AtSLP2), At3g09960 (AtRLPH1), At3g09970 (AtRLPH2). Missing are At3g58500
(AtPP2A-3), and the AtPPKL At1g08420 (BSL3) which was not found in the Genevestigator
database. Expression is indicated by a gradient of white (low expression) to red (high
expression) as a percentage of the top 1% genes expressed in each tissue. The numbers describe
the number of total arrays used in determining the displayed level of relative gene expression.
11
(Templeton et al., 2011)). Other studies employing genetic approaches have additionally
identified RVxF-containing plant PP1 partners I3 (Inhibitor-3 protein) (Takemiya et al., 2009),
RSS1 (RICE SALT SENSITIVE 1) (Ogawa et al., 2011) and PRSL (PP1 regulatory subunit 2like protein) (Figure 1.5; (Takemiya et al., 2013)).
Of the plant PP1 protein regulators identified to date, I2 is thought to be one of the most
ancient (Stubbs et al., 2001; Ceulemans et al., 2002). Mammalian I2 has been studied for over 3
decades and in 2011, the A. thaliana version (AtI2) was characterized, after being captured on a
PP1-Sepharose matrix (Templeton et al., 2011). AtI2 was found to contain a conserved RVxF
motif and to inhibit all nine A. thaliana PP1 isoforms. Utilizing the inhibitory properties of I2,
Takemiya et al., (2006) demonstrated that PP1 regulates stomatal opening downstream of the
blue light sensing kinase phototrophin (Takemiya et al., 2006). Yeast two-hybrid screening for
PP1 interactors also identified several RVxF-containing proteins including PRSL1, which like
GEM, binds PP1 in an RVxF-dependent manner (Takemiya et al., 2013). Although not
completely resolved, these results suggest that PRSL1 targets PP1 to regulate blue light sensing.
Additionally, through a combined approach of genetic screening for salt tolerance in rice and
yeast two-hybrid, Ogawa et al., (2011) identified the RVxF-containing PP1 regulatory protein
RSS1, loss of which, resulted in short-root and dwarf phenotypes under high-salt conditions.
RSS1 accumulates through the progression of S-phase in the cell cycle where it is required for
the maintenance of proliferative cells in meristematic tissues.
12
Figure 1.5: A. thaliana PP1: from non-specific catalytic subunits to specialized cellular
functions.
PP1 phosphatases are encoded in the genomes of all Eukaryotes as bare catalytic subunits that
require the interaction of regulatory protein interactors to drive their specificity within the cell.
Interaction between PP1 catalytic subunits and regulatory proteins is facilitated by the RVxF
protein-binding motif. Depicted are documented PP1 protein interactors which possess an RVxF
motif, and the corresponding cellular process they are implemented in regulating in complex
with PP1. With human PP1 phosphatases maintaining upwards of 200 regulatory proteins, this
list of documented plant PP1 regulatory proteins will likely expand significantly.
13
1.3.2 Type 2A (PP2A) protein phosphatases
The PP2A holoenzyme is trimeric, consisting of a ~35 kDa catalytic (C), ~65 kDa
scaffolding (A) and ~54 - 130 kDa regulatory (B) subunit (DeLong, 2006). A. thaliana encodes 5
catalytic, 3 scaffolding and 17 regulatory B subunits, which form a variety of combinations to
exert different regulatory outcomes (Jonassen et al., 2011; Trotta et al., 2011). The 17 identified
B-subunits group into B (55 kDa), B´ (54-72 kDa) and B” (72-130 kDa) subunit families, and
coupled with recent structural analysis, confirm B-subunits control substrate access to the
catalytic subunit active site (Shi, 2009). Several studies have identified the A. thaliana PP2A-A1
subunit RCN1 (ROOTS CURL IN NAPHTHYLPHTHALAMIC ACID 1) and several Bsubunits as direct players in stress signaling (B´γ) (Trotta et al., 2011), abscisic acid (ABA)
insensitivity, guard cell responses, as well as hypocotyl and root elongation (B’’α) (Blakeslee et
al., 2008). Metabolic links have also established B” subunits (α and β) as negative regulators of
3-hydroxy-3-methylglutaryl Coenzyme A reductase (HMGR), a key enzyme that regulates the
isoprenoid biosynthesis pathway (Leivar et al., 2011) and B55 subunits (α and β) as necessary for
nitrate reductase activation (MacKintosh, 1992; Heidari et al., 2011) as well as TAP46, a key
regulator of cell growth and survival, autophagy, and protein synthesis (Figure 1.6A; (Ahn et al.,
2011)). In vitro, PP2A has been suggested to regulate other metabolic proteins such as
phosphoenolpyruvate carboxylase (PEPC) (Dong et al., 2001) and sucrose phosphate synthase
(Siegl et al., 1990). The B subunits responsible for directing PP2A specificity towards these
targets remain to be uncovered.
Aspects of PP2A function also indicate a regulatory link between different posttranslational modifications, with phosphorylation-acetylation (Tran et al., 2012) and
phosphorylation-methylation (Wu et al., 2011) connections being noted to date. It was found by
14
Tran et al., 2012 that PP2A co-purified with the histone deacetylase HDA14 and the histone
acetyltransferase ELP3. HDA14 deacetylates α-tubulin and is also the ortholog of human
HDAC6, which targets mammalian α-tubulin. The specific function of this interaction remains
unknown; however, PP2A was previously implicated in the control of microtubule function
(Farkas et al., 2007) and may regulate the binding and trafficking of kinesins (Tran et al., 2012).
Current evidence suggests that HDA14 may represent a regulatory 'B' subunit due to its direct
interaction with scaffolding A subunits of PP2A. Further experimentation is required to
specifically resolve this hypothesis (Figure 1.6B). The phosphorylation-methylation link was
made during the examination of brassinosteroid (BR) signaling, where the leucine
carboxymethyltransferase SBI1 (SUPPRESSOR OF BRI1) was found to be responsible for
methylating PP2A (Wu et al., 2011).
Like PP1, PP2A has been implicated in regulating light signaling in plants. In particular,
yeast two-hybrid and in vitro pull-down assays uncovered a direct interaction between RCN1
(PP2A-A1) and PHOT2 (Tseng and Briggs, 2010). This work, along with Tran et al. (2012) and
others (Yu et al., 2008; Herzog et al., 2012; Tran et al., 2012), have highlighted that scaffolding
A subunits do not just bind the B and C subunits of PP2A, but undoubtedly other protein partners
as well. The association of RCN1 (PP2A-A1) and PHOT2 was found to down-regulate
phototropism and stomatal opening through the dephosphorylation of PHOT2 under blue light
conditions (Figure 1.6C; (Tseng and Briggs, 2010)). Furthermore, PP2A-C (isoform C2) has
been implicated as a positive regulator in chloroplast movements mediated by PHOT2. Under
blue light conditions PP2A was shown to dephosphorylate and activate the actin binding protein
15
Figure 1.6: Cellular events regulated by PP2A in plants.
(A) Many key cellular signals converge on target of rapamycin (TOR) in plants as in humans
and yeast. PP2A-C interacts with regulatory subunit Tap46 (α4/Tap42), a target of TOR, to
modulate downstream cellular events: protein translation, autophagy, nutrient cycling and
senescence in plants. Pointed and blunt arrowheads denote activation and inhibition of enzyme
activity or cellular processes, respectively. (B) The plant PP2A-A1 (RCN1) scaffolding subunit
forms a specific complex with HDA14 to deacetylate α-tubulin. Whether HDA14 represents a
novel B targeting subunit or simply a protein interactor has yet to be determined. The role of
histone acetyltransferase ELP3 (elongator complex protein 3) in conjunction with PP2A in
modulating α-tubulin acetylation is also unknown. The identification of an HDA14–PP2A
complex represents an interesting point of cross-talk between protein phosphorylation and
acetylation. Broken arrows denote the possible influence of nearby post-translational
modifications on the ability of a PP2A– HDA14 complex to bind tubulin. (C) PP2A specifically
dephosphorylates light sensor PHOT2 to regulate phototropism and stomatal opening. The
16
regulatory B subunit directing the specificity of these functions remains unknown. (D) PINOID
kinase and PP2A regulate polar auxin transport through auxin efflux carrier PIN via
phosphorylation and dephosphorylation, respectively. (E) Central to BR signaling is methylated
PP2A-mediated dephosphorylation of the brassinosteroid receptor BRI1, which controls either
BRI1 cellular internalization at the plasma membrane or recycling from endosomes leading to
degradation. PP2A methylation results from BR-induced SBI1 expression. Kelch-like
phosphatase BSU1 is also involved in the BR signaling pathway by facilitating nuclear
enrichment of BZR transcription factors via BIN2 kinase dephosphorylation. Evidence for the
potential direct induction of SBI1 transcription by BZR remains to be determined. Solid and
broken arrows represent the activation and subsequent inactivation of BR signaling, respectively.
Abbreviations: Ac, acetylation; BAK1, BRI1-associated kinase 1; BKI1, BRI1 kinase inhibitor 1;
BSK1, BR signaling kinase; CDG1, constitutive differential growth 1; BSU1, BRI1 suppressor
1; BIN2, BR-insensitive kinase 2; LST8, Lethal with Sec Thirteen 8; P, phosphorylation; RPS6,
ribosomal protein S6; S6K, S6 protein kinase. Double black line represents the plasma
membrane. Parts (a), (d) and (e) were adapted from (Ahn et al., 2011; Di Rubbo et al., 2011; Li
et al., 2011).
ADF / cofilin, leading to a rearrangement of the actin cytoskeleton and resulting in light
stimulated chloroplast movements (Wen et al., 2012).
PP2A is additionally involved in regulating aspects of plant hormone signaling, with
recent evidence demonstrating PP2A influence over the auxin transport system and cell polarity
(Li et al., 2011; Ballesteros et al., 2012). PP2A-C, more specifically sub-family 2, and the
corresponding protein kinase (PINOID) were implicated in regulating the phosphorylation state
of the auxin efflux PIN-FORMED (PIN) proteins in roots (Figure 1.6D; (Michniewicz et al.,
2007)). Furthermore, it is hypothesized that PP2A-C (isoform 4) is co-expressed with, and
dephosphorylates, PIN1 to fine tune normal auxin transport to the root tip (Ballesteros et al.,
2012). Interestingly, this regulatory mechanism also seems to be conserved in the leaf epidermis
controlling the differentiation of pavement cells (Li et al., 2011).
Perhaps the most significant contribution to PPP protein phosphatase research in the past
few years is the finding that PP2A is intimately involved in regulating intracellular responses to
17
brassinosteroids (BR). Several key works revealed the action of PP2A at two points in this
pathway. First, dephosphorylation and inactivation of BR-coupled receptor BRI1 (BR-insensitive
1) (Figure 1.6E; (Wu et al., 2011)), and second, upon initiation of the intracellular BR signal
cascade, activation of BR-responsive gene transcription through the dephosphorylation and
subsequent nuclear accumulation of the transcription factors BZR1 and 2 (Figure 1.6E; (Tang et
al., 2011)). The dephosphorylation of BZR1 and 2 specifically employs a PP2AB’ complex and
is critical for the BR signaling cascade; however, it is not known yet if PP2A needs additional
post-translational modifications to execute this function (Tang et al., 2011). In the initial process
outlined above, a genetic screen for suppressors of bri1-5 identified a suppressor (sbi1) that
accumulated BRI1 protein in the mutant plant. SBI1, a leucine carboxymethyltransferase,
specifically methylates the PP2A catalytic subunit C-terminal YFL motif leucine. It is not clear if
PP2A (and SBI1) acts at the plasma membrane or during receptor recycling through the
endomembrane system to mark BRI1 for degradation, and thus switch off BR signaling (Wu et
al., 2011). Although PP2A and SBI1 operate at this level in the pathway, it has yet to be
established if BRI1 is a direct substrate of PP2A.
1.3.3 Type 4 & 6 (PP4 & PP6) protein phosphatases
The PP4 and PP6 catalytic subunits are also conserved across Eukaryotes, including
plants (Dai et al., 2012; Dai et al., 2013). These proteins form a phylogenetically distinct cluster
of PPP protein phosphatases along with PP2A, which is suggestive of a common ancestral
phosphatase (Figure 1.2; (Moorhead et al., 2009)). PP4 and PP6 phosphatases possess 60-65 %
identity to each other in addition to PP2A, while maintaining a much higher ~90-95 % identity
amongst respective subclass orthologs found in other Eukaryotes (Kloeker et al., 2003; Cohen et
18
al., 2005). Close evolutionary relatedness between PP4 and PP2A phosphatases also translates
into a similar sensitivity to MCLR and OA inhibition (Kloeker et al., 2003; Hastie et al., 2005).
As well, both PP4 and PP6 possess protein regulatory (R) proteins named PP4R1-R4 and
PP6R1-R3, respectively, which exclusively bind each respective catalytic subunit and contribute
to their functional specificity (Cohen et al., 2005; Chen and Gingras, 2007; Stefansson et al.,
2008). Similar to the PP2A-A scaffolding subunits, which possess HEAT (Huntingtin, elongation
factor 3 (EF3), protein phosphatase 2A (PP2A), and the yeast kinase target of rapamycin 1
(TOR1)) repeats, PP4R- and PP6R-associating subunits are comprised of "HEAT-like" and
ankyrin repeats, respectively (Cohen et al., 2005; Chen and Gingras, 2007; Stefansson et al.,
2008). Overall, the annotated differences in sequence identity and regulatory binding partners
indicate that PP4 and PP6 largely maintain regulatory roles independent of each other and PP2A
(Cohen et al., 2005; Chen and Gingras, 2007; Stefansson et al., 2008).
Structurally, both PP4 and PP6 catalytic subunits (c) have been suggested to exist in
either trimeric or dimeric protein complexes (Zhang et al., 2005; Douglas et al., 2010; Ahn et al.,
2011). Although no atomic level protein structures have yet been resolved, this is best
exemplified in humans by the identification of a PP4c-R2-HDAC3 (histone deacetylase 3)
complex as well as a PP6c-R1/2/3-DNA-PK (DNA-dependent protein kinase) complex,
accompanied independently by the ability of both PP4c and PP6c to both bind α4 (TAP42 in
yeast; TAP46 in A. thaliana) (Zhang et al., 2005; Douglas et al., 2010). Facilitating protein
interactor commonalities between PP4, PP6 and PP2A may be their maintenance of a C-terminal
YFL motif that allows for C-terminal leucine methylation in PP2A (Sents et al., 2013).
Consistent with this idea was the finding that A. thaliana PP2A, PP4 and PP6 each bind TAP46
with varying affinities independent of their other regulatory subunits (Ahn et al., 2011). As with
19
α4 and TAP42, TAP46 was found to be a substrate of the target of rapamycin (TOR) protein
kinase (Ahn et al., 2011), with RNA-induced gene silencing demonstrating TAP46 to be crucial
for cell growth and survival, autophagy, and protein synthesis (Figure 1.6A; (Ahn et al., 2011)).
To date, no clearly defined roles for plant PP4 have been elucidated; however, with PP4
maintaining multiple regulatory roles in humans and yeast, there will likely be key roles for PP4
in plants. Conversely, PP6 affects PIN phosphorylation and auxin efflux (Dai et al., 2012) as
well as abscisic acid (ABA) signaling in A. thaliana (Dai et al., 2013). Loss of function mutants,
over-expression plant lines and protein interaction analysis suggested that when ABA levels
decrease, PP6 dephosphorylates the transcription factor ABI5 (ABSCISIC ACID-INSENSITIVE
5) leading to its degradation. This allows the initiation of seed germination and post-germination
growth (Dai et al., 2013).
1.3.4 Type 5 (PP5) protein phosphatases
The PP5 subclass of the PPP protein phosphatases is also conserved across all Eukaryotes
(Andreeva and Kutuzov, 1999; Shi, 2009). PP5 protein architecture consists of a conserved Nterminal tetratricopeptide repeat (TPR) domain and a C-terminal phosphatase catalytic domain
(Figure 1.3; (Andreeva and Kutuzov, 1999; Shi, 2009)). Subcellular localization analysis
indicated that PP5 phosphatase largely maintains a dual cytosolic / nuclear cellular location
(Chen et al., 1994; de la Fuente van Bentem et al., 2003). Of the two PP5 phosphatase structural
components, the TPR domain is implicated in facilitating protein-protein interactions as well as
functioning as an auto inhibitory domain (Figure 1.2; (de la Fuente van Bentem et al., 2003; Iki
et al., 2012)). In light of this, PP5 phosphatases are characterized by low basal levels of
phosphatase activity, which increase in the presence of arachidonic acid or following in vitro
20
proteolytic truncation of the TPR domain (Meek et al., 1999; de la Fuente van Bentem et al.,
2005). In the plants A. thaliana and Lycopersicon esculentum, PP5 is encoded by a single gene
that produces two alternatively spliced transcripts, with the larger 62 kDa isoform maintaining
the additional exon and an endoplasmic reticulum localization, while the smaller 55 kDa isoform
exhibits the well conserved dual cytosolic / nuclear subcellular localization (de la Fuente van
Bentem et al., 2003).
In plants, PP5 functions in light detection (Ryu et al., 2005), disease resistance (de la
Fuente van Bentem et al., 2005), thermotolerance (Park et al., 2011) and chloroplast
development (Barajas-Lopez Jde et al., 2013). In particular, PP5 from Lycopersicon esculentum
interacts with cytosolic members of the heat shock protein 90 (Hsp90) family and disease
resistance R protein I-2 through the TPR domain to induce cell death (de la Fuente van Bentem
et al., 2005). This observed interaction between PP5 and Hsp90 represents a phenomenon also
observed in other Eukaryotes (Chen et al., 1996). Most recently, additional protein interactions
between PP5 and Hsp90 were resolved in BY2 tobacco cell culture, where PP5 co-purified with
Hsp90-AGO1 (Argonaute 1) RNA-induced silencing complexes (RISCs) in addition to other
TPR-containing proteins cyclophilin 40 and others (Iki et al., 2012). In vitro binding assays
found that PP5 competes with cyclophilin 40 in binding the Hsp90-AGO1 complex, resulting in
reduced RNA loading onto the RISC complex, while cyclophilin 40 alone enhanced RNA
loading onto the RISC complex. This was proposed as a regulatory mechanism for RISC
complex RNA loading (Iki et al., 2012).
Lastly, AtPP5 regulates both chloroplast development via the tetrapyrrole plastid
signaling pathway (Barajas-Lopez Jde et al., 2013) and light signaling through phytochrome A
(Ryu et al., 2005). Here, mutation in a potential subunit of Mg-protoporphyrin monomethyl ester
21
cyclase complex chl27/crd was rescued by the additional knockout of atpp5, suggesting AtPP5
functions by relaying the chloroplast generated tetrapyrrole signal to the nucleus to stimulate
transcription of chlorophyll biosynthetic genes and other key photosynthesis associated, nuclearencoded genes (Barajas-Lopez Jde et al., 2013). Interestingly, AtPP5 was also found to interact
with phytochrome A through a yeast two-hybrid screen (Ryu et al., 2005). Experiments
employing both atpp5 mutant and AtPP5 over-expression plants related increased AtPP5
abundance to greater photo-responsiveness and enhanced expression of light-inducible genes
(Ryu et al., 2005).
1.3.5 Type 7 (PP7) protein phosphatases
PP7 protein phosphatases are unique to plants, where they maintain a domain architecture
differing from other PPP protein phosphatases. Unlike the comparable class of mammalian
protein phosphatases with EF-hand domains (PPEFs), PP7 protein phosphatases do not maintain
any N- or C-terminal extensions capable of binding Ca2+(Andreeva and Kutuzov, 2009), but
instead possess a charged insertional region of variable length and a C-terminal nuclear
localization signal required for constitutive nuclear localization (Figure 1.3; (Andreeva and
Kutuzov, 2001)). Despite the lack of EF-hand domains, Ca2+ likely still influences PP7 in vivo,
as the insertional region positioned adjacent to the last key PPP protein phosphatase catalytic
motif (HGG) has been shown to bind calmodulin in a Ca2+-dependent manner in vitro (Kutuzov
et al., 2001). As well, this region has alternatively been proposed to be an auto inhibitory domain
(Kutuzov et al., 1998). Across plants, only a single PP7 isoform is encoded by each plant
genome, which, when expressed, is targeted to the nucleus (Andreeva and Kutuzov, 2001).
22
Functionally, PP7 protein phosphatases have been implicated in a number of sensory
functions, in particular light sensing (Moller et al., 2003; Genoud et al., 2008; Sun et al., 2012),
and exhibit preferential expression in the stomatal cells of leaves (Sun et al., 2012). PP7-silenced
A. thaliana indicated AtPP7 involvement in light signaling by revealing that PP7 positively
regulates the cryptochrome (Moller et al., 2003). Subsequently, it was found that atpp7 loss-offunction plants displayed red / far-red light sensitivity, indicating that PP7 also influences
phytochrome signaling (Genoud et al., 2008). Furthermore, this study revealed that phytochrome
regulation by PP7 occurs through a direct interaction with nucleoside diphosphate kinase 2
(Genoud et al., 2008). Most recently, PP7 has been directly tied to stomatal aperture control via
cryptochrome signaling and the dephosphorylation of a nuclear, zinc finger-containing protein
called HRB1 (Sun et al., 2012).
1.3.6 Novel plant bacterial-like PPP protein phosphatases
Novel PPP protein phosphatases include the protein phosphatase kelch-like (PPKL),
Shewanella-like (SLP) and Rhizobiales-like (RLPH) phosphatases (Figure 1.3; (Andreeva and
Kutuzov, 2004; Kerk et al., 2008)). PPKL phosphatases are named after their tandem N-terminal
kelch-repeats which likely facilitate protein-protein interactions (Kutuzov and Andreeva, 2002;
Mora-Garcia et al., 2004), while SLP and RLPH phosphatases were named after their deduced
phylogenetic relatedness to phosphatases from Shewanella and Rhizobiales bacteria, respectively
(Andreeva and Kutuzov, 2004). In A. thaliana there are four PPKL phosphatases, BSU1 (Bri1
suppressor 1), BSL1 (Bri1 suppressor-like 1), BSL2 (Bri1 suppressor-like 2) and BSL3 (Bri1
suppressor-like 3) (Moorhead et al., 2009; Wu et al., 2011), along with 2 SLP phosphatases
(SLP1 and SLP2) and 2 RLPH phosphatases (RLPH1 and RLPH2).
23
BSU1 represents the first, and remains the only, well-characterized PPKL protein
phosphatase (Mora-Garcia et al., 2004; Kim et al., 2009; Kim et al., 2011). BSU1 is an OAsensitive and I2-insensitive protein phosphatase (Mora-Garcia et al., 2004). Investigation of the
other A. thaliana PPKL phosphatases BSL1-3 through RNAi-mediated suppression revealed the
function of these proteins to overlap (Mora-Garcia et al., 2004). Identified as a suppressor of the
BR receptor BRI1, BSU1 was suggested to directly dephosphorylate downstream BR-induced
transcription factors BZR1 and 2, resulting in their nuclear accumulation and activation of BRinduced gene transcription (Mora-Garcia et al., 2004). Subsequently, it was found that BSU1
indirectly fine-tunes the phosphorylation status of both BZRs through the dephosphorylation and
inactivation of GSK3-like kinase BIN2 (Kim et al., 2009), with BZR1 and 2 dephosphorylation
catalyzed by PP2A (Tang et al., 2011). Most recently it was revealed that BRI1 plasma
membrane mediated BR signaling to BSU1 was facilitated by a cytosolic receptor-like kinase
CDG1 (Kim et al., 2011). Transgenic plant experiments revealed that CDG1 positively regulates
BR signaling whereby BR1 phosphorylates CDG1 on S234, which then in turn phosphorylates
BSU1 on S764, resulting in BIN2 inactivation via its dephosphorylation (Figure 1.6E; (Kim et al.,
2011)). Specific disruption of these phosphorylation sites through targeted site-directed
mutagenesis impaired BR signaling (Kim et al., 2011).
1.3.7 Prokaryotic protein phosphorylation and PPP protein phosphatases
Prokaryotic protein phosphorylation occurs on a number of residues including serine,
threonine, tyrosine, histidine and aspartate (Pereira et al., 2011). Historically, prokaryotic protein
phosphorylation was thought to occur solely as part of two component signaling systems
involving pHis and pAsp residues. This required the need for dedicated kinases, but not
24
dedicated phosphatases due to the innate lability of these phosphorylated amino acids (Stock et
al., 1990; Hoch, 2000). However, thanks to advancements in genome sequencing technologies,
numerous eukaryotic-like protein kinases have been uncovered across a multitude of organisms,
leading to a change in this perspective. As of 2008, 383 of the 577 sequenced bacterial genomes
had been found to maintain at least 1 identifiable eukaryotic-like protein kinase, with 25 of those
genomes having greater than 15 protein kinases (Perez et al., 2008). Myxobacteria had the
largest percentage of sequenced genomes maintaining 15 or more protein kinases (~85 %), with
Stigmatella aurantiaca and Plesiocystis pacifica encoding 194 and 230 putative protein kinases,
respectively (Perez et al., 2008).
Correspondingly, genome sequencing of Prokaryotes has revealed homologs from all
four eukaryotic protein phosphatase families (Kennelly, 2002; Pereira et al., 2011). The
identified prokaryotic protein phosphatases qualifying as PPP protein phosphatases have been
characterized to dephosphorylate pHis and pTyr in addition to pSer and pThr in vitro, suggesting
they are DSP phosphatases (Pereira et al., 2011). The prevalence of in vitro dual specificity
amongst prokaryotic PPP protein phosphatases is highlighted by the distant eukaryotic SLP
ancestor CAPTase from Shewanella, which was found to dephosphorylate pTyr residues in vitro
despite maintaining a PPP protein phosphatase catalytic fold and coordinating Mn2+ ions in its
active site (Tsuruta and Aizono, 1999, 2000).
The function of regulatory protein phosphorylation in Prokaryotes seems to parallel that
which is observed in Eukaryotes. In particular, Mycobacterium tuberculosis protein GarA was
found to contain a forkhead-associated (FHA) domain which interacts with phosphorylated PknB
protein kinase (Villarino et al., 2005). FHA domains bind phosphorylated threonine residues
(Hammet et al., 2003), indicating that, even within Prokaryotes, phosphorylation-dependent
25
protein-protein interaction networks exist. With many protein kinases and phosphatases exerting
influence over bacterial cell processes important to human health (e.g. cell division),
understanding the in vivo targets of these regulatory proteins could be very revealing (Pereira et
al., 2011). Much remains to be uncovered regarding the regulatory roles of prokaryotic protein
kinases and phosphatases; however, given the diversity and distant relatedness of these proteins
to those found in Eukaryotes, research in this area will likely uncover many novel findings.
1.4 Research Objectives
With PPP protein phosphatases representing a key regulatory protein family across
Eukaryotes, the focus of my Ph.D. research centered upon the unusual bacterial-like PPP protein
phosphatases of A. thaliana: the SLP and RLPH phosphatase subclasses (Andreeva and Kutuzov,
2004; Kerk et al., 2008; Moorhead et al., 2009). Prior to the work presented in this thesis, little
was known about either SLP or RLPH phosphatases beyond the existence of genes encoding
them in select plants and other Eukaryotes (Andreeva and Kutuzov, 2004; Kerk et al., 2008).
Andreeva and Kutuzov 2004, found the A. thaliana genome to encode two SLP phosphatases,
AtSLP1 (At1g07010) and AtSLP2 (At1g18480), with preliminary analysis suggesting AtSLP1
may be chloroplastic and/or endoplasmic reticulum (ER) localized and AtSLP2 exclusively ER
localized (Andreeva and Kutuzov, 2004, 2008). A. thaliana (At) was also found to encode two
RLPH phosphatases, AtRLPH1 (At3g09960) and AtRLPH2 (At3g09970). Other than their initial
annotation, and a suggested phylogenetic relatedness to phosphatases from Rhizobiales bacteria,
no additional information was known about RLPH phosphatases (Andreeva and Kutuzov, 2004).
26
The goal of the first research chapter of this thesis (Chapter 2) was to resolve the
molecular evolution of eukaryotic bacterial-like phosphatases using a number of bioinformatic
and phylogenetic approaches. This also revealed sequence motifs and characteristics that are
unique to the SLP and RLPH phosphatases, in addition to elucidating their close relatedness to
prokaryotic PPP protein phosphatases. These intriguing findings stimulated additional questions
regarding the cell biological and biochemical properties of AtSLP1 and AtSLP2. This was
addressed in Chapter 3 where the objective was to experimentally verify proposed inferences
about AtSLP1 and 2 subcellular localization and potential inhibitor sensitivities. The objectives
of Chapters 4 and 5 were to explore the cellular and biological function of AtSLP phosphatases
by identifying their protein interactors through the application of protein-protein interaction
techniques, and by identifying plant phenotypes using atslp insertional mutant and constitutive
35S::AtSLP over-expression plant lines. Finally, Chapter 6 shifts from the examination of AtSLP
phosphatases, to examining the AtRLPH phosphatases. The objective of chapter 6 was to fully
characterize the cell biological and biochemical properties of AtRLPH2. Together, the findings
presented throughout this thesis lay a solid foundation for future research endeavours involving
eukaryotic bacterial-like PPP protein phosphatases.
27
Chapter Two: Evolution of bacterial-like PPP protein phosphatases in
photosynthetic eukaryotes features ancestral mitochondrial origin overlaid by
horizontal gene transfer
2.1 Introduction
Reversible protein phosphorylation is a post-translational mechanism central to the
proper function of living organisms (Cohen, 2000, 2002). Governed by two large groups of
enzymes, protein kinases and protein phosphatases, this mechanism has been shown to regulate
upwards of 70% of all eukaryotic proteins (Olsen et al., 2010). Protein phosphatases represent
one half of this dynamic regulatory system and have been shown to be highly regulated proteins
themselves (Roy and Cyert, 2009; Shi, 2009). Recently, the PPP protein phosphatase family has
been expanded to include two novel subclasses which show enhanced similarity to PPP-like
protein phosphatases of prokaryotic origin (Andreeva and Kutuzov, 2004). These bacterial-like
phosphatase subclasses were annotated as Shewanella-like (Shelph; SLP) phosphatases, and
Rhizobiales-like (Rhilph; RLPH) phosphatases based on their similarity to prokaryotic sequences
from these respective sources (Andreeva and Kutuzov, 2004).
Characterization of eukaryotic protein evolution can provide insight into individual
protein or protein class conservation across the domains of life for biotechnological applications,
in addition to furthering our understanding of how multicellular life evolved. In particular,
investigation into the evolution of key signaling proteins, such as protein kinases and
phosphatases from plants, can have wide-ranging agricultural biotechnology and/or medical
potential. This can include the development of disease- or stress-resistant crops in addition to
treatments for parasitic organisms such as Plasmodium (malaria) (Patzewitz et al., 2013) and
other Chromoalveolates (Kutuzov and Andreeva, 2008), that are derived from photosynthetic
Eukaryotes and maintain a remnant chloroplast (apicoplast) (Le Corguille et al., 2009;
28
Janouskovec et al., 2010; Kalanon and McFadden, 2010; Walker et al., 2011). The existence of
proteins that are conserved across diverse eukaryotic phyla, but absent in Metazoa, such as the
bacterial-like PPP protein phosphatases described here, presents unique research and, possibly,
biotechnological opportunities.
Conventional understanding of prokaryotic gene acquisition by Eukaryotes largely
involves ancient endosymbiotic gene transfer events stemming from primary endosymbiosis of
α-Proteobacteria and Cyanobacteria to form the eukaryotic mitochondria and chloroplast,
respectively (Keeling and Palmer, 2008; Dorrell and Smith, 2011; Tirichine and Bowler, 2011).
Over time, however, it has become apparent that alternative modes of eukaryotic gene and
protein acquisition exist, such as independent horizontal gene transfer (HGT) events (Keeling
and Palmer, 2008; Keeling, 2009). Targeted studies of protein evolution have seen a steady rise
in documented HGT events across a wide variety of eukaryotic organisms, including
photosynthetic Eukaryotes (Derelle et al., 2006; Raymond and Kim, 2012; Schonknecht et al.,
2013), Nematodes (Mayer et al., 2011), Arthropods (Acuna et al., 2012), Fungi (Wenzl et al.,
2005), Amoebozoa (Clarke et al., 2013) and Oomycetes (Belbahri et al., 2008). Each instance
documents the integration of a bacterial gene(s) into a eukaryotic organism seemingly resulting
in an adaptive advantage(s) important to organism survival.
Utilizing a number of in silico bioinformatic techniques and available sequenced
genomes, the molecular evolution of two bacterial-like PPP protein phosphatase subclasses
found in Eukaryotes is revealed to involve ancient endosymbiotic gene transfer plus additional
overlays of likely HGT events. Subcellular localization prediction algorithms reveal distinctive
subsets of bacterial-like PPP protein phosphatases, which may correlate with altered functions,
while the large sequence collections compiled here have allowed the elucidation of two highly
29
conserved carboxy-terminal domain motifs, which are specific to each eukaryotic bacterial-like
PPP subclass. Together these findings substantially expand our knowledge of the molecular
evolution of the bacterial-like PPP protein phosphatases, and point the way toward attractive
future research avenues.
2.2 Materials and Methods
2.2.1 Multiple sequence alignments
Protein sequences were aligned using MAFFT (ver 7) ((Katoh et al., 2002);
http://mafft.cbrc.jp/alignment/server/), with the BLOSUM45 scoring matrix, using the E-INS-i
option (very slow, multiple domains with long inserts). Alignments were visualized and handedited in GeneDoc ((Nicholas et al., 1997); http://www.nrbsc.org/gfx/genedoc/). As necessary
for subsequent work, edited sequence alignment formats were converted using an online
converter utility ((Futami et al., 2008); http://biotechvana.uv.es/servers/afc/main.php).
2.2.2 Candidate sequence search, retrieval and validation
Initial eukaryotic sequences of the SLP and RLPH phosphatases were obtained from the
literature (Andreeva and Kutuzov, 2004), and through database searching at NCBI with BLASTP
and PSI-BLAST ((Altschul et al., 1997); http://blast.ncbi.nlm.nih.gov/). These were then used to
generate initial multiple sequence alignments, as described above. Edited multiple sequence
alignments were converted into Stockholm format, and used to generate Hidden Markov Models
(HMMs) by the HMMER (v.3.0) software suite ((Eddy, 1998); http://hmmer.janelia.org/).
Databases of protein sequences from completely sequenced eukaryotic and prokaryotic species
were compiled locally. Eukaryotic sequences were obtained from the Joint Genomes Institute
30
(JGI)
(http://www.jgi.doe.gov/),
Phytozome
(http://www.phytozome.net/),
Metazome
(http://www.metazome.net/), or individual genome project web sites. Prokaryotic sequences
were obtained from UniProt (http://www.uniprot.org/). Databases were searched using HMMs,
candidate sequences extracted, and then placed into further multiple sequence alignments as
described above. Potential candidate sequences were evaluated from the full range of HMM hits
for each sequence class, from the strongest (lowest E value) to the weakest (the statistical
inclusion threshold [E ~ 0.01]). In some instances further iteration was performed with BLASTP
and HHBlits ((Remmert et al., 2012); http://toolkit.tuebingen.mpg.de/hhblits) searching of the
UniProt database comprising all bacterial sequences (HAMAP (High-quality Automated and
Manual Annotation of microbial Proteomes)) ((Lima et al., 2009); http://hamap.expasy.org/).
The rationale was to supplement previously identified candidate sequences from completely
sequenced genomes with closely related homologues (~E<1e-50) from non-sequenced genomes.
Candidate searching for each sequence class was supplemented by using two validated query
sequences per class for NCBI TBLASTN searches against various databases: NCBI Genomes
(chromosome); High throughput genomic sequences (HTGS); Whole-genome shotgun contigs
(wgs). Only candidate sequences with full-length hits to the query and credible matches to
conserved carboxy-terminal conserved sequence motifs (see below) were considered for further
evaluation. The rationale was to search for previously unannotated sequences relevant to each
target sequence class. Candidate sequence identity was confirmed through phylogenetic tree
inference. All gene identifiers for sequences used are located in Appendix A.1. and A.2.
31
2.2.3 Phylogenetic tree inference
ProtTest
(v2.4)
((Abascal
et
al.,
2005)
(http://darwin.uvigo.es/
software/prottest2_server.html) was used with completed multiple sequence alignments to assess
the optimal amino acid substitution model to use for subsequent work. In all instances the LG
model with four gamma categories was optimal. Multiple sequence alignments were subjected
to phylogenetic tree inference at both the CIPRES Science Gateway ((Miller et al., 2010);
http://www.phylo.org/index.php/portal/) and locally. Maximum Likelihood analysis (RAxML (v
7.4.2); ((Stamatakis et al., 2008); http://www.exelixis-lab.org/) was run at CIPRES under the LG
amino acid substitution model, using a maximum of 1000 rapid bootstraps, or until automatic
convergence was reached. Bayesian analysis (Mr Bayes (v3.1.2); ((Ronquist et al., 2012);
http://mrbayes.sourceforge.net/) was performed at CIPRES, using four independent chains, under
the Mixed amino acid substitution model (the LG model is not available with this
implementation), with four discrete gamma categories, running to a maximum of 7.5 million tree
generations, or until automatic convergence (average standard deviation of split frequencies <
0.010) was achieved. Bayesian analysis (PhyloBayes_MPI (v1.3b); ((Lartillot et al., 2009);
http://megasun.bch.umontreal.ca /People/lartillot/www/downloadmpi.html) was run on the
WestGrid system of Compute Canada (https://computecanada.ca/index.php/en/), using two
independent chains, under the LG amino acid substitution model, with four discrete gamma
categories. Maximum Likelihood analysis (PhyML-aBayes (v3.0.1beta); ((Anisimova et al.,
2011); http://www.atgc-montpellier.fr/phyml/versions.php) was run locally, under the LG model,
with four discrete gamma categories, with all other parameters at defaults, through 25 random
starts, employing an initial Parsimony input tree, and SPR moves. For each analyzed protein
sequence class, trees were obtained by all the utilized inference methods which had concordant
32
topologies at all major nodes. Within the body of this report tree figures are presented which
represent a typical topology, with branch support given for each method at the most critical
nodes. The branch support for all the trees is summarized in Appendix B.1. For Bayesian
methods (Mr Bayes and PhyloBayes_MPI), branch support represents the posterior probability
(PP [max value = 1.00]).
For the PhyML maximum likelihood method, branch support
represents a Bayesian-like transformation of the approximate Likelihood Ratio Test value
(aBayes [max value = 1.00]) (Anisimova et al., 2011). For the RAxML maximum likelihood
method, branch support represents rapid bootstrap support (RBS [max = 100]).
2.2.4 Subcellular localization prediction
A battery of methods (10 in total for plant or algal sequences with chloroplast potential, 9
for sequences from non-plant species) was used to infer the probable subcellular localization of
the eukaryotic proteins described in this study. These included TargetP ((Emanuelsson et al.,
2000);
http://www.cbs.dtu.dk/services/TargetP/),
http://wolfpsort.org/),
PREDOTAR
fr/predotar/predotar.html),
((Small
Protein
http://pprowler.imb.uq.edu.au/index.jsp),
WoLFPSort
et
Prowler
PredSL
http://hannibal.biol.uoa.gr/PredSL/input.html),
al.,
((Horton
2004);
((Boden
al.,
2007);
http://urgi.versailles.inra.
and
((Petsalaki
SLP-Local
et
((Matsuda
Hawkins,
et
al.,
et
al.,
2005);
2006);
2005);
http://sunflower.kuicr.kyoto-u.ac.jp/~smatsuda/slplocal.html), iPSORT ((Bannai et al., 2002);
http://ipsort.hgc.jp/), PCLR ((Schein et al., 2001); http://www.andrewschein.com/pclr/),
MITOPROT ((Claros and Vincens, 1996); http://ihg.gsf.de/ihg/mitoprot.html) and ChloroP
((Emanuelsson et al., 1999); http://www.cbs.dtu.dk/services/ChloroP/). The top two in silico
predicted subcellular localizations for each protein sequence are displayed on each respective
33
phylogenetic tree branch. A single subcellular localization is given for those protein sequences
where the prediction methods provided a clear preponderance of that location (80% of methods
used). Protein sequences where no subcellular prediction is given are those which are fragments
lacking a native amino terminus (N-terminal "M") and which therefore could not be properly
assessed. The majority of in silico techniques applied here also have their own internal
thresholds for compartment predictions, and automatically convert the sequence score into a
compartment prediction.
2.2.5 Analysis of sequence motifs and gene architecture
SLP and RLPH sequences were identified by HMM, BLASTP, and TBLASTN analyses
as detailed above, aligned using MAFFT, with each alignment visualized and hand-edited in
GeneDoc. Highly conserved C-terminal regions were manually identified and an amino acid
positional probability consensus was generated using WebLogo 3 ((Crooks et al., 2004);
http://weblogo. threeplusone.com/). Gene models for each photosynthetic Eukaryote were
downloaded from Phytzome v7.0 (www.phytozome.net). Models of representative SLP and
RLPH genes are depicted.
2.3 Results
2.3.1 Eukaryotic bacterial-like SLP and RLPH protein phosphatases are PPP protein
phosphatases
Since the initial documentation of genes encoding SLP1 and SLP2 phosphatases in A.
thaliana (Andreeva and Kutuzov, 2004), numerous genomes from a variety of higher and lower
plants, as well as other organisms, have been sequenced. Preliminary BLASTP analyses of the
34
Arabidopsis thaliana NCBI database with either full-length AtSLP1 or AtSLP2 retrieved
multiple PPP protein phosphatases, with PP1 and PP2A catalytic subunits offering the highest
level of identity. AtSLP1 and AtSLP2 were found to be 31% identical to each other in addition to
both being 10-14% and 9-13% identical to PP1 and PP2A, respectively, suggesting that these
enzymes are PPP protein phosphatases. AtRLPH protein phosphatases, were also found to be
most related to PPP protein phosphatases, maintaining 85% identity to each other in addition to
13% and 12-14% identity to PP6 (AtRLPH1) and AtPP7/AtSLP2 (AtRLPH2).
Consistent with previous findings (Andreeva and Kutuzov, 2004), the vast majority of the
SLP and RLPH phosphatases identified here were found to possess the entire complement of key
catalytic motifs indicative of being PPP protein phosphatases (Figure 2.1 and 2.2; (Andreeva and
Kutuzov, 2004)). These motifs are represented by GDxHG, GDxVDRG, GNHE and HGG
(Chapter 1; (Shi, 2009)), and in some instances can possess conservative substitutions. In a
typical sequence, all four of these motifs can be clearly identified upon individual inspection of
the amino acid sequence or as part of larger computer assisted alignment (Figure 2.1 and 2.2). Of
these sequences, a small proportion in each subclass lacks one or more of the conserved aminoterminal motifs: about 4 % of SLP phosphatases (7/163) and about 6 % of RLPH phosphatases
(3/47). It is possible these represent incomplete or incorrect gene models, but a genuine lack of
one or more amino-terminal motifs cannot be completely ruled out.
35
GDxHG
MgloSLP
UmSLP
PgraSLP
MlarpSLP
RgluSLP
BdenSLP
TgSLP
CparSLP
LbSLP
CneoSLP
SpSLP
Q1D163_MYX
F8CJ73_MYX
Q08Y53_STI
H8MYK0_COR
Q028W8_SOL
B8KKW3_9GA
A4ABU5_9GA
A0Z563_9GA
AlSLPa
AtSLP1
CarSLPa
BrSLPb
ThalSLPb
AcSLPa
AcSLPb
CpaSLPa
CcSLPb
CsSLP
CsSLPb
GrSLPb
MdpSLPd
PperSLPb
MdpSLPe
VvSLPb
MeSLPb
PtSLPa
PtSLPb
PtSLPc
PtSLPd
RcSLPb
MgSLPa
MgSLPb
GmSLPb
GmSLPc
PvulSLPb
MtSLPb
EgSLPa
EgSLPb
CsatSLPa
CsatSLPb
LuSLPc
BdSLPa
PvSLPa
PvSLPb
PvSLPc
SiSLPa
SbSLPa
ZmSLPb
OsSLPa
PpaSLPc
PpaSLPa
SmSLPa
CrSLPa
VcSLPb
CvSLPb
CocSLPa
MpSLPc
MpSLPd
OlSLPa
OrcSLPa
OtSLPc
MpSLPb
MpSLPf
OlSLPc
OtSLPb
OrcSLPb
A9EUH1_SOR
D0LQ64_HAL
A6FXL1_9DE
A6G215_9DE
AlSLPb
AtSLP2
CarSLPb
ThalSLPa
BrSLPa
CpaSLPc
CcSLPa
CsSLPa
MeSLPa
RcSLPa
PtSLPe
GrSLPa
CsatSLPc
GmSLPa
GmSLPd
PvulSLPa
MtSLPa
MdpSLPa
MdpSLPb
MdpSLPc
PperSLPa
LuSLPa
LuSLPb
CpaSLPb
VvSLPa
AcSLPc
MgSLPc
BdSLPb
OsSLPb
PvSLPd
SiSLPb
SbSLPb
ZmSLPa
PpaSLPb
SmSLPb
MpSLPa
MpSLPe
OlSLPb
OtSLPd
OrcSLPc
CocSLPb
CvSLPa
CrSLPb
VcSLPa
ZmSLPd
ZmSLPf
ZmSLPg
CmSLP
EhSLPa
GtSLPe
GtSLPd
FcSLP
TpSLP
EsSLPa
EsSLPb
GtSLPb
GtSLPc
GtSLPa
CpSLP
AaSLPa
TcSLPa
LmSLPa
TbSLPa
TcSLPc
CrSLPc
VcSLPc
MpSLPg
MpSLPh
OlSLPd
OrcSLPd
OtSLPa
PmSLPa
PmSLPb
ZmSLPc
ZmSLPe
EhSLPb
LmSLPc
TbSLPb
TcSLPb
LmSLPb
F4KVD8_HAL
G8T712_NIA
F9YU86_CAP
B8CJM1_SHE
A8FSQ7_SHE
A0KU52_SHE
A6WR58_SHE
A6LG58_PAR
A6L339_BAC
PbSLPa
PySLPb
PchaSLPb
PvxSLPa
PbSLPb
PySLPa
PchaSLPa
PvxSLPb
C7JGL0_ACE
G2I4P6_GLU
A9HIJ4_GLU
Q5FPF8 GLU
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
GDxVDRG
GNHE
HGG
Motif 1
MVVGHT
Motif 2
IDVG
*
20
*
40
*
60
*
80
*
100
*
120
*
140
*
160
*
180
*
200
*
220
*
240
*
260
HRRIVALGDIHGDYEHATSILRAAGILHSWAGGSTIFVSTGDTVDRGDDTIRLYRLPQDLREQSRRVGGNVINVLGNHEMMNA---------------MMD---WRYVTPGDMASFGGPRRQAMSLHGWLGMEWMQNVTMNVQRASFVHGGITPAFAIGVDAMNKDAHSLLTKALWLS----D-GPFWYRGYALD-ACATANRAIDALG-----VSSLIMGHTPHSG-IHARCHGQIFIIDTGMSRAGRLSALEIDSYAYSAVYVPEG
SRRTVAVADLHGDLDHALNVLSMASIVSTWIGGHDTLVSTGDIVDRGDDTIALYRLPVSLRQQARLAGGEVKNCLGNHEVMNA---------------IGD---WRYVTKADVESFGGVRRHAMSDQGWIGQEWLHNVTHTIPRVSFVHGGITPQYALGIDFINTVGHSLLLKGLWSE----H-GPLWYRGYATD-ACANAEKATKSLA-----VNQLVMGHTPHDG-FVTRCNNTILLIDTGISRAGQQSALIIDFDLLTALYKARP
SSRIVAVGDLHGDLDHAVRVLRMAGVVDQWIGGPSILVQTGDIVDRGKATILLYKWMDALRTEAQAAGGAVVSLLGNHEYMNA---------------LGD---WRYVTKEDIETFGSARRKVMSTQGWIGKTWEASVTARIAGTVFVHGGITPEYALGISEINRIGHSLLHRALYAE----H-GPLWERSYALEDICRQIEIATQRLH-----VRRMVMGHTPQKG-ISSRCGGKILLIDTGISSAGPLTALEINYSLIQELADKKK
KQRVVAIGDLHGDLPHAVRVLRLAELIDKWIGKKTVLVQTGDIVDRGRDTIVLYQLMDRLRNEAKAAGGAVVSLLGNHEYMNA---------------LGD---WRYVTEEDIETFGGKRRKLMSSEGWIGESWLKNTTARVSAIAFVHGGITPEYAIGISEINRIGQSFLNRSLYSS----H-GPLWERSYALEEICDQIEKTINLLN-----VRRLVMGHTPQKG-ILGRCQGKILLIDTGISSAGALSALEIQYTLVHGLQEGKL
RQRIVAMGDIHGDLPAATKILRRAEVVDQWIGGDTILVQTGDIVDRGPDTIALYRFFQSLRPQAERAGGAVVSLLGNHEMMNC---------------LGD---YRYVTKEDIASFGGERREAFLH-GWIGQEFRASVTARVSAISFVHGGITPEYSSPITDINRIGHSILESLLWSE----R-GPMWNRDWALE-ICERVEKALEVLN-----VRRMVMGHTPQEG-ILSRCDGKILLIDTGISRAGAHSSLSLTYTLVEALYTAGR
PSRIIAVGDLHGDLAQALKTLKMARIMNEWIAGSSIFVQTGDVVDRGPDTIKLYKMLYDLKVQAEEHGGQVIQLLGNHEVMNM---------------AED---LRYVTEGDYSSFGGHRRKAFDKDGWPG-NYLRNITTWVNGTVFFHGGAHPQWALGIDGMNLRAHNGLIGRSFGG----S-GPLWFRGYAED-ICKQLDKALADMN-----AVRMVVGHTPQDGSVLRRCNGKLYVIDVGISRVGNSAALEI------------GSRILAVGDLHGDIGNTMLLLYGAGVVDNWIAGDTLLIQTGDVVDRGPDGKRIYDYFASLSAQATEQGGKIIQLLGNHDVMNI---------------CGD---FRYAHPSETIEFGGARRRQFMDGGHYG-NMLRPLSIKANGVIFSHAGIPSDFALGLSKLTQQLREELANDCAGS----Q-GPLWTRVYSMG-ICEELDKTLGILD-----SEKMVIGHTVQSGNIEVYCGGRLLLIDTGVSRYDSPRMLEIRDGS--------KGRVLVIGDIHGDLKSLITSLFLSGVINDWIAKNTLLIQLGDVVDRGSHALQIYKLFNKLKSQAPSLGSKFVGLLGNHEVMNL---------------CGQ---LHYVTDEDFQTYGGRRTFEWSKEGFVG-KYLRKLAIRVNDSLYVHAGLLPKYALGLDKLDKLSNDLLEGDFFVE----D-GPLWTRDISLG-ACKLVDETLQILG-----LSRMVVGHTIQDNRINIKCDNKLILADTGFSEAGKPCMLEILYHN--------TRHIVAVGDLHGDLPNARKVLQFSGITDDWTGDVDFFVQTGDIIDRGDDTIPLFFWMDKLRSQAAAVGGTVLSHLGNHEWMNV---------------IGD---WRYVYPTEIKTFGSVRQQMLTT-GRIGRSWAATTASRLSAISFVHGGLSPTYTLTPSRINQISDSLLAKLQYDG----N-GPLWYRGWATD-VCADVDRVLAKTG-----TRRMIMGHTPDKN-IKARCGGKIIIIDTGISHAGALSALSLYYSLVSAIYQHRR
RQRLVAVGDLHGDIDNAKKTLQMARIIDKWVASTDILVQTGDIVDRGAYADDIYRLMQSLRGQAASQGGKVVSILGNHEVMNA---------------IGD---WRYVTKGDIARFGGTRQHALSAEGWLGQEWLASTTALVPTFSFTHGSLRPSYALTPAAINDLGHSLLTKALYAE----G-GPLWWRGLAER-VCEWAKNLKQKIG-----ARRIIGGHTPNEK-IVARCNASVIIIDTGISSAGVLSALEIVYTLVHAIYEHSR
PTVVYAIGDIHGDFPNALDVLSAAGVVSEWTAGNATLVQTGDVVDRGPDTRKLFRWFNDLHKQAEKHGGRVVRLLGNHEFMNA---------------KGD---WRYVHPGDKASYPEPRIIDWGHSGEIGNLLLSNVTYKDNTSHFMHAGLSPEWAYREETVNELGKELLSHFMWAI----E-GPMWYRGLAQL-ACEVALNVTKTLN-----VNRLVMGHTPQHG-IVSRCEGRILLIDTGLCSAGERAVLRISQNDVEAVYRGKI
VERVVAVGDVHGDVDALKEVLRLAGLIDRWIGGKTHLVQTGDVPDRGDQTRAAFDLLMRLEQEALAAGGRVHALLGNHEAMNM---------------LGD---LRYVNPGEMASFADQHRVAYSLQGRYG-QWLRAAVVRINDTLFVHGGVAPGVPGNLAELNRWVRQDFFPDHRDA----Q-GPLWFRGYALG-AEPALDAVLKRYG-----ARRMVMGHTTNDGKVKVRFNGKALLIDTGLSTGRNLAALELRGGKVNALYREGP
VERVVAVGDVHGDVDALKEVLRLAGIIDRWIGGKTHLVQTGDIPDRGDQTRAAFDLLMRLEQEALAAGGRVHALLGNHEAMNM---------------LGD---LRYVSPGEMASFADQHRVAYSLQGRYG-QWLRAAVVRINDTLFVHGGVAPGVPTDLSALNRWVRQDFFPGQRNP----Q-GPLWFRGYALG-AGPALDAVLQRYG-----ARRMVMGHTTNDGKVKVRFNGKALLIDTGLSTGRNLAALELRGGKVNALYREGP
VERVVAVGDVHGDVEALKEVLRLAGLIDQWTGGKTHLVQTGDIADRGARTREAFELMMRLEREALAAGGRVHLLLGNHEVMNM---------------RGD---LRYVTPEELASFAGLHRAAYGLEGRYG-RWLRPAVVRIDGTLFLHGGLHPEVPKTLGALNRWTRQDLFPDATDA----K-GPLWFRGYAQE-WSQGLDAVLERFG-----ARRMVMGHTPTDGRIGVRFGGRAVLIDTGLSTYRHLAALEIRGDRLTALYPDGR
VERIVAVADVHGDVDALKEVLRLAGLIDHWIGGKAHLVQTGDLPDRGDHTRDAFELLMRLETEARKAGGRVHPLLGNHELMNM---------------RGD---LRYVTPGEFASFADQHAAAYAADGRYG-KWLRPAVIRINDTLFLHGGLAPTVPTTLEEVNRWVWQDLTPGQVDP----Q-GPVWFRGYAID-WDAGLTQVLERFS-----ARRMVMGHTPSDGRLSIRFGGRVIVIDTGLSTHRHLAALELRGDRLTALYPEGR
VPRVVAVGDVHGDYNGFVEVLRSAGVIDHWAGGKTHLVQTGDVPDRGPDTRKAMDLLMQLEKEADKAGGHVHALVGNHEAMNV---------------YAD---LRYTTPAEFAAFVDNQRIAFSSEGKYG-KWIRNALVKINDTIYLHGGISPRYAMTVKQINEAVAAELNDLPMGT----D-SPLWYRGIALE-IAAHVDLVLKTND-----AQRIVISHTVTGA-IIERFGGKVVMIDTGMTAVGHRACLLLEGDKVYAIHRGEK
IDRVVAIADLHGDYQSYITVLEQAGLVNRWTGGTTHLVQLGDVPDRGPDTARIIEHLMKLETQAKKAGGKVHALIGNHEVMNM---------------TGD---LRYVHPGEYEALKSRHRQHWLPNGEFG-AWVANTVVRINRSLFVHAGISPDYLRSIGDINDAVRAELAKPNVDE----R-GPLWYRGLALGDVAVEVDALLKYFD-----VDRIVVGHTPGGT-VVPRYDARVIAADSGLAQHGHLASLLIEGGKVYTLQRGQQ
Q
Q
Q
Q
Q
Q
Q QQ
VERVVAVADLHGDYDNYITVLRQAGVIDRWDAGKTHLVQLGDVPDRGPDSDKIIRHLMKLEEQAEKAGGKVHPLIGNHEVMNI---------------TGD---LRYVHPGEYEALTSRHRQHWHPEGQFG-AWVANTVIRINRSLFVHGGIGPDYLASMEDINDTVREELRAPDEDE----E-GPLWYRGLIMGEIAAHVDALMERFD-----VDRIVMGHTPGGT-VVPRYHGRVLAADSGIAEYGNLASVLIENGQAFTLQSGER
SETVIAIGDVHGDHEQFVKLLQATKLIDRWTGGETHLVQLGDLPDRGPDTRKTMDLLLELQTSSIEQGGAVTTLIGNHDMMNV---------------MND---LRYVDPGEYKAFRSRHRLAWAPTGTYG-EWVLPTVAVVGDSLFVHGGISPDYIMSIEDINTAVHSAMGEGYYDP----L-GPLWFRGWSQDLNTMALNSILNKYG-----VARMVVAHTPVPV-VVPRYDGKIIMVDVGLSQHHGFSALKIENGQTMAMVGDRW
ARRIVAVGDLHGDLGKARDALQMAGVLSQWIGQDSVLVQVGDILDRGDDEIAILSLLRSLDGQAKANGGAVFQVNGNHETMNV---------------EGD---FRYVDARAFDECTDFRSILFRPGGRLACE-LAGVILRVNNWIFCHGGLLPHHVYGVERINREVSTWM-RSPRGY----D-SVVWSRLYSRETVNKILHDTLEAVG-----AKAMVVGHTPQSG-VNCEYGCSIWRVDVGMSSGSRPEVLEIRGDKARVIRSNRD
ARRIVAVGDLHGDLGKARDALQLAGVLSQWVGQDTVLVQVGDILDRGDDEIAILSLLRSLDDQAKANGGAVFQVNGNHETMNV---------------EGD---FRYVDARAFDECTDFRSVLLRPGGRLACE-LSGVILRVNNWLFCHGGLLPHHVYGIERINREVSTWM-RSPRGY----D-SVVWSRLYSRETVNKILHDTLEAVG-----AKAMVVGHTPQSG-VNCEYGCGIWRVDVGMSSGSRPEVLEIRGDKARVIRSNRD
ARRIIAVGDLHGDLSKARDALQLGGVLSQWIGQDT----VGDILDRGDDEIAILSLLRSLDVQAKANGGAVFQVNGNHETMNV---------------EGD---FRYVDARAFDECTDFRSVLFRPGGRLACE-LAGVVLRINNWVFCHGGLLPHHVYGVERINREVSTWM-RSARGY----D-SVVWSRLYSRETVSKILRDTLDAVG-----AKAMVVGHTPQSG-VNCEYGCGIWRVDVGMSSGSRPEVLEIIGDKARVIRSNRD
SRRIIAVGDLHGDLSKARDALQIAGVLSQWVGEDTVVVQVGDILDRGEDEIAILSLLRLLDEQAKANGGAVFQVNGNHETMNV---------------EGD---FRYVDTRAFDECIDFRSVLFRPGGRLACE-LSGVVLRVNNWVFCHGGLLPHHVYGVERINREVSTWM-KSSRGQVESSK-TVKSSSAWIECVVGKILRDSLEAVG-----AKAMVVGHTPQSG-VNCEYGCGIWRVDVGMSSGSRPEVLEIRGDKARVIRSNQD
----LAV-------------------------SSQVLVQVGDILDRGDDEIAILSLLRLLDEQAKANGGAVFQVNGNHETMNV---------------EGD---FRYVDARAFDECTDFRSVLFRPGGRLACE-LAGVILRVNDWVFCHGGLLPHHVYGVERINREVATWM-RSYRGY----D-SVVWSRLYSRETVSKILRDTLEAVG-----AKAMVVGHTPQSG-VNCEYGCSIWRVDVGMSSGSRPEVLEIRGDKARVIRSNQD
GRRIVAVGDLHGDLAQTRCALELAGVLSLWTGEETVLIQLGDILDRGEDEIAILSLLRSLSNQAKSKGGAVFQLNGNHETMNV---------------EGD---FRYVDPGAFNECGDFRSALLRPGGPLACE-LAAVVLKVDDWVFCHGGLLPRHVYGIERMNREVSHWM-KGLRGY----D-SVVWNRLYSRDLIQAVLEDTLDAMG-----AKAMVVGHTPQAG-INSKYSSSIWRIDVGMSSGSRPEVLEIIDNEARVIRSNRQ
GRRIVAVGDLHGDLAQTRCALELAGVLSLWTGEETVLIQLGDILDRGEDEIAILSLLRSLSNQAKSKGGAVFQLNGNHETMNV---------------EGD---FRYVDPGAFNECGDFRSALLRPGGPLACE-LAAVVLKVDDWVFCHGGLLPRHVYGIERMNREVSHWM-KGLRGY----D-SVVWNRLYSRDLIQAVLEDTLDAMG-----AKAMVVGHTPQAG-INSKYSSSIWRIDVGMSSGSRPEVLEIIDNEARVIRSNRQ
GRRIIAVGDLHGDLDQARFALQMAGVLSLWTGGETVLVQLGDILDRGEDEIAILSLLRLLDVQAKDVGGAVFQVNGNHETMNV---------------EGD---FRYVEAGAFDECGAFRTALFRPGGPLACE-LASVVLRVNDWVFCHGGLLPNHVYGIERMNKEVSSWM-RGFRGY----D-SVVWNRLYSRDNINAILQDTLQAVG-----AKAMVVGHTPQEG-VNCKYNCSIWRVDVGMSSGSRPEVLEISDDKARVIRSKRE
GRRIVAVGDLHGDLDQARCALAMAGVLSLWTGGESVLIQLGDVLDRGEDELAILSLLRSLDMQAKAEGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECSDFRSVLFRPGGPMACE-LAGVVLKVNDWVFCHGGLLPHHVYGLERMNNEVSLWM-KGLRGY----D-SVVWNRLYSRDIINAVLQDTLRAVG-----AKAMVVGHTPQAG-ANCEYNCSIWRIDVGMSSGSRPEVLEITDNKARVISSKRD
GRRIVAVGDLHGDLDQARCALEMAGVLSLWTGGESVLIQLGDVLDRGEDELAILSLLRSLDMQAKAEGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECSDFRSVLFRPGGPMACE-LAGVVLKVNDWVFCHGGLLPHHVYGLERMNNEVSLWM-KGLRGY----D-SVVWNRLYSRDIINAVLQDTLRAVG-----AKAMVVGHTPQAG-ANCEYNCSIWRIDVGMSSGSRPEVLEITDNKARVISGKRD
GRRIVAVGDLHGDLDQARCALEMAGVLSLWTGGESVLIQLGDVLDRGEDELAILSLLRSLDMQAKAEGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECSDFRSVLFRPGGPMACE-LAGVVLKVNDWVFCHGGLLPHHVYGLERMNNEVSLWM-KGLRGY----D-SVVWNRLYSRDIINAVLQDTLRAVG-----AKAMVVGHTPQAG-ANCEYNCSIWRIDVGMSSGSRPEVLEITDNKARVISGKRD
GRRIVAVGDLHGDLDQARYALEMAGVLSLWTGEDAVLVQLGDVLDRGDDEIAILSLLRSLDIQAKAKGGAVFQVNGNHETMNV---------------EGD---FRYVESGAFDECADFRSVLFRPGGPLACE-LAAVALKVNDWIFCHGGLLPHHVYGIEKMNKEVSQWM-RGLKGY----D-SVVWNRLYSRNIINAILQETLQALG-----AKAMVVGHTPQSG-ANCEYNCSIWRIDVGMSSGSRPEVLEIRDDKARVIRSKRN
----MSVGDLHGDLGQTRCALETAGVLSVWIGGETVLIQLGDILDRGEDEIAILSLLRSLDMQAKAEGGAVFQVNGNHETINV---------------EGD---FRYVDTGGFDECLDFRSILLRPGGPLARE-LAAVVLKVNDWVFCHGGLVPQHVYGVERMNREVSDWM-RGLRGY----D-SVVWNRLYSRDTINYILQQTLQAVG-----AKGMVVGHTPQTG-ANCEYDCSIWRIDVGMSSGSRPEGLSSLNM---------GRRIVAVGDLHGDLDQTRCALEMAGVLSLWTGGETVLIQLGDILDRGEDEIAILSLLRSLDIQAKDEGGAVFQVNGNHETMNV---------------EGD---FRYVETGGFDECIDFRSILLRPGGPLACE-LAAVVLKVNDWVFCHGGLIPNHVYGVERMNREVSHWM-RGLRGY----D-SVVWNRLYSRDTIHSILEETLHAVG-----AKAMVVGHTPQTG-ANCKYNCSIWRIDVGMSSGSRPEVLEIKDNKARVIRSKKD
YRSVLCVGDLHGDLGQTRCALETAGVLSVWIGGETVLIQLGDILDRGEDEIAILSLLRSLDMQAKAEGGAVFQVNGNHETINV---------------EGD---FRYVDTGGFDECLDFRSILLRPGGPLARE-LAAVVLKVNDWVFCHGGLVPQHVYGVERMNREVSDWM-RGLRGY----D-SVVWNRLYSRDTANKLHSSTDATSG-----NGGGAYSSTYGKL-VRCEYDCSIWRIDVGMSSGSRPEVLEIKDNKARVIRSKKD
GRRIVAVGDLHGDLAQARCALEMAGVLSLWTGGDTVLVQLGDILDRGEDEIAILSLLRSLDIQAKASGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECTDFRSILLRPGGLLACE-LAAVVLKIDDWVFCHGGLLPHHVYGIERMNREVSHWM-RGLRGY----D-SVVWNRLYSRDVIYSLLEETLQAVG-----AKAMVVGHTPQVG-VNCKYDCSIWRIDVGMSSGSRPEVLEIRDNTARVIRSKRD
GRRIVAVGDLHGDLDQARCALEMAGVLSLWIGGETVLIQLGDILDRGEDEIAILSLLRSLDIQAKAQGGAVFQINGNHETMNV---------------EGD---FRYVDSGAFDECTDFRSILLTPGGPLACE-LASVILKVNDWIFCHGGLLPQHVYGIERMNSKVSQWM-KGLKGY----D-SVVWNRLYSRDVILSILEETLQAIG-----AKAMVVGHTPQAG-VNCKYNCSVWCVDVGMSSGSRPEVI--------------GRRIVAVGDVHGDLDQARCALEIAGVLSLWTGGETVLIQLGDVLDRGEEEIAILSLLRSLDIQAKAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECSDFRSILLRPGGPLACE-LAAVVLKINDWVFCHGGLLPQHVYGVERMNYEVSHWM-RGLKGF----D-SVVWNRLYSRDMIQSVLEETLQLLG-----AKAMVVGHTPQTG-VNCKYNCSIWCIDVGMSSGSRPEVLEIVENKARVIRSKRD
GRRIVAVGDVHGDLDQARCALEIAGVLSLWTGGETVLIQLGDVLDRGEEEIAILSLLRSLDIQAKAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECSDFRSILLRPGGPLACE-LAAVVLKINDWVFCHGGLLPQHVYGVERMNYEVSHWM-RGLKGF----D-SVVWNRLYSRDMIQSVLEETLQLLG-----AKAMVVGHTPQTG-VNCKYNCSIWCIDVGMSSGSRPEVLEIVENKARVIRSKRD
GRRIVAVGDVHGDLDQARCALEIAGVLSLWTGGETVLIQLGDVLDRGEEEIAILSLLRSLDIQAKAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECSDFRSILLRPGGPLACE-LAAVVLKINDWVFCHGGLLPQHVYGVERMNYEVSHWM-RGLKGF----D-SVVWNRLYSRDMIQSVLEETLQLLG-----AKAMV------------------------------------------------GRRIVAVGDVHGDLDQARCALEIAGVLSLWTGGETVLIQLGDVLDRGEEEIAILSLLRSLDIQAKAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECSDFRSILLRPGGPLACE-LAAVVLKINDWVFCHGGLLPQHVYGVERMNYEVSHWM-RGLKGF----D-SVVWNRLYSRDMIQSVLEETLQLLG-----AKAMVVGHTPQTG-VNCKYNCSIWCIDVGMSSGSRPEVI--------------GRRIVAVGDLHGDLDQARCALEMAGVLSLWIGGETVLVQLGDILDRGEDEIAILSLLRSLDIQAKAEGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECTDFRSVLLRPGGPLACE-LASVILRINDWIFCHGGLLPHHVYGIERINREVSQWM-KGLRGY----D-SVVWNRLYSRDMILSILEETLQSAG-----AKAMVVGHTPQAG-VNCKYNCSVWRIDVGMSSGSRPEVLEIQNDKAKVIRSRKD
GRRIIAVGDLHGDLDKARYALQMAGILSLWIGGQTVLVQLGDILDRGEDEIAILSLLKSLNIQAKLNGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECADFRSVLLRPGGPLASE-LAAVVLKVNDWIFCHGGLLPHHVYGIERINREVSYWM-EGLRGY----D-SVVWSRLYSRDTIESILRETLQAVG-----AKAMVVGHTPQTG-VNCDFNCSIWRIDVGMSSGSRPEVLEIREGRARAIRSKRD
GRRIIAVGDLHGDLDKARYALQMAGILSLWIGGQTVLVQLGDILDRGEDEIAILSLLKSLNIQAKLNGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECADFRSVLLRPGGPLASE-LAAVVLKVNDWIFCHGGLLPHHVYGIERINREVSYWM-EGLRGY----D-SVVWSRLYSRDTIESILRETLQAVG-----AKAMVVGHTPQTG-VNCDFNCSIWRIDVGMSSGSRPEVLEIREGRARAIRSKRD
GRRILAVGDLHGDLKQARSALEMAGVLSLWTGGETVLVQLGDILDRGEDEIAILSLLRSLDKQAKAKGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECNDFRSILFRPGGLLARE-LAAVVLKVNDWVFCHGGLLPHHVYGLERMNKEVSEWM-RGQRGY----D-SVVWNRLYSRDTVCSILDETLQAVG-----AKAMVVGHTPQIG-VNCKYNCSIWRVDVGMSSGSRPEVLEIIDDKARVIRCKRD
GRRILAVGDLHGDLKQARSALEMAGVLSLWTGGETVLVQLGDILDRGEDEIAILSMLRSLDRQAKEKGGAVFQVNGNHETMNV---------------EGD---YRYVESGGFDECNDFRSILFRPGGLLARE-LAAVVLKVNDWVFCHGGLLPHHVYGLERMNKEVSEWM-RGQRGY----D-SVVWNRLYSRDTVCSILDETMQAVG-----AKAMVVGHTPQIG-VNCKYNCSIWRVDVGMSSGSKPEVLEIIDDKARVIRCKRD
GRRILAVGDLHGDLKQARSALEMAGVLSLWTGGETVLVQLGDILDRGEDEIAILSLLRSLDKQAKAKGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDESNDFRSVLFRPGGLLARE-LAAVVLKVNDWVFCHGGLLPHHVYGLERMNKEVSEWM-KGQRGY----D-SVVWNRLYSRDAVCSILDETLQAVG-----AKSMVVGHTPQTG-VNCKYNCNIWRIDVGMSSGSRPEVLEIIDNQAKVIRCKRD
GRTILAVGDLHGDLKQARYALEMAGVLSLWTGGENVLIQLGDILDRGEDEIAILSLLRSLDKQAKSKGGAVFQVNGNHETMNV---------------EGD---FRYVDSGGFDECSDFRSILFRPGGPLACE-LAGVALKVNDWVFCHGGLLPHHVYGLERMNKEVSEWM-RDPRGY----D-SVVWNRLYSRDSVCSVLEETLQAVD-----AKAMVVGHTPQIG-VNCKYNCSIWRVDVGMSSGSRPEVLEIIDDKARVIRSKTD
GRRIVAVGDLHGDLEQARRAFQMAGVLSTWTGKETVLVQLGDILDRGENEIAILSLLRSMDKQAQEDGGAIFQINGNHETMNV---------------EGD---FRYVESGAFEECAGFRSVLLRPGGPLACE-LAAVVLKVNDWIFCHGGLLPHHVYGIERMNFEVSNWM-RGLRGY----D-SVVWSRLYSRDVIQSILEETLQAVN-----AKAMVVGHTPQAG-VNCKYNCSIWRIDVGMSSGSRPEVLEIVDGKARAIKGRRD
GRRIVAVGDLHGDLEQARRAFQMAGVLSTWTGKETVLVQLGDILDRGENEIAILSLLRSMDKQAQEDGGAIFQINGNHETMNV---------------EGD---FRYVESGAFEECAGFRSVLLRPGGPLACE-LAAVVLKVNDWIFCHGGLLPHHVYGIERMNFEVSNWM-RGLRGY----D-SVVWSRLYSRDVIQSILEETLQAVN-----AKAMVVGHTPQAG-VNCKYNCSIWRIDVGMSSGSRPEVLEIVDGKARAIKGRRD
ARRIVAVGDLHGDLKQTRLALEMAGVLGLWTGGQTVLVQLGDILDRGEDEIAILSLLRSLDVQARAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECLNFRSILFRPGGRLARE-LAAVVLKVNDWVFCHGGLLPHHVYGIERMNREVSQWM-KGLRGY----D-SVVWNRLYSRDFINSILKDTLEAVG-----AKAMVVGHTPQAG-VNCKYNCSIWRVDVGMSSGSRPEVLEIRDDKVRVIRSKRD
ARRIVAVGDLHGDLKQTRLALEMAGVLGLWTGGQTVLVQLGDILDRGEDEIAILSLLRSLDVQARAQGGAVFQVNGNHETMNV
EGD
FRYVDSGAFDECLNFRSILFRPGGRLARE LAAVVLKVNDWVFCHGGLLPHHVYGIERMNREVSQWM KGLRGY
D SVVWNRLYSRDFINSILKDTLEAVG
AKAMVVGHTPQAG VNCKYNCSIWRVDVGMSSGSRPEVLEIRDDKVRVIRSKRD
ARRIVAVGDLHGDLKQTRLALEMAGVLGLWTGGQTVLVQLGDILDRGEDEIAILSLLRSLDVQARAQGGAVFQVNGNHETMNV---------------EGD---FRYVDSGAFDECLNFRSILFRPGGRLARE-LAAVVLKVNDWVFCHGGLLPHHVYGIERMNREVSQWM-KGLRGY----D-SVVWNRLYSRDFINSILKDTLEAVG-----AKAMVVGHTPQAG-VNCKYNCSIWRVDVGMSSGSRPEVLEIRDDKVRVIRSKRD
GRRIVAVGDLHGDLDQTRSALRIAGVLSRWTGGQTVLVQLGDVLDRGGGEIAILSLLRSLDVQARLQGGAVFQINGNHETMNV---------------EGD---FRYVDSTGFDECLDFRSTLLRPGGALACE-LAGVLLKINDWVFCHGGILPHHVHGVERMNREVSQWM-KGLRGY----D-SVVWNRLYSQDLIEAALYDTLGSLD-----AKGMVVGHTPQRG-ANCECNGRIWRVDVGMSKGSRPEVLEINESKTRVLRLGRD
GRRIVAVGDLHGDLYQTRAALVMAGVLSLWTGGQTVLVQVGDILDRGEDEIAILSLLSSLNMQAKSQGGAVFQVNGNHETMNV---------------EGD---FRYVDPGSFDECMRYRASLLKQGGPLACE-LAPVVLVVNDWIFCHGGLLPHHVYGLERINKDVSNWM-QGSRGY----D-SVVWTRFYSQDSSSLVAEQTLKSVG-----AKGMVVGHTPQRG-VNCKCDGKVWCVDVGMSYGSRPEVLEIINDRARVIKDRRG
GRRIVAIGDAHGDLSQTRAALVLAGVLSVWTGGRTVLVQVGDILDRGEDEIAILSLLSSLSVQAKSQGGAVFQVNGNHETMNV---------------EGD---FRYVYPGGFDECIRFRSSLFKRGGPLACE-LAPVVLKINDWIFCHGGLLPHHVYGIERMNIEVSTWM-KSSRGY----D-SVVWSRMYSQDSSSIIAEETLKSVG-----AKGMVVGHTPQRG-VNCKCDGKVWCIDVGMSYGSRPEVLEIVNDRPRVLKKRRD
GRRIVAIGDAHGDLSQTRAALVLAGVLSVWTGGRTVLVQVGDILDRGEDEIAILSLLSSLSVQAKSQGGAVFQVNGNHETMNV---------------EGD---FRYVYPGGFDECIRFRSSLFKRGGPLACE-LAPVVLKINDWIFCHGGLLPHHVYGIERMNIEVSTWM-KSSRGY----D-SVVWSRMYSQDSSSIIAEETLKSVG-----AKGMVVGHTPQRG-VNCKCDGKVWCIDVGMSYGSRPEVLEIVNDRPRVLKKRRD
GRRIVAIGDVHGDLSQTRAALVLAGVLSVWTGGRTVLVQVGDILDRGADEIAILSLLSSLNVQAKPQGGAVFQVNGNHETMNV---------------EGD---FRYVDPGGFDECMRFRSSLFKRGGPLACE-LAPVVLKINDWIFCHGGLLPHHVYGIGRMNREVSRWM-KSSRGY----D-SVVWSRLYSQDSSSIVAEETLESVR-----AKGMVVGHTPQHG-VNCKCDGKVWCIDVGMSYGSRPEVLEIVNDSPRVLKKRRD
DRRIVAIGDVHGDLSQTRAALVLAGVLSVWTGGRTVLVQVGDILDRGKDEIAILSLLSSLNLQAKSQGGAVFQVNGNHETMNV---------------EGD---FRYVDPGGFDECIRFRSSLFKRGGPLACE-LAPVVLKINDWVFCHGGLLPHHVYGIERMNREVSMWM-KCSRGY----D-SVVWSRLYSHDPSSIVAEQTLKSVG-----AKGMVVGHTPQGG-VNCKCDGKVWCVDVGMSYGSRPEVLEIVNDRPRVLKKRRD
GRRIVAIGDVHGDLSQTRAALVLAGVLSLWTGGRTVLVQVGDILDRGEDEIAILSLLSSLNMQAKSQGGAVFQINGNHETMNV---------------EGD---FRYCDPGGFDECVRFRSSLLKRGGPLACE-LAPVVLKINDWIFCHGGLLPHHVYGIERMNREVSIWM-KCSRGY----D-SVVWSRLYSQDPSSIVAEQTLEAVG-----AKGMVVGHTPQHG-VNCKCDGKVWCVDVGMSSGSRPEVLEIVNDRPKVLKKRRE
GRRIVAIGDAHGDLSQTRAALVLAGVLSVWTGGRTILVQVGDILDRGEDEIAILSLLSSLNMQAKSQGGAVFQVNGNHETMNV---------------EGD---FRYCDPGGFDECMRFRSSLLKRGGPLAHE-LAPVVLKINDWIFCHGGLLPHHVYGIERMNREVSMWM-KCSRGY----D-SVVWSRMYSQDPSSIAAEQTLEAVG-----AKGMVVGHTPQHG-VTCKCDGKVWCVDVGMSCGSRPEVLEIVNDRPRVLKERRE
GRRIVAVGDLHGDLNQTRAALVMAGLLSVWTGGQTVLVQVGDILDRGEDEIAILSLLSSLNMQAKSQGGAVFQVNGNHETINV---------------EGD---YRYVDPGAFDECIRFRSSLFKRGGPLACE-LAPVVLSVNDWIFCHGGLLPHHVYGIERMNREVSVWM-KSSRGY----D-SVVWSRLYSQGPSSVVAERTLKSVG-----AKGMVVGHTPQRG-VN-------WYLL--FSLG--------------------DRRIIAIGDLHGDFERAQWALQLAGVMSRWTGGTTVLVQIGDVLDRGEDEITILSLLAWLGKQARSKGGAVFQILGNHETMNV---------------AGD---FRYVAPGGFQEAEAFRTKLLSPGGPLARQ-LAGVVLKVNDWLFAHGGVLPHHVYGLERMNREVSLWM-KNIKGF----D-SIVWSRLYSKENACGILTAALSATN-----SRGLIVGHTPQIG-ANSKCDGRIWRIDVGMSSGALPEVLEIVDDKVRILSVAEE
NRRIVSIGDLHGDFERAQEALELAGVLSRWTGGTTILVQIGDVLDRGEDEIAILSLLALLGREARSKGGAVFQLLGNHEAMNV---------------AGD---FRYVAPGGFLEAEVFRTKLLSPGGPLATH-LAGVVLKVNDWLFAHGGVLPYHVYGLERMNREVSLWI-QKEKGY----D-SVVWSRLYSKENACGTLAAALSATN-----SRGLVVGHTPQIG-GNSKCDGRVWRIDVGMSSGALPEVLEIVDDKVRILCAREE
ERRIVAVGDLHGDLRQTKRALRVAGVLSNWTGGTTVLVQVGDILDRGPDEIAILSLLWHLTEQARSKGGAVFQVHGNHETMNV---------------SHE---FNDTPDCGFEDSQHFRVLLFSPGGPLALE-LSGVVLKINDWLFAHGGLLPHHVYGLEKMNSEVSNWM-KGGKGF----D-SIVWSRLYSRKTACAVLQQTLDAVK-----AKGLVIGHTPQNG-ANSECDGKVWRIDVGMSRGATPQVIEIVGDEVKVLSCQKE
TGRIIAIGDLHGDLDKAVEALKLGRVISSWVGGDTVVVQLGDVLDRGDVEIGIINLLRYLDTEARKQGGAVYMLNGNHESLNV---------------CGD---FRYVTPGAFAESALYRYSLYKPGGDLARE-FSPTVLVVNDTVFAHGGLLPTHVYGIERLNSEVAAWM-RGDGDA----N-SVMWNRTLSKERACNALKQALAKVR-----GKRLVVGHTPQGG-VNCECENQVWRIDVGMSYGRPVQVIEIVPPEVRVIRNTPN
TGRIVAIGDLHGDLDKAVEALKLGRVIRAWVGSDTVVVQLGDVLDRGDVEIGIINLLRYLDSEARKCGGAVYMLNGNHESLNV---------------CGD---FRYVTPGAFAESAMYRYSLFKPGGDLAKE-FAPTVLVVNDTVFAHGGLLPIHVYGIERLNSEVAAWM-RGDGDA----N-SVMWNRTLSKERACRALQQALAKVH-----GKRLVVGHTPQSG-VNCECENQVWRIDVGMSYGRPVQVLEITQHEVRIIRSTPT
TGRIIAIGDIHGDLQKALSCLEMAGVLAKWVGGDTVVVQLGDVLDRGDCEIGSVLLLRELDRQARQQGGAVYMLNGNHESLNV---------------AGD---FRYVTPGAFFESARARWYLYQPGGAMARE-LAATVLVVNDVVFAHGGLLPHHLYGLQRINDEVAEWM-NGAGDS----S-SVQWNRTFGKERMNFQLRATLESLN-----ARAMVVGHTPQSG-VNCECDGRVWRVDAGMSSGAAPQV---------------SGRIIAIGDIHGDVQKAITSLKLGGVLVVWCGGNTVVVQLGDVLDRGDSEIGAIILLRELDRQARLQGGAVYMLNGNHESLNL---------------LGAAMWRRYVTPGGFRESGLVRLHLYSPGGRLACE-LAPTVLIINDTVFAHGGVLPNHVYGLERLNAEVAAWM-RGAGGP----D-SVMWNRQFGHERACMDLDATLRLLG-----ARQLIVGHTPQNG-ANCECNGRVWRMDVGMSKGAAPQVLEIEPEDIRLLCPPSV
KGRVVALGDVHGDIGQARRALTIAGVLGTWIGGDTVVVQVGDVLDRGDDEIAILILLQKLHKAAQADGGAVYILNGNHEVLNV---------------SGD---FRYVTQGAFQESTRFRVGLFSPGGPLAQQ-LASTVLIVNDTVFAHGGLMPRHVFGLEKLNNAVADWM-RGAGSK----D-SVVWNRTFGQENACETLGKTLDAID----GAKRLVVGHTPQGG-CNAECDGRIWRIDVGMSFGADPEVIEIVGDEVRVLTSRVP
PGRVVALGDVHGDIGQARRALTIAGVLGEWTGGNTVVVQVGDVLDRGDDEIAILILLQKLHKQAEAQGGAVYILNGNHEVLNV---------------SGD---FRYVTQGAFQESARFRVGLFSPGGPLAQQ-LASTVLIVNDTVFAHGGLMPRHVFGLERLNNAVADWM-RGAGSK----D-SVVWNRTFGTENACEVLGNTLDAISEKYGAAKRLVVGHTPQGG-CNGECDGRIWRIDVGMSFGADPEVIEIDGDEVRVLNSRVP
-GRVVAIGDLHGDIGQARRALRMAGVLDRWVGGDTTLVQLGDILDRGDDEIGILILLQKLDKEAQKQGGRVYVMNGNHEVLNV---------------SGD---FRYVSRGAFGESTRFRIGLFSPGGPLAQQ-LSHTVLIVNDTVFAHGGLVPRHVFGLDKLNRAVTDWM-RGKGGR----D-SIVWHRAYGTEKSCELLGKTLGMID----GVSRLVVGHTPQGG-ANCECNGQIWRIDVGMSFGAEPQVLEIDGDEVRVLSSSSV
PGRVIAIGDLHGDIGQARRALRLAGVLGTWVGGDTTVVQVGDILDRGDDEIGILILLQKLEKEARKQGGGVYVLNGNHEVLNV---------------SGD---FRYVSRGAFGETMRFRIGLFSPGGPLAQQ-MSHTVLIVNDTVFAHGGLVPRHVFGLDKLNRAVTDWM-RGKGGR----D-SIVWHRGYGTERSCALLGETLGMID----GANRLVVGHTPQGG-ANCECDGRIWRIDVGMSFGADPQVLEIDGQEVRVLTPTNM
FGRTVEVSDLAGAMGQARRLPHMRRYRAAWVGGNATLVQLGDILDRGDDEIGILILLQKLDKEAKKAGGAVYVMNGNHEVLNI---------------SGD---FRYVTRGAFGETTRFRIGLFSPGGPLAQQ-LSHTVLIVNDTVFVHGGLVPRHVYGLDKLNRAVSDWM-RGKGGR----D-SIVWHRGYGTENSCELLEKTLGMID----GANRMVVGHTPQGG-ANCECDGKIWRIDXXXXXXAEPQVLEIDGGDVRVITSASS
-----------------MKALECAKVMDKWCGGDTVVVQVGDILDRGDNELAIMRKFRALAKDARAAGGDFIVINGNHEIMNV---------------LGD---FRYVTKGAYGECARYRRALFLPGGEMAVK-MAPTVLQVDDTVFAHAGIDESHVYGFARINAEVSQWM-AGSLEE----K-GVVWTREYGGADACRRLEKALDATG-----AKRIVIGHTPQKG-VNSGCGGKVWRADVGASRGNTPQVIEIVNGRVRVLSA--GRRLVAVGDIHGDFAQAMKALELSKCMNKWIGGTTVLVQVGDILDRGDNELAIMRKFQKLAKEAKEAGGDVVVMNGNHEIMNV---------------MGD---FRYVTKGAFGECRRWRRDLFLPGGEMAVK-MAPTVLQVGDTVFAHAGITENHVYGFQRLNNEVAAWM-VGKLEE----K-GVVWTRDYGGAEACKRLTEALDATG-----AKRLIVGHTPQKG-INSGCGGKVWRSDTGMSRGNTPQVIEIVNGRVRIL----GRRLIAIGDLHGDFLAMVKSLELAQVIDKWNGGDAVVVQVGDVLDRGDSEIAILRKLRSLAKQAKEAGGDLITMNGNHEIMNV---------------MGD---FRYATPGAFEECRRYRQQLFKPGGEIAKW-LAPTVLVVDDTVFAHAGIDLSHVYGFERINKEVAMWM-EGKLES----D-GVVWTRDYGGKDSCRRLASALEGAD-----AKRLVIGHTPQTG-VTSGCKGTVWRVDVGASRGNSAQVIEIIGGKVRVLA---GRRLVAVGDLHGDFLAAVRALELAGVMDKWVGGDAVVVQVGDVLDRGDSEIAIMRKLRALRKQARQVGGDVVCMNGNHEIMNV---------------MGD---FRYATPGAFEECRRYRQQLFKPGGELAMW-LAPTVLVVDDTVFAHAGIDLSHVYGFERINKEVSMWM-QGKLES----D-GVVWTRDYGGKDACRKLNTALDAAG-----AKRLVIGHTPQTG-VTNGCKGALWRVDVGASRGHDVQVIEIVNGRTRVLA---GRRLIAIGDLHGDFLAMVRSLELAGVCDKWIGGNAVVVQVGDVLDRGDSEIAILRKLRALKRQAKEQGGDVITMNGNHEIMNV---------------MGD---FRYATPGAFEECRLYRQKLFSPGGEIAKW-LAPTVLVVDDTVFAHAGIDLSHVYGFERINREVSLWM-QGKLES----D-GVVWTRDYGGKDACRRLGAALDAAE-----AKRLVIGHTPQTG-VTSGCKGAVWRVDVGASRGNAAQVIEVIGGRVRVLA---ADRVVAIGDVHGDLAATRAALRIAGAIDRWIGGKLVLVQTGDELDRGDDEQTIVDLFDRLAVEARAAGGAVYALNGNHEVMNV---------------QLD---FRYVTEGGFKDFEDVRAAAFAPGGLYARK-LADVIIAVGDTVFTHGGVLPAHTYGISRINREVRAWM-EGKAAE----D-SPVWARLYSSDPPCAALDQTLAAMS-----AKRMVVGHTVQHG-ATSACGDKIWRIDVGMASHGKPAALEIVGDTVRVLTPEAT
PARVVAIGDLHGDLDAALRALSLAGAIALWTGGDMVLVQTGDVLDRGDDEQAILDLLARLEREARAAGGAVHALLGNHETMNA---------------AGD---FRYVTPGGFSDFADARAAAFLPGGVYAER-LANTVVVVGDTVFAHGGVVPRWAYGIERVNAELRCWL-AGELAE----D-NPMWSRDFSREPACDALERALAALD-----ATRMVVGHTPQDG-ITSACDGRVWRIDTGMAAHGAVQALELRGDSARILSEDE--------------------------MDKWIGGSLVIVQTGDQLDRGDGEQAILELLARLQHEAKAAGGAIHILNGNHEFMNA---------------MGD---LRYVTPGGLVDFADARVAAFLPGRPWAKE-LGNTVVVVGDTAFVHGGVLPAYAGDITTLNREARAFL-NGEVDP----D-GPVWSRHYSDE-DCALLDQALAKLE-----VKRMVVGHTVHEG-IQSACDEKVWMVDVGMAAHGPTQVLVIAGAEVSVLAG--VPHIIAFGDVHGDFVAMAEVLLGAGIVDHWIAGETWVVQVGDQLDRGYQEEEIMNLFEQLRVEAAEAGGRFLALNGNHEIMQA---------------EGR---MDYVFD--LEAFGGLRVEAFAPGGEWALV-LANVIVKVGRTVFVHGGALPEHALGIENMNDAAKAWL-VGDDGS----G-SIVWDRTYSDDDRCELLTAALAAMD-----ADRIVVAHTIQPG-INAICDDRAWRIDTGMADYGPIEALEITGDVASVIQME-PERLVAVGDLHGDLDKSREAFKIAGLIDRWTGGSTMVVQVGDVLDRGGEELKILYFLEKLKREAERAGGKVLTMNGNHEIMNI---------------EGD---FRYVTKTGLEEFQVWRIAALRPEGPISKRFLTQTVAVVGDSVFVHGGLLAEHIYGLERINEEVRGWI-NGLRGG----N-SVVWLRKFSEEMDCAALEHALSTIG-----VKRMIMGHTIQAG-INGVCNDKAIRIDVGMSKGGLPEVLEIRR-DVRILTSNPL
PERLVAIGDLHGDLEKSREAFKIAGLIDRWTGGSTMVVQVGDVLDRGGEELKILYFLEKLKREAERAGGKILTMNGNHEIMNI---------------EGD---FRYVTKKGLEEFQIWRIAALRPDGPIAKRFLTQTVAVVGDSVFVHGGLLAEHIYGLERINEEVRGWI-NGFRGG----N-SVVWLRKFSEEMDCAALEHALSTIG-----VKRMIMGHTIQAG-INGVCNDKAIRIDVGMSKGGLPEVLEIRR-DVRIVTSNPL
PERLVAIGDLHGDLEKSREAFKIAGLIDRWTGGSTMVVQVGDVLDRGGEELKILYFLEKLKREAERAGGKILTMNGNHEIMNI
EGD
FRYVTKKGLEEFQIWRIAALRPDGPIAKRFLTQTVAVVGDSVFVHGGLLAEHIYGLERINEEVRGWI NGFRGG
N SVVWLRKFSEEMDCAALEHALSTIG
VKRMIMGHTIQAG INGVCNDKAIRIDVGMSKGGLPEVLEIRR DVRIVTSNPL
PERLVAIGDLHGDLEKSKESFRIAGLIDRWTGGSTVVVQVGDVLDRGGEEIKILYFLEKLKREAERSGGKVLTMNGNHEIMNV---------------EGD---FRYVTKKGLEEFQIWRIAALRPEGPIAKRFLTQTVAVVGDSVFVHGGLLAEHIYGLERINEEVKGWI-NGLRGG----N-SVVWLRKFSEEMDCAALEHALSTIG-----VKRMIMGHTIQAG-INGVCNDKAIRIDVGMSKGGLPEVLEIRR-DVRIVTSNPL
PERLVAIGDLHGDLDKSKEAFRIAGLIDKWTGGSTMVVQVGDVLDRGGEELKILYFLEKLKREAEKSGGKILTMNGNHEIMNV---------------EGD---FRYVTEKGLEEFQIWRIAALRPEGPIAKRFLSQTVAVVGDSLFVHGGLLAEHIYGLERINEEVTCWI-NGLRGG----N-SVVWLRKFSDEMDCSALEHALSTIG-----VKRMIMGHTIQAG-INGVCDNKAIRIDVGMSKGGLPEVLEIRK-DVRIVTSNPL
PDRLVAIGDLHGDLEKSKEAFRIAGLIDRWTGGSTMVVQVGDVLDRGGDELKILYFLERLKREAEREGGRVVTMNGNHEIMNI---------------EGD---FRFVTKEGLEEFRVWRIAALRPQGPIAKRFLSQTVAVVGDSVFVHGGLLAEHVYGLERMNEEVTSWI-NGLRGG----N-SVVWLRKFSDERDCAALEHALSTIG-----VRRMIMGHTIQAG-INGVCGDKAIRIDVGMSKGGLPEVLEIRK-DVRIVTSNPL
PERLIAIGDLHGDFDKSKQAFRIAGLIDKWIGGSTTVVQIGDVLDRGGNELKILYFIEKLKREAAKTGGKIITMNGNHEIMNM---------------EGD---FRFVTKEGLDEFRVWRIAALRPEGPISRRFFTVTVLVVGDSIFVHGGLLPEHVYGLERINQEVRNWI-NGSRGR----D-GVVWVRRFSREQDCSALEHVLATVG-----VKRMIMGHTIQSG-INGACNNRAIRIDVGMSSGGLPEVLEIDR-NLQVLTSNTI
VDRLIAIGDLHGDLEKSKQALRLAGLINQWTGGTATVVQIGDVLDRGDDEIKILYLLEKLKREAEKSGGKFITMNGNHEIMNI---------------EAD---FRYATEMGLKEFEDWRIAALRPDGPIARRFLSTTVLVVGDSVFVHGGLLKQHVYGLERINREVRDWI-NGLKGR----H-AVVWLRKFSDE-DCSALEHALATLG-----VKRMIMGHTIQKG-INAVCDNRAIRIDVGLSRGGLPEVLEING-NLLVLTANPL
VDRLIAIGDLHGDLEKSKQALRLAGLINQWTGGTATVVQIGDVLDRGDDEIKILYLLEKLKREAEKSGGKFITMNGNHEIMNI---------------EAD---FRYATEMGLKEFEDWRIAALRPDGPIARRFLSTTVLVVGDSVFVHGGLLKQHVYGLERINREVRDWI-NGLKGR----H-AVVWLRKFSDE-DCSALEHALATIG-----VKRMIMGHTIQKG-INAVCDNRAIRIDVGLSRGGLPEVLEING-NLLVLTANPL
PDRLIAIGDLHGDLEKSKQALRLAGLIDRWFGGSATVVQIGDVLDRGDDELKILYFLEKLKREAVKSGGNLITMNGNHEIMNV---------------EGD---FRYMTKLGLEEFTNWRIAALRPNGPIANRFLSLTVLVVGDSVFVHGGLLAEHVYGLARMNKEVSDWI-TGLRGR----N-AVVWLRKFSDEVDCAALEHVLATIG-----VKRMIMGHTIQAG-INGVCENKAIRIDVGMSRGGLPEVLEING-NLRVLTSNPL
PNRLVAIGDLHGDLEKSKQAFRLAGLIDRWSGGSATVVQIGDVLDRGGEELKILYFLEKLKREAVKSGGNLITMNGNHEIMNV---------------ESD---FRYVTKVGLEEFSNWRIAALRPDGPIANKFLSVTVLVIGDSVFVHGGLLAKHVYGLERMNQEVRDWI-TGLRGR----E-AVVWLRKFSDEVDCSALEHVLATIG-----VKRMIMGHTIQAG-INAVCDNRAIRIDVGMSKGGLPEVLEING-KLRVLTSNPW
PDRLIAIGDLHGDLEKSKQALRLAGLIDKWAGGSATAVQVGDVLDRGDDEIQILYFLEKLKREAMKDGGNFITMNGNHEIMNI---------------EGD---FRYVTKLGLKEFEDWRIAALRPNGPIANKFLSVTVLVVGDSIFVHGGLLAQHVYGLERINEEVRDWI-SGLRGR----N-AVVWLRKYSDV-DCSMLEHVLATVG-----VKRMIMGHTIQDG-INVACNNRAVRIDVGMSKGGLPEVLEINQ-NLRVLTSNPL
PNRLVAIGDLHGDLEKSKQAFRLAGLIDRWCGGSTTVVQIGDVLDRGGEELKILYFLEKLKREAVKSGGQLITLNGNHEIMNI---------------EGD---FRYVTKKALEEFDVWRIAALRPDGPISRRFLALTVLVVGDSVFVHGGLLEKHVYGLQRINDEVRDWI-NGLRGR----N-AVVWLRKFSDEFDCSLLEHVLATIG-----AKRMIMGHTIQIG-INGACDNRAIRIDVGMSKGGLPEVLEIDR-NLRVLTSNPM
PDRLIAVGDLHGDLSKSKEALRLAGLIDRWIGGSATVVQIGDVLDRGGDELKILYFLEKLKREAAKDGGMIITMNGNHEIMNV---------------EGD---FRYVTKEGLEEFRAWRIAALHPNGPISGRFLSTTVLVVGESVFVHGGLLPGHVYGLQRINEEVRDWI-KGLRRS----N-AVVWLRKFSDESDCSLLKHVLDTIG-----AKRMIMGHTIQAG-INGVCNNQAIRIDVGMSKGGFPEVLEFVG-NPRILTSNPY
PPRLVAIGDLHGDLEKSKEALRLAGLIDRYTGGSATVVQIGDVLDRGGDELKILYFLEKLKREAARRGGRIITMNGNHEIMNV---------------EGD---FRFATLPGVEEFRVWRVAALRPNGPIAKRFLSVTVLVVGDSIFVHGGLLPQHTYGLEKINEEVRDWV-NGSRGA----D-GLVWVRKFSRGDDCSALEHVLSTVG-----VKRMVMGHTIQVG-INGVCDDKAIRIDVGLSKGGLPEVLEISG-SLRILTGNPL
PPRLVAIGDLHGDLEKSKEALRLAGLIDRYTGGSATVVQIGDVLDRGGDELKILYFLEKLKREAARRGGRIITMNGNHEIMNV---------------EGD---FRFATLPGVEEFRVWRVAALRPNGPIAKRFLSVTVLVVGDSIFVHGGLLPQHTYGLEKINEEVRDWV-NGSRGA----D-GLVWVRKFSRGDDCSALEHVLSTVG-----VKRMVMGHTIQVG-INGVCDDKAIRIDVGLSKGGLPEVLEISG-SLRILTGNPL
PPRLVAVGDLHGDLEKSKQALRLAGLIDRYIGGSATVVQVGDVLDRGGEELKILYFLEKLKREAARSGGRIVTMHGNHEIMNV---------------EGD---FRFATESGVEEFRVWRVAALRPNGPIATRFLSVTVLVVGDSIFVHGGLLPEHSYGLEKINEEVRDWV-KGLRSA----D-GLLWLRKFSRSKDCSALEHVLATVG-----VKRMVMGHSIQVG-INGACDDKAIRIDVGLSKGGLPEVLEISR-NLRILTEDPL
PSRLIAIGDLHGDLKKSKEALSIAGLIDNYTGGSATVVQIGDVLDRGGDEIKILYLLEKLKRQAAIHGGNFITMNGNHEIMNA---------------EGD---FRFATKNGVEEFKVWRVAALRPNGPISKRFFTVTVLVVGDSIFVHGGLLKEHVYGLEKINGEVSDWY-KGLRGR----N-ALVWLRKFSD--DCSSLEHVLSTIG-----VKRMIMGHTIQEG-INGVCENKAIRIDVGMSKGGLPEVLEIDR--VRILTSNPL
QDRLVAIGDLHGDLEKTKESLRLAKLIDKWVGGSTTVVQIGDVMDRGGDEIKILYYLEKLKREAARCGGTVITMHGNHEIMNV---------------EGD---FRFVTRKGLEEFRVWRIAALRPSGPISSRFLSVAVLVVGDSVFVHGGLLSQHVYGLEKINEEVRDWI-HGLHGA----N-GVVWLRKFSLGLDCSALEHVLATIG-----AKRMIMGHTIQFG-INGVCNERAIRIDVGMSKGGLPEVLEIKG-NMRILTSNPL
QDRLVAIGDLHGDLEKTKESLRLAKLVDKWVGGSTTVVQIGDVLDRGGDEIKILYYLEKLKREAARCGGTVITMHGNHEIMNV---------------EGD---FRXVTRKGLDDFRVWRIAALRPSGPISSRFLSVAVLVVGDSVFVHGGLLGQHVYGLEKINEELREWI-HGLRGA----N-SVVWLRKFSHEYDCSALEHVLATIG-----AKRMIMGHTIQFG-INGVCNERAIRIDVGMSKGGLPEVLEIKG-NLRILTSNPL
QDRLVAIGDLHGDLEKTKESLRLAKLVDKWVGGSTTVVQIGDVLDRGGDEIKILYYLEKLKREAARCGGTVITMHGNHEIMNV---------------XXD---FRXVTRKGLDDFRVWRIAALRPSGPISSRFLSVAVLVVGDSVFVHGGLLGQHVYGLEKINEELREWI-HGLRGA----N-SVVWLRKFSHEYDCSALEHVLATIG-----AKRMIMGHTIQFG-INGVCNERAIRIDVGMSKGGLPEVLEIKG-NLRILTSNPL
QDRLIAIGDLHGDLEKTKESLRLAKLIDKWVGGSSTLVQIGDVFDRGGDELKILYYLEKLRREAARCGGTVITMHGNHEIMNV---------------EGD---FRFATHKGLDEFRGWRVAALRPNGPISSRFLALTVLVVGDSVFVHGGLLAAHVYGLEKINAEVRDWV-HGLRGR----N-AVVWLRNFSHEFDCSALEHVLSTIG-----AKKMIMGHTIQFG-INGVCEERAIRIDVGMSKGGLPEVLEING-NMRILTSNPL
PSRLIAIGDLHGDLDKTKQAFHLAGLIDRWSGGSSTVVQVGDVFDRGGDELKILYFLEKLKREAAKSGGELITMNGNHEIMNV---------------EAD---FRFVTRSGLQEFEDWRIAALRPGGPVSTRFLTQTVVVVGDSVFVHGGLLSNHVYGLERINDEVREWL-SGSRGR----D-AVVWLRRFSEEADCSALEHVLATIG-----VKRMIMGHTIQSG-INGICGNKAIRIDVGLSKGGLPEVLEIDGTNLRILTANPL
PSRLIAIGDLHGDLEKTKQAFRLAGLIDRWSGGSSTVVQVGDVFDRGGDELKILYFLEKLKREAAKSGGELITMNGNHEIMNV---------------EAD---FRFVTRSGLQEFEDWRIAALRPGGPVSTRFLTQTVVVVGDSVFVHGGLLSNHVYGLERINDEVREWL-SGSKGR----D-AVVWLRKFSEEADCSALEHVLATIG-----VKRMIMGHTIQGG-INGICENRAIRIDVGLSKGGLPEVLEIDGTNVRILTANPL
PDRLIAIGDVHGDLEKTKEAFRIGGLIDRWIGGSTTVVQVGDVLDRGGEEIQILYFLEKLKREAARSGGKIITMNGNHELLNV---------------DGN---FKFTTADSLKEFKKWRIAALRPGGPITRRFLSATVLVIGDSIFVHGGLSSKHLYGLDRINEEVKDWM-NGL-GE----D-GVVWMRKFSVKKDCSALEHLLTTVG-----VQRMIMGHTPQ-R-INDECDGKAIRIDVGMSRGRLPEVLEITR-GLKILSSSPA
PDRLIAVGDLHGDLQKSKEALRLAALIDRWTGRTATVVQIGDVLDRGGDELKILYFLEKLKREAEKSGGTIITMNGNHEIMNV---------------DGD---FRFVTQAGLDEFRVWRIAALRPEGPISVRFLSQTVVVVGDSVFVHGGLLPKHVYGLERINEEVRDWI-NGLRGK----H-SMVWLRKFSHELDCSTLEHVLATIG-----AKRMIMGHTIQTG-INGACGNRAIRIDVEADR---------------------PNRLVAIGDLHGDLDKSKQAFKIAGLIHRWIGGNTTVVQIGDVFDRGDDELKILYFLEKLKREALRSGGSIITMIGNHEIMNI---------------DGD---FRYITPSGLQEFKAWRIAALRPNGPISTRFLSPTALVVGDSVFVHGGLLQDHVYGLERINEEVRDWI-NGLRGR----N-SVVWLRKFSDQVDCSTLQHVLATIG-----AKRMIMGHTIQMG-INGVCDNQAIRIDVGMSKGGLPEVLEIIG-NVRILTSEKV
PSRLIAIGDLHGDLPKAKQALRLAGLVGRWSGGSATVVQVGDIFDRGGDEIKLLYFFEKLKREAAGAGGLVITMNGNHEIMNV---------------EGD---FRFAAREGFEEFENWRIAALRPNGLISSRFLSQTVVVVGDSVFAHGGLLQNHVYGLDLINEEVRDWI-LGLRGR----D-SIVWLRSFSNELDCSMLEHVLETIG-----VKRMIMGHTIQNG-INTVCGNRAVRIDVGMSRLRFPEVLEIGE-NLKVLTSNPL
PSRLVAIGDLHGDLAKSLAALRLAGLLPSWAAGPTLAVQLGDILDRGGDELRLLYFLRRLSISAAAQGGALLPILGNHEVMNV---------------AGD---YRFVTPEGLREFSAWRLAALRPEGPIARRFLAPTVLVVGDSVFVHGGLLEAHVYGIERINAEVSDWI-RGGSGR----D-AVVWLRRFSEGFDCQRLQGVLGMLG-----TKRMVMGHTIQEG-INAVCGAQAVRVDVGLSRGGLPEVLEINGGGVRVITTDPA
PTRLVAIGDLHGDLPKSLSALRLAGLVPSWSAGPTLAVQLGDILDRGGDEIRLLYLIRRLAISAAGQGGALLPIMGNHEVMNV---------------SGD---FRFATPQGLREFSAWRLAALLPDGPIARRFLAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGR----D-AVVWLRRFSDGVDCQRLEGVLGMIG-----AKRMIMGHTIQEG-INAVCGAQAVRVDVGLSRGGLPEVLEINGGGVRVITTDPA
PSRLVAIGDLHGDLPKSLSALRLAGLVPSWAAGPTLAVQLGDILDRGGDELRLLYLLRRLALSAEARGGALLPILGNHEVMNV---------------SGD---FRFATPQGLQEFSAWRLAALRPDGPIARRFLAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGR----D-AVVWLRRFSDGFDCQKLEGVLGMIG-----AKRMVMGHTIQVG-INAVCGAQAVRVDVGLSKGGLPEVLEINGGGVRVITTPPS
PSRLVAIGDLHGDLPKSLSALRLAGLVPSWAAGPTLAVQLGDILDRGGDELRLLYLLRRLALSAEARGGALLPILGNHEVMNV---------------SGD---FRFATPQGFQEFSAWRLAALRPDGPIARRFLAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGR----D-AVVWLRRFSDGFDCQRLEGVLGMIG-----AKRMVMGHTIQVG-INAVCGAQAVRVDVGLSRGGLPEVLEINGGGVRVITTPPS
PSRLVAIGDLHGDLPKSLSALRLAGLVPSWAAGPTLAVQLGDILDRGGDELRLLYLLRRLALSAEARGGAFLPILGNHEVMNV---------------SGD---FRFATPQGLQEFSAWRLAALRPDGPIARRFLAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGR----D-AVVWLRRFSDGFDCKRLEGVLGMIG-----ARRMVMGHTIQVG-INAVCSAQAVRVDVGLSRGGLPEVLEINAGGVRVITTPPS
LSRLVAIGDLHGDLPKSLSALRLAGLVPSWAAGPTLAVQLGDILDRGGDELRLLYLLRRLSLSAESRGGAFLPILGNHEVMNV---------------SGD---FRFATPQGFQEFSAWRLAALRPDGPIARRFLAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGR----D-AVVWLRRFSDGFDCKRLEGVLGMIG-----AKRMVMGHTIQVG-INAVCSAQAVRVDVGLSRGGLPEVLEINAGGVRVITTPPS
PKRLIAVGDIHGDLAKARAALHVAEVIDHWIGGETVVVQVGDLLDRGGEEIKVIYLLEKLRGEAQKVGGNVHIMNGNHEIMNI---------------EGD---FRYATPLGLDEFQRWRIAALRPGGPLASRFLAPTVLVVGSSVFVHGGLLPVHVHGLERINQEVSEWM-LGTHGG----N-ALVWLRKYSDVKDCDLLKRCLGSIG-----AKRMVVGHTIQIG-LNGACDNKVIRVDVGLSKGGMPQVLEIRG-DLRILSSRLP
VERLVAIGDIHGDLAKTRESLRIAGVVDRWIGGKTVVVQVGDVLDRGGQELKVLYLLERLKGEAQRHGGDLHIMNGNHEVMNI---------------EGD---FRYATKEALDEFSAWRYAALRPGGPISSRFLAPTILIVGSTVFAHGGILPSHLYGLEKINAEVRDWI-QGRRGK----D-AVVWARQYSIPVDCKLLDEVLTGIG-----ASRMVVGHTIQFG-INSACQNQVFRVDVGMSSGTAPEVLEIRH-DVRVLS---VERLVAIGDIHGDLAKTRESLRIAGVVDRWIGGKTVVVQVGDVLDRGGQELKVLYLLERLKGEAQRHGGDLHIMNGNHEVMNI
EGD
FRYATKEALDEFSAWRYAALRPGGPISSRFLAPTILIVGSTVFAHGGILPSHLYGLEKINAEVRDWI QGRRGK
D AVVWARQYSIPVDCKLLDEVLTGIG
ASRMVVGHTIQFG INSACQNQVFRVDVGMSSGTAPEVLEIRH DVRVLS
VRRLVAVGDLHGDLAKTKEALVAGGLMDRWSGGTTTAVQVGDQLDRGGDEVAILLLLERLRKEARDAGGELVVMNGNHETLNV---------------AGR---FRYATADGLEDFRRWRLAALAPGGPMARRFLAPLVVAVGSTVFAHGGVLPHHVYGLERINKETSDWV-RGNSGR----E-SVVWARDYSMPEDCDVLEASLSKMG-----MKRVVVGHTIQHG-VNAACDGKVLRVDVGMSKGGKPEVLEILDDGGVFRLSLDE
-ERLVAIGDLHGDLNKATRAFRAGGLIDKWIGGATVAVQVGDQLDRGGDEVAILYMLERLRKEARDAGGELIVMNGNHETLNV---------------AGR---FRYAFEPGIEDFRRWRLAALAPGGPMAKRFLAPVVVAVGSTLFAHGGVLPHHVYGLERINQETSEWI-RGDSGG----R-SVVWARDYSWPQDCGVLRRALDGLG-----IDRVVVGHTIQEG-VNAACDGRVLRVDVGMSEGSEPEVLEIL-----------VERLVAIGDVHGDLAKTREAFRAAKLTNEWVGGTTTCVQVGDQLDRGKDEVAILHFLERLRGEARAAGGELVVMNGNHETLNV---------------SGR---YRYSLAEGNADFTRWRWRALAPGAPLALRFLAPVVVAIGSTLFVHGGVLPEHVYGLDTLNEEISQWI-KRGQGR----D-SVVWARDYSHVQDCDLLEETLRMIG-----VERVVVGHTIQHG-ITSACDGKVIRIDVGMSKGAKPEALEILKDGIAKMKLGDD
VERLVAIGDVHGDLSKVREAFRAAGMTNEWVGGSATCVQVGDQLDRGKDEVAILHFLERLRGEARDAGGELVVMNGNHETLNV---------------SGR---FRYSLPEGRADFARWRWRALAPGSPMTMRFLAPVVVAIGSTLFVHGGVLPEHVYGLDTLNAEISAWM-QRGQGR----D-SLVWARDYSHVQDCDLLKETLAMIG-----IERVVVGHTIQYG-ITKACDGQVIRIDVGMSKGAKPEALEILNDGI-------VERLVAIGDVHGDLDKTREAFRAARLTNEWIGGTATCVQVGDQLDRGKDEVAILHFLERLRTEARNAGGELIVMNGNHETLNV---------------AGR---YRYALTEGSADFARWRWRALAPGAPLTLRFLAPVVVAVGSTLFVHGGILPEHVYGLDSLNAEISTWM-KMGQGR----D-SLVWARDYSHVQDCDLLKETLKMIG-----VERIVVGHTIQHG-ITSACDGKVIRVDVGMSKGAKPEALEILRDGLTKLKVGAD
VPRLVAIGDLHGDLKKARRAFRLGGLTDRWIGGTTTAVQVGDQLDRGNDEVRILYFLERLEREAERAGGKLHILNGNHETMNV---------------AGR---FNYATLPGLADFYHHSEALHCSAGAVTARFLAPIALQIGSTVFVHGGVLPQHALGLERINQETRAWM-RGETGR----Q-AIVWAREYSAGADCAALEQVLGRLG-----AERMVVGHTIQAG-INSACEDRVFRIDVGLSKGGEPQV---------------VPRLVAIGDLHGDLRKARRAFRLAGLIDRWAGGTTTV--VGDLLDRGDQELPLLFFLERLQGQAAAAGGALHVLNGNHETMNV---------------AGQ---FRYVTHRGMHSFADWRILALQPGGEVTRRFLAPVVLQVGSTLFVHGGVLRQHVYGLERINRETQEWMLKGSSGR----G-AVVWSRHYSAAADCGQLAEVLGRVG-----AQRMVVGHTIQGG-INSACQGRVLRIDVGLSRGGSPEVLEIVN-DVRRLSESQQ
PPRLVAIGDIHGDYHKAVRALRLAGLMDRWAGGSTVAVQVGDILDRGDHEIRILILLERLAAEAAAAGGRLYLLNGNHETMNV---------------MGD---HRYATPGANLEFLGFR--ALMPGSELARRFFAPTVLQLGGNVFVHGGVLPAHVYGLEKINSETQSWM-LAPRGG----S-AIVWARAFSASDDCDTLKSVLESVG-----AQRMVVGHTIQRG-INSACESRVVRVDVGMSHGGPVEVLEVLK-DVLRLREHTP
ADRLVAIGDIHGDYHKAVRAFRLAGLTDRWSGGSTVAVQVGDILDRGDHEIRTLIMLERLAREAEAAGGRLYLLNGNHETMNV---------------MGD---HRYATPGANLEVLGLR--ALRPGSEISRRFFAPTVLQVGDNLFVHGGVLPAHVYGLERINRETQSWL-LGGLGG----S-AIVWARAFSASDDCETLQNVLESIG-----ARRMVVGHTIQRG-INSACESRVIRIDVGMSHGFPVEVLEVLK-DVRRLREHGP
-----------------------------------------------------------------------------------------------------------------------CLTVLRPDGPISRRFMAPTVLVVGDSVFVHGGLLEANVYGLERINAEVSEWI-RGERGW----D-IVVWLRRFSDGFDCKRLEGVLGMIG-----AKRMVMGHTIQVG-INTVGRGPPPSTSVSVGRGAMAQTLATVG---RTLPQRGG
-----------------------------------------------------------------------------------------------------------------------RLTALRPDGPISRRFLAPTVLVVGDSVFIHGGLLEANIYGLERINAEVSEWI-RGERGR----D-TVVWLRRFFDGFDCKRLEGVLGMIG-----AKRMVMGHTIQVE-INTVGRGPPPSTSVSVGRGAMAQTLATTG---RTLPQRGG
-----------------------------------------------------------------------------------------------------------------------RLTALRPDGPISRRFLAPTVLVVGDSVFIHGGLLEANIYGLERINAEVSEWI-RGERGR----D-TVVWLRRFFDGFDCKRLEGVLGMIG-----AKRMVMGHTIQVE-INTVGRGPPPSTSVSVGRGAMAQTLATTG---RTLPQRGG
VRRLLALGDVHGDLASLRKCLSLANVINEWIGGDAVVVQVGDVFDRGDAERAVLHFLDTLDRQARERGGAVYRLLGNHEVMNV---------------DLD---FRYVTPGGFAEFRRRRAMALAPGCPTARLLAEQLVLIIGDTLFVHGGLRPDHVYGLERLNAETRAWMERRAKGS----R-SPVWMRTYSAPHACRELEETLRRAG-----VRRMVVGHTPQ--VINGACRGRVWRVDTGMSAAGACEVLEISETHIRIFTDHGV
PGRVVALGDLHGDAAAAVTALTAAGLIDHWSGGDATLVQLGDVLDRGGEEFALWGLLERLRLEARSAGGRVVTLLGNHEVLNA---------------LGKA--ATYVHPAGHAQFGDDRCAAFRPGGALAARLADPVVAVVGDSVFAHASLPRGATGSLARLNAETRAWL-LGEGGR----D-SPVWDRTFSAPGDCEALRDTLRGLG-----ASRLVVGHTPQ--RVNGACGGAVWRCDTGCSRWGPVEALEIQGGAVRVLRRGTA
PKRIVAVGDLHGDIHATRQALRLAGVLHEWIGGDTFLVQVGDQVDRGDDEIQVMSLLHRLGKQARAEGGRVEVLVGNHELMSA---------------QGN---FRYATKGAMRNFQRWRYLSLRSGGTFASKFLAKAILIVGDTVFVHAGLLHKHFEAINVLNQEVAAFL-KGEWGN----D-GILWTRRFSKIDTCKQLKKTLNRLN-----VRRMVVGHSVQDG-ISPACDASVWRIDVGMSKGTSPQVLEIKQDGVNILGKSIE
PDRLVAIGDLHGDIVATRRALRLAEVLHEWVGGKTVVVQVGDQLDRGDDELQILSLLRKLAVQARQHEGALHVLIGNHEILS----------------THT---ARYATRGALENFFRWRYMALHPGGMISRTILATTALIVGQTLFVHAGIDLDHIEALKKINEEVSAFF-RGESTP----D-SMVWMRRYGGTHTCATLNATLKALD-----VRRMVIGHTIQSKTINSACDGAVWRVDIGMSSGTEPQILEMWKNGVNVKKEAAM
NQRLICIGDVHGDLWALQDFLEISGVYDEWIGGNTIVVQCGDILDRGVEELACYKLLCKLSQQAIHTDGKVILLVGNHEAMNS---------------MGL---FQYAINDLEHERKIGRWSSYEPGGLLATSLLAKVAIRVGRTVCVHAGLRPSHLGGIEGMNKQFRDWITLET---------GPIWMRDYSYPHTTRMIEETLLLLD-----CDRMIMGHTIQ--QINCVLHGRAWRIDVGASRGGTPE-IKIKYRY--------NQRIVSFGDVHGDITALRTFLITARVLDRWSGGDTICVQTGDVLDRGDDELACFRLLATLSRQAKESGGALLLLYGNHESLNA---------------AGL---FQYANPGGNAEFESTRWASFEPGGLLAENMLGLVACVVGRTVFVHAGLQAVHLNGISKLNMEARDWILKDCIGA----S-SPVWMRDYSQPNKNPYAKTMIGTLN-----VQRMVMGHTPQ---INAAMNGRAWRIDVGASQGGTPEVLEI------------AERLVVIGDVHGDIDAFRSCLQMADLVDKWAGGETVVVQMGDIFDRGDDDLPIQEWVYKLAQEAGRANGALYSVMGNHEMLNA---------------MGD---HSMATRKAFVPFLALRLAAMRPGGPVA-RLMAAVSMKVGDNLFVHAGLLPEHIGGMERLNADTCAWM-LGKWQP----E-GPLWTRVYSTPDARAQLEEVLRLTG-----TKRMVVGHTPQAG-INSAADGQVWRVDTGMTAMGRPEALEIRGEEMTILTEYKS
--MIPFIGNRVLD--AFAETLLAAGLVDNWAAGETVLVQAGDVFDRGDADLEVEEWLWTLQEQATESGGAVYHLLGNHEIMNA---------------MGD---HSTASPNSFKPFQDLRLAAMAPGGPVS-KMLASVAMKIGDTLLVHGGLRPVN-FDLESLNRWTHEWL-VGQWNR----D-SPVWTRFYSSPGAEADLQKVLDATG-----TVRMIVGHTPQAG-INSALGGRLWRTDTGMTAMGQPEALEIVDGVATIITAGGK
AERIIAIGDVHGDVDALHECLKVANLVDNWIGGAAHLVQVGDILDRGMEERRCLQSLLDLKQKAADAGGAVHVLLGNHEVMNV---------------DLD---FRYVSPRENAWFGWERVYAFRPGGGAA-RCLSPIAIQIGDSVFVHGGLRLQHVYGLEKLNQETAAWL-YGSDDI----N-SPIWSRIYSVPSAELELEQVLEKLN-----AKRMVVGHTPQRG-INAFVGYEVWRTDTGMSQGGPIECLEILEDGVHVLTQAGI
PKRIIALGDIHGDVRALATSLHMSHLIDNWIGKDSVLVQLGDVLDRGPNDYWCMRLLIKLQEQARASGGDVICLLGNHEVMNV---------------QLD---FRYVDPAAWAGWELERVNALRRGGTLS-KTLSPVCVVIGDTVFCHGGLMPEAVYGLQRLNDETSNWL-AGENP-----QFSPIWHRAFATANTLSAADCTMRLLH-----VSRMVIGHTPQEG-VNCLVGREIWRVDAGMSTGGPVECLEILAAKVNILSEDGV
AARIIAIGDVHGGFNELHHALKISGCADRWTGGDTVVVQMGDFLDRGSDESRSIEMLRDLKVQAKDAGGDVITLLGNHEIMNA---------------DLD---FSYAP--DIYSFDSWRAQLMQRGGKLA-MLMSPVVVQIGDNVFVHGGLTRETIHGIDKLNQEVADWLRKDADPPKGGRTVSPLWERVYGMPIALETLDGMLESMD-----AKRMIVGHTPQYGGVGTEKEKEVWRIDTNLNDKGRVECLEILTDLVRVLSEDGR
ARRIIAIGDL--------ASLRLAGVIDKWIGGDTVVVQVGDVLDRGDEESAVYDLLRRLASSAARKGGAVHMLLGNHEVMNV---------------GWD---FRYCEMYEKVDGDCPERGGDRPGGPVARK-LGNTVLVIGENVFVHAGLHGRHVYGLERINREVRDWM-RGT---------DPDATKPACVT-NCAKL--TLEDIP-----AKRMIVGHTDN-G-INCACSGAVWRLDVGMSRVAAAEVLEIRGDTVTALRRGVR
TDRVVAIGDVHGDLEALRSCLRLSGLVDAWVAAGATLVSCGDVLDRGDGDWDCLTYLADLKTRATDAGGDVHLVLGNHEVLNV---------------LGD---VRFASRGALVRC---RKRAFAPGSGEGARLLAPVARVCGDTLFCHGGLHAR--GGLDALNGDAARWL-GGAPSP----S-SPVWSRSYSHPAQCGDARSALDALG-----CARMVVGHTPQQG-INACCDDSVFRIDTGLSAYGPKEVLELRPGR--------EPRLVAFGDIHGDVFRCRQILQLANITDRWIAGSSIVVQLGDIADRGLHPHEIYDLFASLERQAMKAGGEFIFLVGNHELMNI---------------LGI---FYYVHPDVMSAFGGEYAAAFGPEGPYGLYILQPVTVVREGVVFAHAGITPEYAKGVEGINAELMNGF-RGESNS----S-SPLWSRAVLDE-NCSLLMESLRLLP-----VRVMVGGHTVQGGVMAVECNGSLVGADVGLSRFGYVAYVEFLLDD--------VHRIIAVGDVHGDADNFLKILRIANLIERWTSVRTTLVQVGDLIDRGEQDLEALNIAISLQEQTAQSGDEVVLLIGNHELLNI---------------QGH---YHYVNKHNYGGFLSKRAEGMKATGAFGKYIVDKAAHMDEGVLFIHAGIETSMNKDVEALNADVREALRQGILGS----S-GPLWTRKMIIE-ECSDVRAALKQLN-----ATRIVVGHTPQSGHIGQHCDGQVLAIDVGMSRWDKVAALELVFSK--------IHRIVAVGDVHGDAERFRQILEMSGVISRWTRLRTTLIQTGDLIDRGEEDLEVLEMAVSLFNEVRTNYDKVVLLMGNHELLNL---------------QGH---FHYVHSKSMGGFLTRRKRAFELDGTFGGFILETVAYAVADTLFVHAGIDEHVVDGIERLNREAKQAIRTKNLGS----T-GPLWSRKMFLD-RCADTKKALASLG-----VKRVVVGHTPQSGRVETFCGGSVIAIDVGMSRWGNIAALEITVTS--------IHRIIAVGDVHGDTENFRQILSMAGIVSMWTKLRSTLIQMGDLIDRGEDDLGVLEMAFSLFEQVKSNHDNIVLLLGNHELLNL---------------QEQ---FYYVHPETMGGFLSKRKRAFEPSGTFGRFLLDNVLYFDAETVFVHAGIDQRFAVGVEMLNKKTMQAIREKDLGT----S-GPLWTRKMITD-RCTGIQKMLSFIG-----AKRIVVGHTPQSGHVEVFCNDSVIAIDVGLSRWGNLAALELMVTS--------HSRLVLLPDLHGDARQAARALQLAGLISQWTGGDAMLVQLGDVVDRGHDSLALLGLLQRLGQAAEAAGGRVQAILGNHELMLV---------------HHD---YRYVSREELRAIGAAWQAGLAPSQPLGALVRRPLALLAKGVLVVHAGAFPWMRAGVDAWNAVALEALVHCDLGG----E-GAVWSRRYIQA-VCAEVAAVAERLG-----VQRVVVGHTVQGGRVSSRCGGQLLMVDVGLSRAGEMAVLTCSNGV--------PSRLVVLPDIHGDGPQALRALHLAGLISQWSGGDTVLVQLGDMVDRGPDSLALLTWLESLRGQARATGGDVVALLGNHELMNI---------------HHD---FRYVAPAEIVALAEAWREALAADAPLGSLVRQPLAALIDAVLAVHAGAFPWMLGGVAAWNAATAGALRFCELGE------GAVWSRHLAHGPVCLEVAAVVSRLR-----VERLLVGHTIQGGRVSSRCGGQLLLADVGISRAGEMAVLQCSSGKTDRQTEEEQ
FARIVAIPDIHGDLHHYRQSLRLAGVVDEWTAGSTHLVQTGDVVDRGQHSLLIMDMLANLTVRAKRVGGKVTALMGNHELMSL---------------MDD---TRYVHKDEILLLGTKWHRSFDPDAKQGRRLRRPLATVAGSSLFSHAGVRSRHLGGVDAMNEAAAAAI-QGKYDN----E-SPVWNRFYSREDVCDEVNRVLTAAR-----ANRMVIGHTVQGG-MRTKCGGKLHLIDVGMSSAGRGAAWVCEGGETELLEKEAD
ARRLVALPDLHGDLDLARRSLILARVINAWSGGETILVQTGDVLDRGDASVALMRLLNKLARAAKDAGGEVVGLLGNHELMTL
ARRLVALPDLHGDLDLARRSLILARVINAWSGGETILVQTGDVLDRGDASVALMRLLNKLARAAKDAGGEVVGLLGNHELMTL---------------QGD---LRYVSKRELVKLGKAWRDLFRAGADLGETLRFSMLHVAGESLFSHAGIRAHHL---EGVNGALRAASAGDGAFG----D-GPLWNRFWSTPRVCDELGAVLAKVG-----ARRMVIGHTIQRG-MATRCGGGLHLIDVGVSGKGRPAAWACEEGVVEVLDDGVE
QGD
LRYVSKRELVKLGKAWRDLFRAGADLGETLRFSMLHVAGESLFSHAGIRAHHL
EGVNGALRAASAGDGAFG
D GPLWNRFWSTPRVCDELGAVLAKVG
ARRMVIGHTIQRG MATRCGGGLHLIDVGVSGKGRPAAWACEEGVVEVLDDGVE
-------------MF---EALALAGVTRGWGAGRATCVQTGDLVDRGERSIEAVDEVERLTREANAVGDEFVSLLGNHELMTL---------------QGD---HRFASRDELTALGRSWRQTFAKGSTRGDVLREPWAAVRGRTAFSHAGLLPEHLFGVDELNARGAKLLAVDELLD----R-GPMWTRTISMG-ACALAAEVIRRLG-----VRRMVVGHTVTSGKIETRCDGLIHMIDVGMSKAGSPSVWMCTESESRVPLEN-RRKRLA------------VGRRLAGVVDSWGGGATTVVQTGDATDRGARSIETIDAIERLRVEAKEVGDEVVGLLGNHELMTL---------------QGD---YRFVAREELLALGRRWGKMFARGSERGEVVREPWAVVRGRTAFAHAGLLPEHLLGVDALNAEGKRLLAVECLVG----D-GPVWTREISTG-ACDAALEVTRRLG-----VRRLVVGHTVTSGRIETRCDGLIHMIDVGMSRLGSPSAWTCVESQGERVALE----------------------------------------------------------MDLGVRALREGGT----------------------------TGD------------------WMRAFARGSARGDEVRSPVAVTRGETVFVHAGLTSRHLFGVDALNARARELFDVDVTGG----D-GPLWTREISMG-VCKEVEETLARLN-----VKRMVVGHTPTSGSIETRCEGMVHMIDVGMSSAGVPSVWMCTESEGERVALE-LKRIVAVADVHGDRRNLMQALENGRVLVEWVMEGTQVVQLGDLVDRGPLGLQCYRLMQDLY--VAEGANEVVRVLGNHEVLNL---------------LGMA--GRYVTDEDVAEFGGERRESWSPGGEIWTILKDELVHVYGGHRFVHGGVMPALTRSIDELNEQASRMIKNGALS-----ESSPLWSRVYALG-ACPPLLNVLRHYG-----VARMVVGHTPSDGRMKVRCGGRAILADVALSRWGHPAALEMTLVN--------LKRIVAVADVHGDRRNLMQALENGRVLVEWVMEGTQVVQLGDLVDRGPLGLQCYRLMQDLY--VAEGANEVVRVLGNHEVLNL---------------LGMA--GRYVTDEDVAEFGGERRESWSPGGEIWTILKDELVHVYGGTLFVHGGVMPALTRSIDELNEQATRMIRDGALS-----ESSPLWSRVYALG-ACPPLLNVLRHYG-----VARMVVGHTPSDGRMKVRCGGRAILADVALSRWGHPAALEMTLVN--------PSRLVAIGDLHGDLPKSLSVLRLAGLVPSWPAGPTLAVQLGDILNRSDDELRLLYLLHRLSLSAETRGGAFLPILGNHEVMNV---------------SGD---FRFATPQGFHDRAVCKLPAFLPGGFLASNQTSQRLIKV---LFVHSGLLMKNRSDLEEYIQETGRAGRDGRLSCHLFLDSTTFYKSEYAMSKICSLVKESTSRKV-----LTQLEIGHLQQSGEISFGCGGRYLEVDPVVMEAAKAELVKLVGKG--------PSRLVAIGDLHGDLPKSLSALRLVGLVPSWTAGPTLTVQLGDILDRGGDELRLLYLL-RLSLSAETRGGAFLPILGNHEVKNV---------------SSD---FRFATPQGFHDRAVNRGGGLDPSRLCACAGFAGAVLIVSRDFNLHQAFKFECAYAYTEENTKPGKYI------TKPRTNNSLIW--------KCDQVLS-----------NNLFGLGYAIQ--------ERRVERASVCVTT---------------------PSSVCAVGDLHGDLQHALAALALCGAVDSWVGGAMTVVQTGDVLDRGNNSLGVLRALWRLQAEAEAAGGELVLLLGNHELMNM---------------QGK---VHYVHKAELAAEGGAWKRRMQPTGDLGAALLRDAAAVRGGTLFVHAGVRLSVAFGVERLNEA-----------------------------------------------------VGHNIV--WVSSRCGGTLALLDVGMSSAGLPAACDVGEEGYHGAATRGP
ARRLVAVGDLHGDYEQTVSVLRLTRLIDHWIGEDALLVQLGDILDVGPDDILIVRLLMRLQQEAHAKGGDVIELLGNHELRNF---------------RGD---YKAVDKASLAASGGQRDVLLSNATDLGRY-LRKAVFHYGPFLFMHGGFSTATATSVEQFNSELTKALLNGTEDDVDDVA-NPILVRSILTV-KCNALSKVLDKKG-----IQSVVVGHVPHPRDFDGLCGGRLIDIDFGMSRWGHVAALEIEEATVQLIETSTA
GRQIVVVGDLHGDLNQTLAILKITGLVDHWIGGDSFFVQLGDIFDVGPDDISIVKLLMKLEKEAQSVGGDVIELLGNHEIRNL---------------LGD---YSAVDPGSLAGSGGVRDRLLSNRTSVGMY-LRKAIFHHNEFLFMHGGLSTATATGIHEFNKDLRKALTNGTEGGGQEVV-NPILVRSILNV-RCKDLIKVLQNKG-----IKSVVVGHVPHHQDFKDLCGGHLIAIDFGLSRWGHVAALQIDDTTVQLLESTVR
GRRIVAVGDLHGDLNQTLSILHLAGLVNHWIGKDTYFVQLGDILDVGPDDLMIVRLLMRLEKEAQAEGGDVIQILGNHEIRNL---------------LGD---FSAVDPVSLAQSGGKRRELLSNRTPLGIY-LRRAIFHHKEFLFMHGGLSTATGTGVEEFNKALRDTLINNTENEAKKVA-NPILVRSILNV-RCSELEKVLSKKG-----IKSVVVGHVPHTDDFSDLCGGRLIAIDFGLSRWGHVAALEWDDVSVQLMESTGK
NQRIIAIGDLHGDIDRLRSTLRAANVLEAWRKGTDVVVQLGNVVGYGPDAPEMLQLLSGLKPQALASGGRLITLSGNQELLTL---------------SGV---LEYVHPRQLNLSAGYLRYLYGPGGRYGRMMAELAVAIVSDIVFVHGGLTAAYARGVNKLNAE---WY-EGAHDE----E-SPLWDHSVVRA-NCGPLSAGMAALD-----INLMVVGHTTMDGKVGTWCAGKLMTIDVAMSRYGGYEAFVSFLPMLVLGTAERV
VEKLIAISDIEGNFEAFRRFLLSHKVIDNWIFGKGHLVCVGDFFDRGANVTECLWLIYDLEEKAKAAGGYVHFILGNHEIMNM---------------SND---YRYVSPKYMRNADLYYGNWFKSSTELG-RWLGNVVEKIGDMIFVHGGLAPDLNKTLIDINNAMRPFYFIQEFDA----N-GPLWNRSYVMANPESDVAESLRKFG-----GKRIIVGHTLV--HAKMLYGGKVIAIDTRHVDPNSTALFFEDGKL--------PKQIFVLSDIEGNFKSLFLLLRNYKIIDKWSFKDNHLVILGDCFDRGEEVMECLWLIYSLEERAQRDGGHVHFILGNHEIMNM---------------NGD---WRYIHPKYAITPSGSATALYDGNSELW-RWLRNIIEQIGDILFVHGGISEALLLSVTEINELARPYYTRANNS-----DESPFWYRGYYRAASEGQVDTILKKFD-----AKMIITGHTIV--KVTSFFNGKIINVDTNHAAGVSEALFIKGNNF--------PEKLIAISDIEGNFDAFCGFLQKNKIIDNWVFGKGHLVLNGDFVDRGQHVTPTLWLIYKLEEQALQQGGKIHYVLGNHEIMNF---------------QGN---HKYADAKYIKLAQIIYKILYSKKSEIG-RWLRNVVTKIGDYLFVHAGFSPEILLSLENINEITRKNWDKDLLG-----RKGPFWYRGLVKSYNAKDVQEILKTYQ-----AEKIVVGHSIV--AVSADYHGKVIRIDVKHGNE-QGLLVENGIEY--------IKKVVALSDVHGQFDVLITLLTNQKIIDNWAFGNGHMVMTGDIFDRGHQVNEVLWFMYKLDQQARDAGGMVHLLMGNHEQMVL---------------GGD---LRYVHKRYDVAAKLLYDKLYGADTEIG-QWLRNTIIKINDVLYMHGGISSEWILTLARANKLYRQHVDASKFFG----N-GPTWYRGYFSEETETELDTILNHFD-----VKRIVVGHTSQ--RILGLFNNKVIAVDTSIKKGGELLLLENKQLT--------IKKVVALSDVHGQYDILITLLRNQNIIDNWAFGDGHMVMTGDMFDRGHQVNEVLWFMYQLDQQAIEAGGKLHLLMGNHEQMVM---------------RGD---LRYVHERYQVAAKLLYDELYDKSSEIG-QWLRHTLVKINDTLFLHGGISGEWLLTLDVANDLYRKNIDRSKFFG----N-GPTWYRGYFKPGDEAELDKILSYFD-----VKHIVVGHTSQ--RVLGLYNNKVIAVDSSIKEGGELLLMEDNTLT--------AGKIVALSDVHGQFDVMINLLKAHKIIDHWAFGDGHMVMTGDMFDRGHQVNEVLWFLYELDKEAQAAGGRLHLLMGNHEQMVF---------------RGD---LRYINERYKTSAELLYDALYNKDTEIG-RWLRNTLVKINNLLFMHGGISPEWVLNISDANQLFRQHLDDKKFFT----N-GPTWYRGYFKDASEQEIDQILSYFK-----VDHIIVGHTSQ--RVLGLYHNKIIAIDSSIKNGGELLLIDKDKLT--------VSKIVALSDVHGQFDVMINLLKAHKIIDHWAFDDGHMVMTGDMFDRGHQVNEVLWFLYALDQEAQAAGGRLHLLMGNHEQMVF---------------RGD---LRYVNERYQVSADLLYDALYNKDTEIG-QWLRHSLVKIINILFMHGGISPEWVLHISEANQLFRQHLDDKKFFT----N-GPTWYRGYFKDEKESDIDSILSYFK-----VEHIVVGHTSQ--QVLGLYHNKIIAIDSSIKNGGELLLIDAGKLS--------PEKTFVLSDPHANWSCFASLLKAGKVIDNWIFGTNQLVIIGDVFDRGVDVLPIYWLIYKLEKEAEDAGGKVTFLIGNHETMVL---------------GND---LRYTKKKYTQLADTLYPELWQK-SELG-HWLKNSIQVVGDNLFVHAGLSKEFLYDIPTVNEIVSDGLFLTKFA-----TYGPIWYRGMVRSADRDDLRKILEKYN-----VNRIFVGHTIF--DITTFYHYKVIAVNVDNQENGRGVMIEKDGSM--------ADKVFVMSDPHGRLDCVISLLQGNHIIDKWSFGKNHLMIIGDIFDRGKDVPQIFWLFYKLEEEAAKAGGHVSFILGNHEPMVL---------------AND---LRYTKEKYKILAEKLYPRLFGPDTELG-RWLGNTMQMIGNDLYVHAGLGKDFYLSIPTVNEEMSKGLFMTKYG-----NSGPIWYRGLVRTDAKDSLEMIMDRYK-----AKHIIVGHTIF--DISTFYNGKVIGVNVDNKENGRAMLIENNQYF--------NGKIIAIGDIHGDIESLKLILRHSKLIGNWIGDNVLLVQNGDVFDRGIYGPIIYNFLFKLQKEAIKKNSRVILIMGNHEQLNL---------------CGY---FNYVNPKEIEMFFHNRYHSFVNPGEYHKRLIRPPMVKVNNIIFTHGGLNLLISLSINDINLKTRLQIENNCLS-----RDGVLWSDAMSRNVKCSELFQILDKYD-----AKYLVVGHTRQSHQIGSYCNNHYFLIDTGMSLFPYPNYLKIDDHK--------NGKIIAIGDIHGDIESLKLILRHSKLIDNWIGNNVLLVQNGDVFDRGIYGPIIYNFLFKLQKEAIKKNSRVILIMGNHEQLNL---------------CGY---FNYVNPKEIEMFFYNRYHSFVNPGEYHKRLIRPPMVKVNNIIFTHGGLNLLISFSINDINLKTRLQIENKCLS-----RDGVLWSDAISRSVKCSELFQILDKYD-----AKYLVVGHTRQSHQIGSYCNNHYFLIDTGMSLFPYPNYLKIDDHKPSHQIGSYC
NGKIIAIGDLHGDIDSLKLILRHSELIDNWIGDNVLLVQNGDVFDRGIYGPIIYSFLFKLQKEAVKKNSRVILIMGNHEQLNL---------------CGS---FHYVNPMETKIFFDNRYYSFVSPGAYHKRLIRPPMVKVNNIIFTHGGLNSLISFSINDINLKTRLQIENNCLS-----RDGVLWSDVISRSVRCSELFHILDKYD-----AKYLVVGHTRQSHKIGSYCNNHYFLIDTGMSLFPYPSYLKIDDNK--------EGKIIAIGDIHGDIESLKLILRHANLINEWVAENVLLVQVGDVLDRGIYGPLIYDYLFKLQKEAPLKKSKVLLIMGNHEQLNL---------------CGY---FDYVNEKEVEVFFKKRLFHFVYSGEYFKKLIRPAIAKVNDILFVHAGISTQISLSLNTIRLKTRLQIENMCVS-----REGVLWHDHISRTAACSILSQIFNNYK-----AKHLVVGHTRQTHEISSYCNGGFFLIDTGMSLFPYPNYLVVQNGT--------EYDFYSIGDLHGDKDAFIRILLNESIIDKVIRNNVLTVITGDVLDPTYDDIDIILFIKNYNESGKSLNSKIILLLGNHEVNNL---------------CLK---FK-TPQSNIENY---RNDMFRKGETIYNYLIDPFVVNVNNIIFSHAGVLPFYSYGIDFINEEGKREIINNC-------EYGPTLNRYYSYVTICSSLYKSLGLLK-----SSRMVIGHTVQNKQVNSFCQDKLLLADTGISRWGVISYIQYFNDG--------EYDFYSIGDLHGDKDAFIRILLNESIIDKVIRNNVLTVITG
36
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
238
238
239
239
237
225
229
229
237
238
238
238
238
238
238
237
238
238
238
237
237
233
241
208
237
237
237
237
237
237
237
223
237
237
237
222
237
237
189
222
237
237
237
237
237
237
237
237
237
237
237
237
237
237
237
237
237
237
237
207
237
237
237
237
237
221
240
238
242
237
238
238
217
232
233
233
233
237
236
207
233
237
237
237
237
237
237
236
236
237
237
236
237
237
237
237
237
234
237
237
237
237
238
238
235
216
237
237
238
238
238
238
238
238
237
233
238
225
238
230
238
222
236
235
235
134
134
134
238
238
238
238
224
224
237
232
237
237
242
221
224
229
230
230
230
230
239
238
236
Figure 2.1: Alignment of SLP phosphatases from both Prokaryotes and Eukaryotes.
Candidate SLP phosphatase sequences identified as detailed in Materials and Methods were
aligned using MAFFT. The alignment was visualized in GeneDoc and manually edited to
remove the extreme N- and C-terminal regions outside the protein phosphatase domain, plus
poorly aligned regions, prior to phylogenetic tree inference. Two conserved bacterial-like
phosphatase motifs (red) were identified in addition to the known canonical PPP-family
protein phosphatase motifs (blue). Of the two bacterial-like PPP-phosphatase motifs, Motif 1
represents a novel region of conservation. Motif 2 was previously reported (Andreeva and
Kutuzov, 2004). The gene identifiers of each sequence used here are located in Appendix A1.
37
GDxHG
AlRLPHc
AlRLPHa
AtRLPHb
CarRLPHa
ThalRLPH
BraRLPH
AtRLPHa
CarRLPHb
AlRLPHb
CpaRLPH
CcRLPHa
CcRLPHb
CsRLPH
LuRLPH
MeRLPH
RcRLPH
VvRLPH
PperRLPH
PtRLPHa
PtRLPHc
PtRLPHb
CsatRLPHa
MdpRLPHa
GmRLPHa
PvulRLPH
GmRLPHb
GrRLPHa
GrRLPHb
AcRLPHa
AcRLPHb
MgRLPH
EgRLPH
BdRLPH
PvRLPH
SiRLPH
SbRLPH
ZmRLPH
OsRLPH
CsatRLPHb
PpaRLPH
SmRLPH
MdpRLPHb
MpRLPH
MpRLPHb
SrRLPH
BnRLPH
NgRLPH
F0SP41_PLA
D5SSG4_PLA
E8R2G2_ISO
GammataRLF
B0C4K2_ACA
B2J0C0_NOS
Q8YP31_NOS
D7E399_NOS
Q31LV8_SYN
Q5N017_SYN
C7QL34_CYA
B1WQZ5_CYA
B1X333_CYA
B0JSQ6_MIC
E0UA25_CYA
A8ZSZ1_DES
Q3A7H2_PEL
A7HLU4_FER
H9UCV4_FER
C5CDU8_KOS
D0MK25_RHO
D5H4I3_SAL
Q2S6K7_SAL
B8DK70_DES
E1YER6_9DE
I2Q722_9DE
G7Q752_9DE
E1K2K4_DES
H2J819_MAR
A9BIC6_PET
E3I2B3_RHO
G8API0_AZO
D3NZT3_AZO
G7ZAB6_AZO
H5YEZ7_9BR
E8L7W5 _ 9RH
F2IW59_POL
E0TGJ0_PAR
Q11UR6_CYT
B7IWU6_BAC
B7HV78_BAC
C6GXU9_STR
E8UQ78_STR
D3QFL2_STA
B9DJA7_STA
Q3M5P0_ANA
Q8YZT4_NOS
F4B184_KRO
E4NEL1_KIT
F8JTJ0_STR
G8WZV1_STR
F2RD99_STR
E4N448_KIT
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
GDxVDRG
GNHE
HGG
Motif 1
VVSGH
Motif 2
IDEG
*
20
*
40
*
60
*
80
*
100
*
120
*
140
*
160
*
180
*
200
*
220
*
RTVICVGDIHGYISKLNNLWLNPQSAIDPSEFSSALVIFLGDYCDRGPETGKVIDFLISLPEKHPDQTHVFLAGNHDFAFSGFLG----EGWYRGSVKGMAYKGSIYDLESVKLFVGLPLDDYNNV--NH----------------------------LKAITAGL----KALKLLGVEWGVVEKEAAGKLAVAETHH-TLRIT------------------------------RTVLCVGDIHGYISKLNNLWLNLQSAIDPSEFSSALVIFLGDYCDRGPETGKVIDFLISLPEKHPDQTHVFLAGNHDFAFSGFLG----EGWYRGSVKGMAYKGSIYDAGSTFESYGVPHGLMKAVPESHKKFLTNMVWVHEEDDVCIDTEEGLKHCKLIAVHAGLNNVEEQLKLLRAK--KIQHLSGRKTVVVSGHH-KLHIRLIIDEGGGFPPVAAIVLPSKKIIRDTDNLSS
RTVICVGDIHGYISKLNNLWLNLQSAIDPSDFSSALVIFLGDYCDRGPETRKVIDFLISLPEKHPDQTHVFLAGNHDFAFSGFLG----EGWYTGSVKGMAYKGSIYDAGSTFESYGVPHGLMKAVPESHKKFLTNMVWVHEEDDVCIETEEGLKHCKLIAVHAGLNNVEEQLKLLRAK--KIQHLSGRKTVVVSGHH-KLHIRLIIDEGGGFPPVAAIVLPSKKIIRDTDNLSS
RTVICVGDIHGYISKLSNLWLNLQSAVDPSDFNTALVIFLGDYCDRGPETRKVIDFLISLPEKHPDQTHVFLAGNHDFAFSGFLG----EGWYRGSVKGMPYKGSIYDAGSTFESYGVPHGLMKAVPESHKNFLTNMVWVHEEDDVCIETEDGLKHCKLIAVHAGLDNVEEQLKLLRAK--KVQHLSGRKTVVVSGHH-KLHIRLIIDEGGGYPPVAAIVLPSKKIIRDTDNLSS
RTVICVGDIHGYISKLNNLWSNLQSSLNPSDFSSALVIFLGDYCDRGPETRKVIDFLISLPEKHPDQTHVFLAGNHDFAFAGFLG----EGWYRGSVKGMAYKGSIYDAGSTFESYGVPHGLMKAVPESHKKFLTNMVWVHEEEDVCIETEEGLKHCKLIAVHAGLNGIDEQLKLLRVK--KIQYLSGRKTIVISGHH-KLHIRLIIDEGGGYPPIAAIVLPSQKIIRDTDNVSS
RTVICVGDIHGYITKLTNLWLNLQSALDPSQFTSALVIFLGDYCDRGPETRKVIDFLISLPEKHPEQTHVFLAGNHDFAFAAFLG----EGWYKGSVKGMAYKGSIYDAGTTFESYGVPHGLMKAVPESHKKFLTNMVWVHEEDDVCVETEEGVKRCKLIAVHAGLDSVEEQLKLLRAK--KVPYLSGRRTVLVSGHH-KLHMRLIIDEGGGYPPVAAIVLPSKMIIRDTDNVSS
RTVICVGDIHGNISKLNKLWLNLQSDIQNSDFSSALVIFLGDYCDRGPETRKVIDFLISLPEKHPDQTHVFLAGNHDFAFAGFLG----EGWYKGSAYGVLYNGSIYDAASTFESYGVPHGLIKAVPESHKKFLTNMVWVHKEDDVCIETEEGLTHCKLIAVHAGLNNVEEQLKLLRDK--RIQPLTGRKTIVVSGHH-KLHIRLIIDEGGGYTPLAAIVLPSKKIIRDTDNFSN
RTVICVGDIHGNISMLSKLWLNLQSALK-SDFSSALVIFLGDYCDRGPETRKVIDFLISLPEKHPNQTHVFLAGNHDFAFAGFLG----EGWYKGSAYGVVYNGSIYDAASTFESYGVPHGLMYAVPESHKNFLTNMVWVHEEDDVCIETEEGLKHCKLIAVHAGLNNVEEQLELLRAK--RIQPLVGRKTIVVSGHH-KLYIRLIIDEGGGYAPLAAIALPSKKIIRDTDDISN
RTVICVGDIHGNISLLNKLWLNLQSDIEQSDFNSALVIFLGDYCDRGPETKKVIDFLISLPEKHPEQTPCLSCRKPRLCFCWVLG----EGWYKGSAYGVLYNGSIYDAASTFESYGVPHGLINAVSESHKKFLTNMVWVHEEEDVCIETEKGLKHCKLIAVHAGLNNVEEQLKLLRAK--RIQHLTGRKTLIVSGHH-KLHIRLIIDEGGGYAPLAAIVLPSKKIIRDTDNFSN
RTVCCIGDIHGYFTKLKNLWSNLESIIDPPDFQSALVIFLGDYCDRGPDTRRVIDFLLSLPARYPNQTHVYLSGNHDLAFAAFVG----EGWYKGTVKGTDYKGSIYDAAPTFESYNVPHGLVKAVPDDHKKFLADMVWVHEEDDVCIETDEGIKHCRLIAVHGGLVKVEEQLEFLRAR--KVEALSGRKTIVVSGHH-ELHIRLIIDEGGGLEPVAAIVLPSMKVIRDTDNIAK
RRVCCIGDVHGYISKLQNLWKNLETHIGPSDFNSAIIIFLGDYCDRGPNTREVIDFLISLPTKYPNQKHVFLSGNHDLGFAAFVG----EGWFKGAAKGTEYKGSIYDAAPTFESYGVAHGLVKAVPDHHKKFLADMLWVHEEDEVCVETNDGIKHCKLIAVHAGLKKVEEQLELLKAK--KVEALSGRKTIVVSGHH-KLHIRLIIDENGGLEPVAAIVLPSMKIVRDINNLVRRVCCIGDVHGYISKLQNLWKNLETHIGPSDFNSAIIIFLGDYCDRGPNTREVIDFLISLPTKYPNQKHVFLSGNHDLGFAAFVG----EGWFKGAAKGTEYKGSIYDAAPTFESYGVAHGLVKAVPDHHKKFLADMLWVHEEDEVCVETNDGIKHCKLIAVHAGLKKVGEQLELLKAK--KVEALSGRK--------------VII---------------------------RRVCCIGDVHGYISKLQNLWKNLETHIGPSDFNSAIIIFLGDYCDRGPNTREVIDFLISLPTKYPNQKHVFLSGNHDLGFAAFVG----EGWFKGAAKGTEYKGSIYDAAPTFESYGVAHGLVKAVPDHHKKFLADMLWVHEEDEVCVETNDGIKHCKLIAVHAGLKKVGEQLELLKAK--KVEALSGRK--------------VII---------------------------RTVICVGDIHGYITKLQNLWTNLEALID--DFPTALVIFLGDYCDRGPDTKKVIDFLITLPSRYPNQTHVFLAGNHDLAFSAFLG----EGWYEGVAKGTEYQGSIYDAAPTFESYGVPHGLMKAVPESHREFLANMVWVHEEEAVCVEKEGEIKHCKLIAVHAGLKDVQEQLKLLKAK--KIEALSGRKTVVVSGHH-KLHIRLIIDEGGGLAPVAALVLPSMKLVRDTDSLLS
RVVICIGDIHGYLAKLQNLWSNLQSVINPEEFSSALVIFLGDYCDRGPDTKKVIDFLLNLPSAYPNQKHVFLSGNHDLAFAAFLG----EGWYKGATKGTEYKGSIYDAGPTFESYGVPHGLMKVVPDEHKRFLADLVWVHEEENVCIESEEGIKHCKLIAVHAGLKNVEEQLKFLKAK--KVEALSGRKTIVVSGHH-KLHIRLIIDEGGGLEPVAAIVLPSMKLVRDTDDLTK
RVVICIGDIHGYFSKLQKLWSNLEAIVNPQDFNSALVIFLGDYCDRGPDTKKVLDFLIKLPSFYPNQKHVFLSGNHDLAFAAFLG----EGWYKGVAKGTEYKGSIYDAGPTFESYGVPHGLIKVVPDEHKKFLVDMVWIHEEEDVCIENEEGMKHCKLIAVHAGLKNVEEQLAFLKVK--KIEALSGRKTIVVSGHH-KLHIRLIIDEGGGLEPVAAILLPSMKLVRDTDNFNK
-----------------------------------------------------------------------------------------------VTKGTEYQGSIYDARLTFESYGVPHGLVKAVPDEHKKFLADMVWVHEEDDVCIQTEEGIKHCKLIAVHAGLEAVDEQLKTLKAK--KVGPLSGRATIVVSGHH-KLHIRLIIDEGGGLEPVAAVVLPSMQVVRDTDKLAK
RVVCCIGDTHGFYTKLQNLWSNLQTTIDPSDFNTALIIFLGDYCDRGPDTKKVLDFLISLPSKYPNQRHVFLSGNHDLAFAAFVG----EGWFKGAAKNTQYKGSIYDAEPTFASYGVPHGLVKAVPDEHKKFLADMVWVHEEDDVCVETEDGIKHCKLIAVHAGLKDVKQQLEFLRAR--RIEALSDRQTVVVSGHH-KLHIRLIIDEGGGKEPVAAVVLPTMKIVRNTDVLAN
RLVICIGDIHGHITKLQNLWSNLETQFDPQHFNAATIIFLGDYCDRGPDTKKVLDFLIDLPSRYPNQKHVFLSGNHDFAFAAFVG----EGWYKGTIKGTECKGSIYDAGPTFASYGVPHGLLKAVPDDHKKFLADTVWVHEEDDVCIEDEEGIRHCKLIAAHAGLKNVGEQLRFLKAK--KIEALSGRKTIVVSGHH-KLHIRLIIDESGGFEPLAAIALPSMKLVRDTDNLTK
RLVICIGDIHGYITKLQNLWSNLETQFDPQHFNAATIIFLGDYCDRGPDTKKVLDFLIDLPSRYPNQKHVFLSGNHDFAFAAFVG----EGWYKGTSKGIEYKGSIYDAGPTFTSYGVPHGLLKAVPDDHKKFLADTVWVHEEDDVCIEDEEGIRHCKLIAAHAGLKNVGEQLRFLKAK-----------IIVVSGHH-KLHIRLIIDEGGGFEPVAAIALPSMKLVRDTDNLTK
RLVICIGDIHGHITKLQNLWSNLETQFDPQHFNAATIIFLGDYCDRGPDTKKVLDFLIDLPSRYPNQKHVFLSGNHDFAFAAFVG----EGWYKGTIKGTECKGSIYDAGPTFASYGVPHGLLKAVPDDHKKFLADTVWVHEEDDVCIEDEEGIRHCKLIAAHAGLKNVGEQLRFLKAK--KIEALSGRK-------------LLIFLSETFYPPFSPAFFSGKGLGC------RVVCCIGDVHGYITKLQNLWSNLESSIHPSDFNSALIIFLGDYCDRGSNTREVIDFLVNLPSKYPNQKHVFLAGNHDFAFAAFLG----EGWFRGLAKGTDYQGSIYDAGPTFESYGVPHGLVKAVPDEHKKFLSNMAWVHEEDDVCLDTEDGIKHYKLIAVHAGLKDVQEQLNSLKAK--KIECLSGRRTMVVSGHH-KLHIRLIIDEGGGLQPVAAVVLPPMKIVRDTDNMKQ
---------------ISNPPSTHQISPPPSSFSSAITATA------AXDTKKVIDFLVSLPSRYPNQKHVFLTGNHDLAFAAFVG----EGWFKGXAKGTEYKGSIYDAGPTFESYGVPHGLVKAVPDEHKKFLADMVWVHEEDDVCIETADGIKHXRLIAVHAGLKDVKQQLAFLRAR--KIQALSGRKTVVVSGHH-KLNIRLIIDEGGGMAPVAAVVLPSVKIVRDTDALTN
RAVICVGDIHGFITKLQSLWKNLEGSLDRSEFETATLIFLGDYCDRGPATRQVIDFLISLPSRYPRQKHVFLCGNHDLAFAAFLR----EGWFKGTVKGTEYQGSIYDAGPTFESYGVPHGLVKAVPDDHKKFLADLVWVHEEDDVFVNTDDGVKCCKLIAVHAGLKDVKEQLKLLKAR--KVEALSGRKAIVVSGHH-KLHVRLIIDEGGGYKPVAAIILPSMKIIRDTDELAK
RVVICVGDIHGFVTKLKNLWSNLERSIDRSEFETATVIFLGDYCDRGPDTRHVIDFLVALPSRYPRQKHVFLSGNHDLAFAAFLH----EGWFNGTVKKTEYQGSIYDAGPTFESYGVPHGLVKAVPDEHKKFLADLVWVHEEDDVFINTEDGGKCCKLIAVHAGL-DVNEQLKLLKAR--KVEALSGRRTIIVSGHH-KLHTRLIIDEGGGFKPMAAIVLPSQKVIRDTDVLAK
RLVICMGDIHGFITKLQILWSNLEASLDRSEFETATLIFLGDYCDRGPGTRHLIEFLVSLPSRY------LLGG---------------------TVKGTEYQGSIYDAGPTFESYGVLHALVKAVPDDHKKFLADLVWVHEEDDVFVNTDDGVKCCKLIAVHAGLKDLKEQLKLLKAR--KVEALSGRKQKHLIRHH-KLHVRLIIDEGGGYKPVAAIILPSMKIIRDTDVLAK
RTVICVGDIHGYVTKLLNLWSNLQSQIDPDSFNTATVIFLGDYCDRGPDTRKVIDFLITLPKRYSNQKHVFLSGNHDFAFGGFVG----EGWYKGAAKGIDYKGSIYDAAPTFESYGVAHGLMKAVPEAHKKFLADMVWVHEEDDVCIETQEGVKHCKLIAVHAGLKNVREQLEFLKAK--KVTDLSGRKTILVSGHH-KLHIRLIIDEGGGLEPVAAIVLPSMKIVRDTDNFSRTVICVGDIHGYVTKLLNLWSNLQSQIDPDSFNTATVIFLGDYCDRGPDTRKVIDFLITLPKRYSNQKHVFLSGNHDFAFGGFVG
EGWYKGAAKGIDYKGSIYDAAPTFESYGVAHGLMKAVPEAHKKFLADMVWVHEEDDVCIETQEGVKHCKLIAVHAGLKNVREQLEFLKAK KVTDLSGRKTILVSGHH KLHIRLIIDEGGGLEPVAAIVLPSMKIVRDTDNFS
RTVICLGDIHGYLTKLLNLWSNLQSQIDPDSFNTATIIFLGDYCDRGPDTRKVIDFLISLPKRYPNQKHVFLSGNHEFAFARFIG----EGWYKGVAQGIDCKGSIFDAAPTFGSYGVSHGLMKVVPEDHKKFLADVVWVHEEDDVCIETQEGVKHCKLIAVHAGLKNVREQLEFLKAK--QVTGLSGRKTIVVSGHH-KLHIRLIIDEGGGLEPLAAIVLPSMKIVRDTDNLSRVVCCIGDLHGYISKLQNLWSNLESEIDPVSFQSALIIFLGDYCDRGPDTHKVLDFLISLPSKYPNQNHVFICGNHDFAFSAFLR----EGWYKDHAKGTEYKGSIYDAGTTFESYGVLHGLVKAVPDEHKKFLANLVWVHEEDNVSIQTSEGKRNYKLIAVHAGLKGVEEQLKLLKGK--KVAALSGRATVVVSGHH-KLHIRLVIDQGGGLEPIAAIVLPSLKIIRDTDAKVA
RVVCCIGDLHGYISKLQNLWSNLESEIDPVSFQSALIIFLGDYCDRGPDTHKVLDFLISLPSKYPNQNHVFICGNHDFAFSAFLR----EGWYKDHAKGTEYKGSIYDAGTTFESYGVLHGLVKAVPDEHKKFLANLVWVHEEDNVSIQTSEGKRNYKLIAVHAGLKGVEEQLKLLKGK--KVAALSGRATVVVSGHH-KLHIRLVIDQGGGLEPIAAIVLPSLKIIRDTDAKVA
RLVICVGDIHGYITKLRNLWTNLETAVGPSDFRSALVVFLGDYCDRGPRTDEVIDFLISLPSKYPDQSHVFLCGNHDLAFAAFLG----EGWYKGHAKNTEYKGSIYDAGPTFEAYGVPHGLMKAVPEEHKKFLRDLVWVHEEDDVSIEMKEGLQKCKMIAVHAGLKGVEEQLNYLKNK--KVEPLSGRKTIVVSGHH-KVHVRLIIDRGGGLEPVAAIVLPSKKMVLDTDEFAT
RTVVCVGDVHGHFTKLRALWANLESSIPPHAFRAALVIFLGDYCDRGPDTRRVIDFFLSLPSAYPGQTHVFLAGNHDFAFAAFVG----EGWYRGEAKGTEYKGSIFDAAPTFESYGVAHGLIKAVPEEHKKFLANMVLVHEEDDVCITTPEGTKHCRLIAVHAGLKDVGEQLRSLKAK--KIEAIAGRKTIVVSGHH-KLHIRLIIDEGGGYPPVAAVVLPSLKIIRDTDILPT
RTVICVGDVHGFISKLESLWANLQSALPADAFATALVIFLGDYCDRGPHTRRVLDFLLALPARHPAQRHVFLCGNHDLAFAAFVG----EGWFHGPKKGLPYKGSIYDAQPTFESYGVAHGLTKAVPEEHKRFLHDLVWIHEGENVPIDTDEGQIVCKLIAVHAGLIDLNEQLRVLRTR--KVQMLSGRQTVIVSGHH-KLHIRFIIDEGGGYEPIAAVVFPSKQLIRSTEGTAP
RTVICVGDVHGHITKLESLWSNLQSALPAGAFATALVIFLGDYNDRGPHTRGVLDFLLALPRRHPAQRLVFLCGNHDLAFAAFVG----EGWFRGPKKGLPYRGSIYDAQPTFESYGVAHGLAMAMPEEHKRFLRDLVWIHEEENVPIDTDEGQIICNLIAVHAGLIDLNEQLRVLRTR--KVPMLSGRQTIVVSGHH-QLHIRFIIDEGGGYEPIAAIVFPSKALIRSTEGTTS
RTVICVGDVHGYIAKLESLWSNLQSALPADAFATALVIFLGDYNDRGPHTREVLDFLLALPGRHPAQRHVFLCGNHDLAFAAFVG----EGWFRGPKKGLPYRGSIYDAQPTFESYGVAHGLAKAVPEDHKMFLHDLVWIHEEENVPIDTDEGQIICNLIAVHAGLIDLNEQLRVLRTR--KVPMLSGRQTIVVSGHH-QLHIRFIIDEGGGYEPIAAIVFPSKTLIRSTEGAAS
RTVICVGDVHGYVTKLESLWSNLEAALPADAFATALVIFLGDYNDRGPDTRRVLDFLLELPTRHPGQRHVFLCGNHDLAFAAFVG----EGWFRGPKKGLPYKGSIYDAQPTFESYGVAHGLAKAVPEEHKRFLHDLVWIHEEENVPIDTDEGQIICNLIAVHAGLIDLNEQLRVLRTR--KVQMLSGRQTIIVSGHH-KLHIRFIIDEGGGYAPIAAIVFPSKTLIRSSEAH-RTVICVGDVHGYITKLESLWSNLQAALPADAFATALVIFLGDYNDRGPHTRRVLDFLLALPTRYPAQRHVFLCGNHDLAFAAFVG----EGWFRGPKKGLPYKGSIYDAQPTFESYGVAHGLAKAVPEEHKRFLHDLVWIHEEENVPVDMDGGQIICNLIAVHAGLIDLNEQLRVLRTR--KVQMLSGRQTIIVSGHH-KLHIRFIIDEGGGYEPIAAIVFPSKTLIRSTEEAGT
RVVICVGDVHGYISKLESLWANLQSALPPDAFATALVVFLGDYCDRGPSTREVIDFLLALPSRHPAQRHAFLCGNHDLAFAAFVG----EGWYRGPKRGSSYMGSIYDARPTFESYGVAHGLVKAVPEEHKKFLRELVWIHEEENVPIDTNEGQIICKLIAVHAGLIDLNEQFRILRTK--KVAMLSGRQTIVVSGHH-KLHIRFVIDEGGGYAPIAAIVFPSKELIRSTEGTSS
GVLCCIGDIHGYFTKLQNLWRNLESAIGASDFASATVIFLGDYCDRGPNSREVIQFLVSLPFRYPDQKHVFLAGNHEFGLAGFLG----EGWYKG-AYGIEFMGSVYDAAPTFESYGVPHGLMNAVPDEHKKFLSNLVWVHEEDDVCLETKDGIKTYRLIAVHAGLKDIEEQLKFLKAK--KIMGLSGRKTILVSGHH-RLHMRFIIDEGGSAPPLAAIILPSIKIVRDTDLL-RMTICVGDIHGHLDRLKALWRNLELKLGSEVFASSTVIFLGDYNDRGPDTKGVLEFLVGLPERYPKQRHVFLCGNHDFAFAAFLG----EGWWSGTTRNEPYKGSIYDARPTFESYGVSHGLIAAVPDNHKEFLRNLAWVHEQEGEETEDPE-TSYTKLIAVHGGLNSLDNQMQMLRTK--RIEPLAGRKVLLVSGHH-KLHFRLIIDESGGFDPIAAMILPAREIVRDTDNFSE
RPTVCIGDVHGHLIRLRALWRNLEAGLGA-LFASATVIFLGDYCDRGPDTAGVLDFLVALPASYPSQKHVFLCGNHDFGMSAFLG----EGWWSGVKKNMEYRGSIFDSEPTFSSYGVEHGLLGAVPEDHKAFLRKLVWIYEQ-------DGSESCGKLVAVHAGLRPLEEQLRVLREK--RHENMLGRSILSLSGHH-SLHMRLIIDESGGQDPLAAVVLPGRQIVRDTDSIGH
SSRIS--------GPISNPPSTHQISPPPSSFSSGITAFPPDIRIRSTSSSPITTTWPSPPSWASCSR---------------------KGGLREATKGTEYKRSIYNDGPTFESYGVPHGLVKAXPDKHKXFLSDMVWVHEEDNVCIETADRIKHCRLIXVHAGLKDVKQQLAFLKAK--KIEAIFG--VVVLIVTD-ETNYKLTIDEGGGMAPVAAVVLPSMKIVLDTDALTN
RATVLVGDVHGHRAKLVSLFENLEREIGAERLSTSRVVFLGDLVDRGPETSGVLDFIASLPAVHPSMEVRILGGNHDLGMATFCS----PLWVDA------GTRNAFDSEATFASFGVDPGLLSSLPESHAVLLSSLEFVVELDDV--EDDMHPEVNKLIAVHAGLRPIPEQLEALRER--WIEALQGRTVLVASGHH-ILKTWLVVDECAGLDQLAAVVLPERAVVKSH--------------------------LKLELGTERFETSTVIFLGDLCDRGPETSGVFNFLSSLKDKHPRQDVRHLAGNHDFAFATFLG----PLWEDS------DGINAFDSHTTFTSYSAAPGLLKKVPVEHKKILESLEFVVEVEDA--EEHG----KKLVAVHAGLCPFDVQMTALRQR--WVEALQGRAVIIASGHH-ILEMRIIIDGCGGHRRLAAVILPERKVVRSSS---DMCLCIGDLHGRYLRLQQLWTNASVYFGP-WMAGVDVVFLGDLCDRGSDTRQVVDFLLWLQRTRPNTTHI-IAGNHDFGLSSFLR-------------------SVFEAAATFESYGCKFPLLAAMPAAHLSFFRDLPWLVDL---------RFSFGRVICLHAGLKDMDPQMQLARER--FLEVIAGRGVIEVSGHF-FLDVRCIVDEGGGRDALAALVLPSRHILRHNEHVQT
KRVVCIGDLHGNIDKITNLWANLQDQLGKEGLKKATVIFLGDYCDRGPDTKGVISWLIDLKKKRDASGTYFLCGNHDFSMAAYLG
KRVVCIGDLHGNIDKITNLWANLQDQLGKEGLKKATVIFLGDYCDRGPDTKGVISWLIDLKKKRDASGTYFLCGNHDFSMAAYLG----PGYTSG--------CSIYNADSTFSSYGVKWGFIAAVPEAHKLFLKSLDWVCDI-------EIGFGPGRLIACHAGLGSLKDQLEGLKKR--RYFSFSARDAYLVSGHH-YREVRIICDRSGGYALLEAVILPSQPLNFVNDVREN
PGYTSG
CSIYNADSTFSSYGVKWGFIAAVPEAHKLFLKSLDWVCDI
EIGFGPGRLIACHAGLGSLKDQLEGLKKR RYFSFSARDAYLVSGHH YREVRIICDRSGGYALLEAVILPSQPLNFVNDVREN
KYIVAIGDLHGHLSALKLLMLKLEEALGNDLHQKYHLVFLGDYVDRGPKIKETIEYLIQLEKSRKPGTTHFLMGNHDFAMAMFLGLFDDELWKGN---------FTYSSFNTFYSYCTKDGLLRRMPESHKQFFRNMPWMLYN-------------EKNIFVHGGFSSLKEQMLPLMRRVPRIKPISERNRRIVSGHMQRVKVRALVDVSGGLTELACVVLPSCKVITTQPPVAA
GPIAVVGDLHGQVDKLGVVLDAIQDA---PQFDDRWVVFVGDFVDRGPDTKACLDMVLEFMDYHPK--TTAVAGNHDFAMCSSLG----GRWL-----------DHYDSQATFRSYGVEFGLAAAMPLQHKKFLASLPWVVEH-------------PYTIIVHAGMTPTDMQLKILRQRLNRPQWLCEKKKTVVSGHVWRVTYRILCDTTGGLENLSAVLLPELQVVDSGNSSFP
GPVAVIGDVHGQLDLLNFILSQLQQL---PNYDELWIVLIGDLVDRGPDPAGVIQRIVELREAHRR--TTVVFGNHELAMLGALG----PRWV-----------QFYDSEPTFRSYGVEPGLARAIPPEHREFLTTMPWVVEH-------------PENIFVHAGLQPTEMQLRILHAKLVRPPWLCDKARRIISGHVPEVVIRILTDTTGGLRELSCVILPECRILQSRGGVDR
YPILAIGDLHGQSEEFDRLLDRVDRL---AEWPDCAVVFLGDFVDRGPDSRKVIDRVLEILERPPG--GAAVMGNHDLALVHAARLRSPHYWLER-------YLSVYDAATTVRSYVGRTWLRAAMPETHRRFLAELPWVVEA-------------SGHLFLHCGLADALAQVEAMKRRWDRPDYWLGADKIQVTGHCQRPDARIRIDTSGGYGPLTACLLPTDPPHFISSWDDL
YPVVAIGDLHGRVEWLDKLVAKLRRR---PEWPHAKLVFLGDLVDRHFEVRALVSRVLELIAEKPG--STCVAGNHDLALVRAAG---LVEWAQR-------YGRNYDHQWTFRSYLGRTPLKAAMPAEHRAFLAGLPWVAEA-------------EGHVFLHNGLCPAAVQLECLHRKWDRPEYWLGADKVQVSGHVHAPDARIRIDTSGGVTPLTACVLPNAEPVFVFSNE-LRRFVIGDIHGHYQGLLDLVSLLEIG------ESDQIYFLGDLIDRGPDSSKVVDFVREQ-----S--YTCLLGNHEQLMVAALANLSPQMWL-Q---------A--GGRETLQSYSSK-----QHLWEHLSWIKTLPNHLDL-------------GDYWLVHAGILPLERQTAQEFC-WIRREFHNMP-RMIITGHTMFSGVWLGIDTGAYHPWLTALELSSSTVYQINVHSNV
PRRIVIGDVHGHYEGLMTLLEAIAPT------SDDQIYFLGDLIDRGPHSSQVVNFVKRH-----N--YPCLLGNHEQMLLNILTNERTQAWL-Y---------S--GGQATVASYHEA-----SIPDDHLDWFKSLPTYLDL-------------GDIWLTHAGVKLVTEQTADQLC-WIREEFHSIE-KLIIIGHTILPGVWLDIDTGAYHPWLTGLDVTNNLVYQVNIFKNY
QRRIVIGDVHGHYEGLVTLLAAIAPG------GDDQVYFLGDLIDRGPHSSYVIDLVRDN-----N--YFCLLGNHEQMLLNILSGDISQAWL-H---------S--GGHATIASYPDQ-----SIPKEHLEWLRSLPTYIDL-------------GDVWLTHAGLLPLSEQTSEQFC-WIREDFHSIP-KLVIIGHTIFPGVWLDIDTGAYHPWLTALDITNNLIYQINIFTNC
PRRIIIGDVHGYYQGLMNLFDKIAPS------SADQVYFLGDLIDRGPQSAQVVDFVKQN-----D--YCCLLGNHEQMLLNVFGNRSHQAWL-Y---------S--GGQATITSYQAA-----QIPQEHIDWFLTLPTFIDL-------------GDYWLTHAGVKSVSEQTAEELC-WIREEFHSME-KLIIVGHTILPGVWLDIDTGAYHPWLTGLDITNNLVYQVNVFNNS
GRRLIIGDVHGHYRSLYRLLELADPD------TDDQVYCLGDLIDRGPDSAKVVALVRSQ-----G--YTSLMGNHEHLLSQVLAFPDL-HWF-Q---------A--GGQATLASFGSW-----EALEELRPWLQQLPLYLDL-------------GEIWLVHAGVLPLSRQDANEFC-WIRDQFLARS-KTILVGHTIMAGIWLGLDTGVYHPWLTALDWDNQRVFQVQAQSNE
GRRLIIGDVHGHYRSLYRLLELADPD------TDDQVYCLGDLIDRGPDSAKVVALVRSQ-----G--YTSLMGNHEHLLSQVLAFPDL-HWF-Q---------A--GGQATLASFGSW-----EALEELRPWLQQLPLYLDL-------------GEIWLVHAGVLPLSRQDANEFC-WIRDQFLARS-KTILVGHTIMAGIWLGLDTGVYHPWLTALDWDNQRVFQVQAQSNE
MRTLAISDIHGCSIALETLLETVHLT------PKDTLITLGDYIDKGPDSKGVLDKLINLYKNY-R--LIPLKGNHEIKMLQARQSDPDCFWL-E---------F--GGRETLNSYS----TLANVPEEHWNFIQNLCVYWQT-------------DNHIFVHANLLSLKRQSEYDLF-WKKFNYQAP--KTMICGHTSKPINRICLDTWVCGGWLTCLELESGKIWQANQRGQI
MRTLAVGDIHGCATAFDKLLEAIHLR------PQDRLITLGDYVDKGPNSKEVLERLLYLYENH-R--LVPLKGNHELMMLDAFQGKKKDFWV-I---------A--GGKTTLQSYP----PLLNVPDSHRDFIKQHCLWYET-------------DNHIFVHANLLPLHKQSNHDLF-WQKLYPRHG--KIVVCGHTSSPVNQICIDTWACGQWLTCLDVDSGKIWQTNQQGEV
MRTLAVGDIHGCTTAFDQLLEAIDLR------PEDKLITLGDYVDKGPNSKGVLERLLFLYENH-Q--LIPLKGNHELMMLDAFQGKRKNFWL-L---------T--EGKTTLESYP----PLLNVPDSHWDFLKQCCLWYET-------------DNHIFVHANLLPLHKQSNHNLF-WQKLYPHHG--KIVVCGHTSCPVNQICIDTWACGQWLSCLDVDTGKIWQTNQQGEV
MRTLAIGDIHGCSKALDHLLEIVNPK------PQDTLITLGDYVNKGRDSKGVIDRLISLHKQG-N--LIPLKGNHEIIMLQARNNPLKRLWL-E---------K--GGKATLKSYS----HLIKIPESHWDFLGKVCISYET-------------ANHLFVHASLLPLARQPEYKLF-WEKFQYPAA--KTMICGHTSKPINAICIDTWAYGQWLSCLDVETGKLWQVNQRGNF
MRTLAIGDIHGCLEAFNLLLDVVALR------PNDTIITLGDYINKGPQSKGVIDQLIALHERG-Q--LVALKGNHELMILEARKSLEKFEWL-K---------K--YGTSTLLSYS----TLEDIPPHHWDFLQNICLWWET-------------KEHFFVHANILPLDQQLEDKLF-WAKFVDPQP--KVMICGHTSLPLNAICLDTWVCGEWLTCLDIDSGQIWQTNQQGKV
GKIFAVGDIHGCYKKLRVLMDRIPINY-----KEDTLVFLGDYIDRGDESFEVVAYLAELRKKHPG--IVFLKGNHEELFFNYLSGEDEVSFL-F---------N--GGEQTLKGYMSPD-GDISVPKEHLNFFNALQLYYET-------------DDYIFVHAGLVPLEQQKPEDLL-WIRKPFIESS-KIVIFGHTPEVVVKIGVDTGAVYGKLTCIELPARKFYEA-----GRLLAIGDIHGCHRALRSLLARLSPN------RGDRMVFLGDYIDRGPDPRGVIDTLLAVRRRVMR--CTFLMGNHERMLLDVLEGRNLPLYL-A---------N--GGLVTLLAYMTE--GQLRLPASHRRFFNGLQRFHAT-------------GSHIFVHAGLCPLSQQTEEDLL-WIRDAFLASD-KTVVFGHTPKPLLRIGLDTGAVYGVLTACDLHTRRLWQAREPNRD
-MLWAIGDIHGCLNALETLINEISPT------PNDKLVFLGDYIDRGPDSKGVVDFLIELSKRT-D--CIFLRGNHEQMLLDVIDNGDDYLWV-I---------N--GATATWRSYGNL--QNLLYNDEHLEFFRNTQYYFIE-------------DKYLFVHGGVIPIEKQDKRDLI-WIREEFISKK-YIVIFGHTPDVFIKIGIDTGCVYGKLTAYNLTLSKKVEVECLNGMGLWAIGDIHGCLRALERVIERISPS------EHDKLIFLGDYIDRGPDAKGVVDFLLLLSKMT-Q--CVFLRGNHEQMLLDVIDNNDDFLWN-L---------N--GAQATIRSYGNL--IQLETNEEHMSFYRNTKYYHIE-------------GKYLFVHGGVVPIEKQEPRDLI-WIREEFILKR-FVVVFGHTPEVYFKIGIDTGCVYGKLTAIEVNQKVVIQEDCRNVR
-MIYAIGDIHGCLESLKKLIEKINPS------RTDMLIFVGDYIDRGPNSKGVVDFLIKLSRSR-K--CIFIRGNHEQMLLDYLNGGKTKLWH-F---------N--GMEYTINSYG----GIDKIPEKHIRFFEKTILFHET-------------DEYVFVHAGVIPLNKQKDGDLL-WIRDEFIYAE-KVVIFGHTPEPLIKIGIDTGCVYGKLTALRLDDMCFFSVKSIF-MALIAIGDIHGCARTLDALLDRLAPT------RDDQLVFIGDYIDRGPDARGVIERLLRLREEI-S--CVFLRGNHEALMLNYLDRGEADLWF-I---------N--GGLTTLNSYRND---GIRIPEEHEAFIRETLLYYDT-------------PDFFFVHGGLLTIAEAHTDLFL-WEREHLNAPR-KPVVCGHTPEPINLIAIDTGCVYPRLTAVRLPERTFISIPRQDLI
MGLIAVGDIHGCLESLNALLDRLNPS------SDDHLLFVGDYIDRGPDSRGVIDRLLDLRESI-S--CTFLRGNHEAMMIDYLDSGAFSLWR-M---------N--GGVSTLQSYLKGEGSEVHIPAAHAEFVRDTKLYHET-------------DDFLFVHAGLLTVEEPDEEVLL-WERGHLEASG-KTVVCGHTPDPISLILIDTGCVYHRLTAVHLPQREFIDVPYADGMGLIAVGDIHGCLESLNALLDRLNPS------SDDHLLFVGDYIDRGPDSRGVIDRLLDLRESI-S--CTFLRGNHEAMMIDYLDSGAFSLWR-M---------N--GGVSTLQSYLKGEGSEVHIPAAHAEFVRDTKLYHET-------------DDFLFVHAGLLTVEEPDEEVLL-WERGHLEASG-KTVVCGHTPDPISLILIDTGCVYHRLTAVHLPQREFIDVPYADGTRTFAIGDIHGHRDRLEALLRRLPLDR-----ERDTVVFLGDYVNRGPDSRGVIDLLLGLQRTCPG--AVFLRGNHEQILLDYADTGDALLPL-L---------RFLGVEATLRSYGVD--GLACLPPEHAAFLRGLAFAHET-------------ATHVFVHAGLPRSETQRLDSRR-LVRRDMAPHD-RTVVFGHSSTPLVRIGIDTGAGGGMLTALELPALRFHHA-----GKIFAIGDIHGCYDKLISLMDRLPFDL-----HSDTLIFLGDYINRGSQSKEVIEYLLKLKQSSEN--IIFLKGNHEHALLEYAQTKDQYLRL-L---------RSMDVEPTLKSYSDA--DLGFLPPAHLDFLNGLLTYYKT-------------DDYLFIHAGVEDPDGCSLDRLL-SVRDTFLRYE-YTVVFGHTPMPLVMIGIDTGAVYGMLTAVLLPDLRFFHA-----GKLIAIGDIHGQSDALRRLLDDLPYRP-----GRDRLIFLGDYINRGPDTRGVLEVLSALRRDDPG--AVFCLGNHEEALLRYAAGGDPDLRL-L---------RTLGIETTLESYGDL--GLSFLPEEHREFLLGLESFRRI-------------GPYVFVHAGLLPPEACPPDCLL-SVRGAFLTGP-LTVVFGHTTTPLVKIGLDTGAAWGALTALVLPDMVFLHAPGDRFL
GKLIAIGDVHGQSDALRRLLDDLPYTP-----GRDRLIFLGDYINRGPDTRGVLEILATIRRDDPG--AVFCLGNHEEALLRYAAGGDPDLRL-L---------RTLGIETTLESYGDL--GLAFLPEAHREFLLGLESFRRI-------------GPYVFVHAGLLPPEACPPDCLL-SVRGAFLTGP-LTVVFGHTTTPLVKIGLDTGAAWGALTALVLPDMVFLHAPGERFL
ARLIAVGDIHGQADALRRLLDDLPYRP-----GTDRLIFLGDYINRGPDTRGVLDLLTDIARQDPG--AVFCLGNHEETLLRYADEGEPDLRL-L---------RGLGIETTLRCYGDP--GLSFLPPEHQAFLRALVPYARI-------------GDMLFVHAGLLPPEECPTDCML-SVRGAFLSGP-LTVVFGHTTTPLVKIGLDTGAAWGALTACILPDMELRHVFI----MIWAISDIHGMYDNLIKVLNQIP--------ENDKIIFLGDYVDRGPGSRQVLDLLFKLKNR-----AVFLKGNHEDMMLDYFENTGKGAWF-R---------N--GAQATLDSFNND------IEEKYLDFIRNLKLYHGT-------------QKYLFVHGGIISLSDQAERDLL-WIRDDFYMSD-YIVIHGHTPYITGSIDIDTGCVYGKLSALGISNDNKYDIISI---MIWAISDIHGMYDTLISLLKRIQIK------DSDTMIFLGDYVDRGPDSKKVLDLLITLSKQRN---RIFLKGNHDDMMVDYCQKTHEGVWF-Y---------N--GALSTIKSFNNN------IGEEYINFLKDLPLYYEN-------------EKYLFVHAGVKSLSKQDKWDLL-WIRDEFLSMS-YTVIHGHTPYLTGSVDIDTGCVYGKLTAFGITEDNRHVVLQAV-MRVYAVGDIHGRLDLLEIMLDLVDADQHERGIRPALIVFLGDYVDRGPSSKEVVDAFIGELGEALS--PVFIKGNHEALLMSFLYDATPNFWL-K---------N--GGDAALLSYGLDPDFREALPASHLSFYCNLKPSFRI-------------GGYFFVHGGVVPLDQQREEDLL-WIRDPFLTSD-AVVVHGHTPEPQLRIGVDTYAFKSRLTAVGLEGTERWILSTAS-VRVYAVGDIHGRLDLLEQLLAQIDRDAASGADLVKYLVFLGDYVDRGPDSAMVIERLCREPLPGFG--AIHLRGNHEAAMMDFIEKPEAPDWL-E---------Y--GGRATLASYGVPAPFAAALPPHHRTFLAGLRSSVAI-------------GDYLFVHAGILPLHRQRDEDLL-WIRREFLTSH-KLVVHGHTIQPDIRIGIDTGAYASRLTALVLEGGERRFLCTI--MRVYAVGDIHGRLDLLDQMMGQIARDAAQAPNLLKYIVFLGDYVDRGPDSRLVIERLACGLPPVLG--AVFLRGNHEDTLLGFLSDLRVPGWL-T---------Y--GGDATLESYGIPAPLNSLLPAHHRAFLTGLRCHLTI-------------GDYHFVHAGVVPLDRQEDKDRL-WIRDAFLASR-KVIVHGHTIEPELRIGIDTGAYATRLTALVLEGAERRFLCTV--MRVYAVGDIHGRLDLLDQMLGQIAHDAGQAPNLLKYLVFLGDYVDRGPDSRLVIERLACGLPPVFG--AVFLRGNHEDTMLSFLSDLRIPGWL-T---------Y--GGDATLESYGIPAPLNGLLPPHHRAFLTGLRNHLTI-------------GDYHFVHAGIIPLDRQEDKDRL-WIRETFLASR-KIVVHGHTIEPELRIGIDTGAYATRLTALVLEGTERRFLCTV--VRIYAVGDLHGCADLLAGAFDLIDADLARSRPEQAIQVFLGDYIDRGPDAKRTIDLLIDRGQRHE---TVFVRGNHEALLLRLLDDPKMPNWI-K---------L--GGMTTLMSYGITISLAAAIPSAHTDFLANLVPFFSC-------------GDYFFVHAGVVPLDKQSEEDLM-WIREPFLSSD-KVVVHGHTPEPEIRINIDTGAYATLLTVLSIQDDKVEFHSSSRSR
LRLYVIGDVHGRLDLLDDLSKQIAADLDDAP-ADVLTIFVGDYIDRGRESAGVIDRLNRGEFPTP---FHALRGNHEEILLKFLEDESVESWR-K---------F--GGLETLHSYGVDVSLLELMPETHRRFLEGTDLFASF-------------GDYLFVHAGVVPVHLQNADDLL-WIREEFLRFR-KVVVHGHTPAPDVRINVDTGAFATVLTALVLEGADRRFLQTRAPQ
Q
Q
TRVYAVGDIHGRFDLLWRLAGAIEADLAARPVARVVEVYLGDYVDRGPDSARVVDWLSRPAAGGRE--RICLMGNHEHALLSFLADPGLAQWR-Q---------F--GGGETLISYGIDLPLRAALPVSHLRFLRALPALHRI-------------GGYAFVHAGVVTLDAQVETDLL-WIRQEFLDYP-AVVVHGHTPTIDLRINIDTGAYMTRLTCAVLDADGVRFLQTTREG
KILVAIGDIHGRLDLLDRLLPKISEHLDG---RAARLVFLGDYIDRGPDSAGVLDRLIGVRESFSD--CVFLKGNHEEALLDFLAAPEEASWL-G---------W--GGDATLLSYGLDTDFAAALPDDHFRFLLDLELFHRA-------------APYQFVHAGLLPFDRQIERDLL-WIRDRFFNAN-WVIVHGHTPAPDNRINVDTGAVWSQLTAVVLDGEDRRFLTS---KRTFVIADIHGCYKSLKALVENLQPT------HEDQFIFLGDYIDRGPDSKAVINYLIYIKEHYKN--CVFLRGNHEQMFLDAHQSIAAERWI-I---------N--GGEEVMISYGLK--NHDHVPYHVQDFISNTFYYFQD-------------ERFIFVHAGIAESPLKDYNSMM-WIRNAEITHT-RIVVHGHTPLDTIEICLDNGCVFKQRTGLGLHSNRLYVQNNIDFKRILVISDIHGEIEKFEQLLKEAQYDA-----KQDQLILLGDYVDRGPNARAVIEKVKELKEE--G--ALILKGNHEDMMIKALTTNEENHWVKR---------N--GGDKTLYSYGFLEELQSSVLEEHIKFIQELDHYIET-------------EEYIFVHAGVKRVSESEPYTLM-WIRNEFHNG--KVVVFGHTETLHSIIGIDGGAVYGQLNCLELPSKKVYVIKN---KRILVISDIHGEIEKFEQLLEEAQYDA-----KKDQLILLGDYVDRGPNARAVIERVKELKED--G--ALVLKGNHEDMMIKALTTDEENHWVKR---------N--GGDKTLYSYGFVEELQSHVLEEHVKFIQELDHYIET-------------EKYIFVHAGVKRVSECEPYTLM-WIRNEFHNG--KVVVFGHTETLHGIIGIDGGAVYGQLNCLELPSKKVYVIKS---KEFFVIGDVHGKFELLLDILKKWNE-------KRQQLIFLGDLIDRGENSKACLELVWSLVREK-G--AICLTGNHERMFLAWLRDPIDDHYR-R---------N--GGDTTINSLLGRPLLEQY--EDLIDFVKSCPYHLET-------------EDYIFVHAGVLPDWRTSNHDKV-WLRTPFHEAE-KMIVFGHTPNLYQKMGIDGGAVYGVLHGIVLDGTGLVQDYIVGAV
KEFFVIGDVHGKFELLLDILKKWNE-------KRQQLIFLGDLIDRGENSKACLELVWSLVREK-G--AICLTGNHERMFLAWLRDPIDDHYR-R---------N--GGDTTINSLLGRPLLEQY--EDLIDFVKSCPYHLET-------------EDYIFVHAGVLPDWRTSNHDKV-WLRTPFHEAE-KMIVFGHTPNLYQKMGIDGGAVYGVLHGIVLDGTGLVQDYIVGAV
QRILVASDIHGHGDALIKLLSEAVYNA-----ETDQLVLLGDYVNNGPDSVGTLRLVQKLVNKG----AIALVGNHELRWLERQD---------------------------------------SEAQAWHKFLSGLPYIKVL-------------DSYIFVHAGIVPIDKQQCEHVT-AHHSVALNQH-KTIIHGHVPEIHIMIDIDTGAGHNYLTLVDLTNACSYSVNVSHKD
GRLFVASDIHGHGETLRQLLETAEYSP-----GNDQLVLCGDYVNNGPDSEGTLELIQKLQKD--G--AVVLFGNHELRWLEEIA----TEFIMH---------H--KMKVTYQRIQKD-----WGKLKWRSLLESFDKYYMT-------------DDFLFIHAGFVPLEKQTAAEVT-GYDKTKDLQH-KYMVHGHVPEIKVSVNIDTGAGHGYLTLLDLTNSKQYCQRVVMKE
MRTIVVGDIHGCYAELLQLLAKVEIA------EEDCLVSLGDIVDRGIDSVKVYDFLKNRPN------TIVLMGNHERKHLRQTLSYSQ--------------------------------QFGDSYDEFLEWISHLPYYHET-------------ESAIFVHAAVIPIEEQREEVLC-WSQ--------KPVIFGHRVHPLITYGIDTGACHGTLTAIILPSFEIVQVQAAKDY
MRTIIVGDIHGCYEELLQLLTKAQIT------KEDCLISLGDIVDRGVDSVRVYDFLKNRPN------TIVLMGNHERKHLRQTLSYSQ--------------------------------QFGDRYPEFLEWVSHRPYYHET-------------DAAILVHAAVIPIGEQRPEVLC-WSQ--------KPIIFGHHVIPLIIYGIDTGACHGKLTGLILPSFEVVQVQAPQDY
MRTLVFGDIHGGYRALVQVMKRANVT------RDDHLIFLGDYVDGWSESDAVIDELMRIKKEYKK--VTLIRGNHDDLVQQWLEGKTMPKWL-Q---------H--GGQSTIDCYEKR---SAQEIQWHIEFYKTFEDYLDD-------------ENRFFCHAGFGPEYEWHSTAFY-WDRDPLYPKRLEEIYIGHTPVPVNIWNLDTGAAFKSLTVMDVDTKEFWQSDALPSL
GPLFVIGDVHGYLDELLAALHQLIDTEGHWSAGRSRIWFLGDFTDRGPDGIGVIDLVMQLAAEAAAAGCRALMGNHELLFLGASR----AAWRLN------------GGQQH---------DLERLQPHHISWLSRLPAMALE-------------DGHLLLHSDTLEYGETIADVNDAW--DAFFTKRFYRVVHGHSPGPYIAIAMDGGVTMDRLLVARLPLN----------GPLYVVGDVHGYLDELKAALRELIDAEGHWSAGQSRLWFLGDFTDRGPDGVGVIDLVMQLSAEAAAAGAKALMGNHELLLIGAKR----AAWRLN------------GGQPT---------DMERLQDHHIAWMSRLDAIALE-------------DNHLLLHSDTLEYGSSIEEVNDAW--ELFFTKRFGRVVHGHSPGPHLALAMDGGVTMERLLVAQLPLRG---------GPLYVVGDVHGYLDELKAALRELIDAEGHWSAGQSRLWFLGDFTDRGPDGVGVIDLVMQLSAEAAAAGAKALMGNHELLLIGAKR----AAWRLN------------GGQPT---------DMERLQDHHIAWMSRLDAIALE-------------DNHLLLHSDTLEYGSSIEEVNDAW--ELFFTKRFGRVVHGHSPGPHLALAMDGGVTMERLLVAQLPLRG---------GPLYVVGDVHGYLDELVAALRALIDENGGWAAGNARLWFLGDFTDRGPDGIGVIDLVMRLSAEAAAAGCKALMGNHELLLIGAKR----AAWLLN------------GGQKH---------DMDRLQDVHLQWMSRLDAVVRE-------------DDHLLVHSDTLEYGQTIEDVNETW--DVFFTKRFHRIVHGHSPGPHVAIAMDGGVTMAKLLVVQLPLNG---------TPLYVAADIHGHRTEFRAVLRALADENGHWSGGRARLWLLGDHVDRGPDGIGVIEDVRRLAAQARDEGVGALLGNHEVQLLAAHR----GGWARF------------GGQEA---------DLRGLTPSHLEWLAALPAAALV-------------DGHLLVHSDTLELGTSVAAVNAAWL-EFSMSSRGSVLVHGHSTAALRVLAIDGGAYLGRILLTRL--------------
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
:
165
228
228
228
228
228
228
227
228
228
227
187
187
226
228
228
137
228
228
219
209
228
207
228
227
205
227
227
228
228
228
228
228
228
228
226
228
228
225
227
220
201
215
190
202
213
213
202
202
210
205
190
190
190
190
189
189
194
194
194
194
194
194
198
195
197
192
196
198
198
195
195
201
201
197
185
190
204
203
203
203
205
203
206
199
197
195
195
196
196
172
191
169
169
198
184
185
185
185
182
Figure 2.2: Alignment of RLPH phosphatases from both Prokaryotes and Eukaryotes.
Candidate RLPH phosphatase sequences identified as detailed in Materials and Methods
were aligned using MAFFT. The alignment was visualized in GeneDoc and manually
edited to remove the extreme N- and C-terminal regions outside the protein phosphatase
domain, plus poorly aligned regions, prior to phylogenetic tree inference. Two conserved
bacterial-like phosphatase motifs (red) were identified in addition to the known canonical
PPP-family protein phosphatase motifs (blue). Of the two bacterial-like PPP-phosphatase
motifs, Motif 1 represents a novel region of conservation. Motif 2 was previously reported
(Andreeva and Kutuzov, 2004). The gene identifiers of each sequence used here are located
in Appendix A.2.
38
2.3.2 Distribution and interrelationships of bacterial-like PPP phosphatases
2.3.2.1 SLP phosphatases
After multiple sequence alignment and phylogenetic tree inferences using sequences from
Shewanella-like phosphatases (SLPs), a final depiction of SLP phosphatase molecular evolution
was obtained (Figure 2.3). Here, SLP representatives from numerous eukaryotic supergroups
(Opisthokont, Chromalveolate, Rhizaria, Excavates, Plantae) were identified. It is clear from
inspection of the sequence composition of this tree that organisms which are now photosynthetic
(Green and Red Algae, Plants, diverse Chromalveolates), or which are thought to have been
derived from photosynthetic ancestors (Apicomplexa, Oomycetes, possibly Euglenozoa),
predominate. Fungi are the only non-photosynthetic group represented in strength. A single
sequence was found in Apusozoa (an animal ally), but none in Animals. Thorough TBLASTN
searching failed to reveal any additional SLPs amongst previously unannotated sequences in
Animals, or any other eukaryotic group.
The previously described SLP1 and SLP2 forms of plants and their associated Green
Algae are seen here to represent the terminal, most derived aspect of a broad SLP radiation
which spreads across Eukaryotes (Figure 2.3). The SLP1 and SLP2 sequences have presumably
arisen by gene duplication and divergence from the deeper group of SLP sequences (which we
here term SLP3) which are present as a distinct lineage in Green Algae. At the base of the SLP
tree is a cluster of bacterial sequences from δ-Proteobacteria ("Outer Myxobacteria" sequences),
plus γ-Proteobacteria (including the genus Shewanella). Within the structure of the eukaryotic
SLP radiation itself is a second cluster of δ-proteobacterial sequences ("Inner Myxobacteria"
39
40
Figure 2.3: Orthogonal phylogenetic tree depicting SLP phosphatase distribution and
interrelationships across both Eukaryotes and Prokaryotes.
Phylogenetic tree inference was performed as outlined in the Materials and Methods. The most
crucial nodes are labelled. Branch support values with the four inference methods (PhyML
[aBayes]; RAxML [RBS]; MrBayes [PP]; PhyloBayes_MPI [PP]) are as follows (see Materials
and Methods for details). Node A: (0.998, 75, 0.86, 1.00); Node B: (0.995, 36, 0.80, 0.52); Node
C: (0.755, 58, 0.86, 0.52); Node D: (1.00, 80, 0.93, 1.00); Node E: (1.00, 100, 1.00, 1.00); Node
F: (0.999, 88, 0.93, 1.00). Branch support values for all trees are summarized as Appendix B.
Predicted in silico subcellular localizations are represented by Ch (chloroplast), Mt
(mitochondria), Cy (cytosol), Nu (nuclear), ER (endoplasmic reticulum), Px (peroxisome) and
SP (signal peptide). Gene identifiers for the sequences used in phylogenetic tree generation are
listed in Appendix A.1, while compiled in silico subcellular localization data can be found in
Table 2.1.
sequences). Intensive searching revealed only four Archaeal SLPs, all in the family
Halobacteriaceae. Finally, closely associated with the SLP sequence assemblage is a group of
sequences from α-Proteobacteria (Figure 2.3). In another, more basal cluster, are representative
distantly related sequences from a diverse group of bacterial phyla, including Cyanobacteria,
Bacteroidetes, and Actinobacteria.
Through the application of 10 different in silico localization programs, SLPs were found
to maintain differing consensus subcellular localizations in plants, with SLP1s being
chloroplastic and SLP2s being cytosolic (Table 2.1); however, the potential for tissue-specific
subcellular localization differences still exists. SLPs in the Green Algae associated with each of
these two groups show predicted localizations in accord with their related plant sequences. This
suggests that these differing protein isoform localizations may have been established early in
evolution, before the advent of land plants. Furthermore, it is interesting that in the group of
green algal sequences deeper in the tree ("SLP3 phosphatases"), the predominant predicted
localization is mitochondrial (Figure 2.3). This is also true of the sequences from other
photosynthetic organisms in the deeper SLP radiation and suggests that protein retargeting may
41
Table 2.1: In silico subcellular prediction data for eukaryotic SLP phosphatases used in
phylogenetic tree and alignment construction.
Prediction consensus is highlighted in yellow. Tree labels, gene identifiers, organism group
and organism information are also provided.
SLP Phosphatases
Predict ion Progr am Res ults
Predotar
Wolf pSORT
Target P
ChloroP
Tree
Designation
Gene Identifier
AlSLPa
470724|PACid:16040444
Streptophyta
Arabidopsis lyrata
0.01mTP 0.92cTP 0.01ER 0.08other
chlo: 6.0, cyto: 3.0, E.R.: 2.0, nucl: 1.0, mito: 1.0
0.929cTP 0.075mTP 0.031SP 0.073other RC=1
YES
AlSLPb
472067|PACid:16060211
Streptophyta
Arabidopsis lyrata
0.01mTP 0.00cTP 0.01ER 0.99other
cyto: 8.0, chlo: 4.0, nucl: 1.0
0.171cTP 0.071mTP 0.282SP 0.572other RC=4
NO
AcSLPa
Aquca_030_00216.1|PACid:22035664
Streptophyta
Aquilegia coerulea
0.02mTP 0.92cTP 0.00ER 0.07other
chlo: 12.0, mito: 1.0
0.916cTP 0.062mTP 0.020SP 0.072other RC=1
YES
AcSLPb
Aquca_030_00216.1|PACid:22035665
Streptophyta
Aquilegia coerulea
0.02mTP 0.92cTP 0.00ER 0.07other
chlo: 12.0, mito: 1.0
0.916cTP 0.062mTP 0.020SP 0.072other RC=1
YES
AcSLPc
Aquca_042_00158.1|PACid:22033007
Aquilegia coerulea
0.01mTP 0.01cTP 0.00ER 0.99other
nucl: 7.0, cysk: 6.0
Arabidopsis thaliana
AtSLP1
Organism Group
PredSL
SLP-Local
MitoProt
PCLR
iPSORT
mTP, No
cTP, No
mTP, cTP, No
No
cTP
mTP / cTP
nucleus or cytosol/ chloro
No
No
No
Cyto
0.999914 0.000215 0.00014
chloro / SP
mTP
cTP
mTP / cTP
Chloro
0.999914 0.000215 0.00014
chloro / SP
mTP
cTP
mTP / cTP
Chloro
0.666418 0 0.621623
nucleus or cytosol/ chloro
Consensus
mTP, cTP, ER, Cyto
At1g07010
Protein Prowler
Organism
Streptophyta
0.406TP 0.030mTP 0.114SP 0.567other RC=5
Yes/NO SP, mTP, cTP, other, PTS1
NO
0.00 0.01 0.98 0.01 0.00
cTP, mTP, SP, Other
0.999844 0 0.000418
chloro / SP
0.113195 0 0.092027 other
0.00 0.01 0.99 0.00 0.00
0.00 0.01 0.99 0.00 0.00
0.03 0.10 0.01 0.86 0.00
0.03 0.14 0.03 0.80 0.00
No
No
Chloro
No
Cyto
cTP
Chloro
0.01mTP 0.90cTP 0.05ER 0.10other
chlo: 5.0, nucl: 2.0, cyto: 2.0, E.R.: 2.0
0.816cTP 0.067mTP 0.039SP 0.155other RC=2
YES
0.00 0.03 0.94 0.03 0.00
0.999876 0.000122 0.000309
chloro / SP
AtSLP2
At1g18480
Streptophyta
Arabidopsis thaliana
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 8.0, nucl: 3.0, chlo: 2.0
0.152cTP 0.072mTP 0.277SP 0.443other RC=5
NO
0.03 0.12 0.02 0.84 0.00
0.510013 0 0.222184 other
nucleus or cytosol/ chloro
No
No
No
Cyto
BdSLPa
Bradi3g25370.2|PACid:21831821
Streptophyta
Brachypodium distachyon
0.11mTP 0.11cTP 0.95ER 0.04other
chlo: 13.0
0.751cTP 0.476mTP 0.104SP 0.006other RC=4
YES
0.51 0.11 0.32 0.06 0.00
0.512254 0.013118 0.579544
chloro / mito
mTP
cTP
cTP
Chloro
Bradi4g20750.1|PACid:21811426
Streptophyta
Brachypodium distachyon
0.022cTP 0.019mTP 0.257SP 0.438other RC=5
BdSLPb
Streptophyta
cTP, mTP, SP other
0.01mTP 0.13cTP 0.04ER 0.83other
cyto: 7.0, pero: 4.0, chlo: 2.0
Bra015544|PACid:22693638
Streptophyta
Brassica rapa
0.05mTP 0.12cTP 0.06ER 0.78other
nucl: 4.0, chlo_mito: 4.0, chlo: 3.5, mito: 3.5
BrSLPa
Bra025903|PACid:22696925
Streptophyta
Brassica rapa
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 10.0, mito: 2.0, pero: 1.0
0.120cTP 0.380mTP 0.029SP 0.690other RC=4
CpaSLPa
evm.model.supercontig_2.360|PACid:16412742
Streptophyta
Carica papaya
0.01mTP 0.34cTP 0.02ER 0.64other
chlo: 13.0
0.906cTP 0.032mTP 0.009SP 0.050other RC=1
CpaSLPb
evm.model.supercontig_9.374|PACid:16428284
Streptophyta
Carica papaya
0.01mTP 0.01cTP 0.00ER 0.99other
CpaSLPc
evm.model.supercontig_9.376|PACid:16428286
BrSLPb
0.727cTP 0.324mTP 0.005SP 0.016other RC=3
No
cTP
YES
0.33 0.15 0.03 0.49 0.11
YES
0.00 0.28 0.70 0.02 0.00
0.997418 0.00217 0.003871
chloro / mito
NO
0.01 0.09 0.00 0.89 0.00
0.004621 0.000792 0.012195 other
nucleus or cytosol / mito
No
No
No
Cyto
YES
0.00 0.01 0.99 0.00 0.00
0.999675 0.000306 0.00068
chloro / SP
mTP
cTP
cTP
Chloro
0.10 0.09 0.00 0.80 0.00
0.001743 0.000256 0.040843other
nucleus or cytosol / SP
0.15 0.12 0.02 0.71 0.00
0.000979 0.000309 0.0862 other
NO
0.592426 0.001214 0.060819
chloro / SP
No
mTP
No
cTP
No
cTP
mtp / cTP
No
No
Cyto
Chloro
cyto: 12.0, cysk: 1.0
0.018cTP 0.048mTP 0.782SP 0.554other RC=4
0.01mTP 0.00cTP 0.12ER 0.87other
cyto: 10.0, cysk: 3.0
0.142cTP 0.041mTP 0.333SP 0.475other RC=5
NO
nucleus or cytosol/ chloro
No
No
No
Cyto
CarSLPa
Carubv10012402m|PACid:20892489
Streptophyta
Capsella rubella
0.01mTP 0.82cTP 0.12ER 0.16other
chlo: 12.0, vacu: 1.5, E.R._vacu: 1.5
0.934cTP 0.065mTP 0.064SP 0.047other RC=1
YES
0.999885 0.000128 0.000278
chloro / SP
mTP
cTP
mTP / cTP
Chloro
CarSLPb
Carubv10017403m|PACid:20887668
Streptophyta
Capsella rubella
0.01mTP 0.00cTP 0.02ER 0.98other
cyto: 10.0, chlo: 2.0, nucl: 1.0
0.089cTP 0.070mTP 0.472SP 0.421other RC=5
NO
0.03 0.08 0.00 0.89 0.00
0.04467 0 0.087162 other
nucleus or cytosol/ chloro
No
No
No
Cyto
CcSLPb
clementine0.9_012529m|PACid:19282462
Streptophyta
Citrus clementina
0.06mTP 0.94cTP 0.01ER 0.06other
chlo: 14.0
0.947cTP 0.031mTP 0.010ER 0.100other RC=1
YES
0.00 0.01 0.99 0.00 0.00
0.999485 0.000595 0.000972
chloro / SP
mTP
cTP
mTP / cTP
Chloro
Streptophyta
Carica papaya
0.01 0.04 0.94 0.02 0.00
Cyto
CcSLPa
clementine0.9_028360m|PACid:19276388
Streptophyta
Citrus clementina
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
CsSLPb
orange1.1g016381m|PACid:18111291
Streptophyta
Citrus sinensis
0.06mTP 0.94cTP 0.01ER 0.05other
chlo: 14.0
0.955cTP 0.032mTP 0.011SP 0.099other RC=1
YES
0.00 0.01 0.99 0.00 0.00
0.999757 0.000391 0.000455
chloro / SP
mTP
cTP
mTP / cTP
Chloro
CsSLPa
orange1.1g041373m|PACid:18124504
Streptophyta
Citrus sinensis
0.01mTP 0.00cTP 0.08ER 0.92other
cyto: 12.0, nucl: 1.0
0.046cTP 0.087mTP 0.257SP 0.634other RC=4
NO
0.02 0.06 0.00 0.92 0.00
0.00987 0 0.612498
nucleus or cytosol/ chloro
No
No
No
Cyto
CrSLPa
Cre03.g185200.t1.1
Chlorophyta
Chlamydomonas reinhardtii
0.14mTP 0.02cTP 0.01ER 0.83other
chlo: 10.0, mito: 4.0
0.846cTP 0.738mTP 0.000SP 0.013other
YES
0.00 0.93 0.07 0.00 0.02
0.001124 0.999607 0.000346
mito / chloro
mTP
cTP
mTP / cTP
Chloro/Mito
Chlorophyta
Chlamydomonas reinhardtii
CrSLPb
Cre17.g718800.t1.1
0.041cTP 0.235mTP 0.250SP 0.689other RC=3
NO
0.05 0.11 0.00 0.83 0.00
0 0.82683 0.290684
nucleus or cytosol / mito
No
No
No
Cyto
CocSLPa
jgi|Coc_C169_1|30480|estExt_Genewise1Plus.C_140169
Chlorophyta
Coccomyxa sp.
0.15mTP 0.30cTP 0.01ER 0.59other
chlo: 10.0, mito: 4.0
0.763cTP 0.113mTP 0.006SP 0.085other
YES
0.01 0.85 0.12 0.02 0.00
0.000474 0.999785 0.000608
mito / chloro
mTP
cTP
mTP / cTP
Chloro/Mito
CocSLPb
jgi|Coc_C169_1|35854|fgenesh1_pm.4_#_256
Chlorophyta
Coccomyxa sp.
0.01mTP 0.00cTP 0.00ER 0.98other
mito: 5.5, chlo_mito: 4.5, cyto: 4.0
0.066cTP 0.420mTP 0.029SP 0.706other RC=4
NO
0.02 0.65 0.01 0.32 0.00
0 0.102375 0.017014 other
nucleus or cytosol / mito
No
No
No
Cyto
CsatSLPa
0.01mTP 0.00cTP 0.01ER 0.98other
cyto: 9.0, mito: 3.0, nucl: 1.0
Cucsa.151840.1|PACid:16964486
Streptophyta
Cucumis sativus
0.949cTP 0.037mTP 0.010SP 0.068other RC=1
YES
0.00 0.00 0.99 0.00 0.00
0.999885 0.000287 0.000173
chloro / mito
mTP
cTP
mTP / cTP
Chloro
CsatSLPb
Cucsa.151840.2|PACid:16964487
Streptophyta
Cucumis sativus
0.01mTP 0.96cTP 0.02ER 0.04other
chlo: 12.0, nucl: 1.0
0.949cTP 0.037mTP 0.010SP 0.068other RC=1
YES
0.00 0.00 0.99 0.00 0.00
0.999885 0.000287 0.000173
chloro / mito
mTP
cTP
mTP / cTP
Chloro
CsatSLPc
Cucsa.075800.1|PACid:16955776
Streptophyta
Cucumis sativus
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 7.0, chlo: 3.0, nucl: 2.0, cysk: 1.0
0.185cTP 0.055mTP 0.074SP 0.509other RC=4
NO
0.02 0.07 0.00 0.91 0.00
0.013107 0 0.246241 other
nucleus or cytosol / chloro
No
No
No
Cyto
CvSLPb
jgi|ChlNC64A_1|50727|fgenesh3_pg.C_scaffold_4000226
Chlorella variabilis
0.01mTP 0.05cTP 0.00ER 0.94other
chlo: 10.0, cyto: 4.0
0.721cTP 0.050mTP 0.047SP 0.274other RC=3
YES
0.05 0.26 0.31 0.38 0.00
0 0.998924 0.004293
CvSLPa
jgi|ChlNC64A_1|9678|gw1.24.110.1
Chlorophyta
Chlorophyta
Chlorella variabilis
0.01mTP 0.96cTP 0.02ER 0.04other
chlo: 12.0, nucl: 1.0
mito / chloro
mTP
cTP
cTP
Mito/Chloro
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
EgSLPa
Eucgr.B04000.1|PACid:23569453
Streptophyta
Eucalyptus grandis
0.02mTP 0.78cTP 0.04ER 0.21other
chlo: 10.0, vacu: 3.0
0.837cTP 0.089mTP 0.003SP 0.056other RC=2
YES
0.00 0.08 0.88 0.04 0.00
0 0.241401 other
nucleus or cytosol / chloro
mTP
cTP
mTP / cTP
Chloro / Mito
EgSLPb
Eucgr.B04000.2|PACid:23569454
Streptophyta
Eucalyptus grandis
0.02mTP 0.78cTP 0.04ER 0.21other
chlo: 10.0, vacu: 3.1
0.837cTP 0.089mTP 0.003SP 0.056other RC=3
YES
0.00 0.08 0.88 0.04 0.00
0 0.241401 other
nucleus or cytosol / chloro
mTP
cTP
mTP / cTP
Chloro / Mito
GrSLPa
Gorai.006G232000.1|PACid:26833651
Streptophyta
Gossypium raimondii
0.01mTP 0.01cTP 0.07ER 0.92other
extr: 4.0, chlo: 2.0, nucl: 2.0, cyto: 2.0
0.207cTP 0.098mTP 0.145SP 0.537other RC=4
NO
0.13 0.22 0.20 0.46 0.00
0.49021 0 0.517934 other
mito / chloro
No
No
No
Cyto
Streptophyta
Gossypium raimondii
0.00 0.01 0.98 0.00 0.00
GrSLPb
Gorai.013G271300.1|PACid:26789935
0.02mTP 0.52cTP 0.00ER 0.47other
chlo: 12.0, nucl: 1.0
0.886cTP 0.059mTP 0.044SP 0.156other RC=2
YES
chloro / SP
mTP
cTP
mTP / cTP
Chloro
GmSLPc
Glyma02g39710.1|PACid:26288354
Streptophyta
Glycine max
0.01mTP 0.87cTP 0.05ER 0.12other
mito: 6.0, chlo: 4.0, cyto: 2.0, nucl: 1.0
0.918cTP 0.143mTP 0.026SP 0.046other RC=2
YES
0.00 0.02 0.98 0.01 0.00
0.999915 0.000198 0.000159
chloro / SP
mTP
cTP
mTP / cTP
Chloro
GmSLPa
Glyma19g13655.1|PACid:26327286
Streptophyta
Glycine max
0.01mTP 0.01cTP 0.04ER 0.95other
cyto: 9.0, nucl: 2.0, cysk: 2.0
0.377cTP 0.028mTP 0.054SP 0.642other RC=4
NO
0.03 0.09 0.01 0.87 0.00
0.314858 0 0.392796 other
nucleus or cytosol / chloro
No
cTP
No
GmSLPd
Glyma19g13655.2|PACid:26327286
Streptophyta
Glycine max
0.01mTP 0.01cTP 0.04ER 0.95other
cyto: 9.0, nucl: 2.0, cysk: 2.0
0.377cTP 0.028mTP 0.054SP 0.642other RC=4
NO
0.03 0.09 0.01 0.87 0.00
0.314858 0 0.392796 other
nucleus or cytosol / chloro
No
cTP
No
Cyto
GmSLPb
Glyma14g37780.1|PACid:26287143
Streptophyta
Glycine max
0.01mTP 0.97cTP 0.03ER 0.03other
chlo: 14.0
0.964cTP 0.019mTP 0.057SP 0.032other RC=1
YES
0.00 0.01 0.99 0.00 0.00
0.999914 0.000171 0.000181
chloro / SP
mTP
cTP
mTP / cTP
Chloro
GmSLPe
Glyma16g07840.1|PACid:26351113
Streptophyta
Glycine max
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
LuSLPc
Lus10031284|PACid:23151137
Streptophyta
Linum usitatissimum
0.14mTP 0.46cTP 0.00ER 0.46other
chlo: 9.0, nucl: 1.0, mito: 1.0, vacu: 1.0, E.R.: 1.0
0.728cTP 0.102mTP 0.041SP 0.158other RC=3
YES
0.00 0.05 0.93 0.01 0.00
0.999835 0.000327 0.000265
chloro / SP
No
No
mTP / cTP
Chloro
LuSLPa
Lus10038865|PACid:23150278
Streptophyta
Linum usitatissimum
0.01mTP 0.00cTP 0.04ER 0.95other
nucl: 7.0, cyto: 4.0, cysk: 2.0
0.175cTP 0.066mTP 0.146SP 0.656other RC=3
NO
0.03 0.09 0.01 0.88 0.00
0.643592 0 0.4377
nucleus or cytosol / SP
No
No
No
Cyto / Nuc
LuSLPb
Lus10014985|PACid:23152752
Streptophyta
Linum usitatissimum
0.01mTP 0.01cTP 0.02ER 0.97other
nucl: 8.0, extr: 2.0, chlo: 1.0, cyto: 1.0, cysk: 1.0
0.087cTP 0.061mTP 0.161SP 0.695other RC=3
NO
0.02 0.07 0.00 0.91 0.00
0.560296 0 0.513057
nucleus or cytosol/ chloro
No
No
No
Cyto / Nuc
MdpSLPa
MDP0000769617|PACid:22639836
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 8.0, nucl: 2.0, E.R.: 2.0, mito: 1.0
0.748cTP 0.018mTP 0.153SP 0.434other RC=4
YES
No
Cyto / Chloro
MdpSLPb
MDP0000465067|PACid:22671458
Streptophyta
Malus domestica
Streptophyta
Malus domestica
0.01mTP 0.00cTP 0.00ER 0.99other
0.06 0.24 0.21 0.50 0.00
0.999843 0 0.0004
0.045992 0.241069 0.015069other
chloro / SP
No
No
Cyto
cyto: 9.0, E.R.: 3.0, nucl: 1.0
0.395cTP 0.029mTP 0.061SP 0.823other RC=3
NO
0.03 0.16 0.05 0.75 0.00
0.020244 0.000857 0.806373
chloro / nucleus or cytosol
No
No
No
Cyto
MdpSLPc
MDP0000899519|PACid:22657341
Streptophyta
Malus domestica
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 9.0, E.R.: 3.0, nucl: 1.0
0.395cTP 0.029mTP 0.061SP 0.823other RC=3
NO
0.03 0.16 0.05 0.75 0.00
0.020244 0.000857 0.806373
chloro / nucleus or cytosol
No
No
No
Cyto
MdpSLPd
MDP0000941407|PACid:22628975
Streptophyta
Malus domestica
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 5.0, nucl: 3.0, extr: 3.0, cysk: 3.0
0.081cTP 0.132mTP 0.140SP 0.852other RC=2
NO
0.01 0.05 0.00 0.93 0.00
0.000143 0.002346 0.060857 other
nucleus or cytosol / SP
No
No
No
Cyto
MdpSLPe
MDP0000244675|PACid:22655284
Streptophyta
Malus domestica
0.01mTP 0.50cTP 0.08ER 0.45other
cyto: 9.0, nucl: 3.0, cysk: 1.0
0.209cTP 0.103mTP 0.171SP 0.633other RC=3
NO
0.09 0.11 0.00 0.80 0.00
0.010932 0 0.78466
nucleus or cytosol / SP
No
No
No
Cyto
chloro / SP
mTP
cTP
mTP / cTP
Chloro
MeSLPb
cassava4.1_009955m|PACid:17994174
Streptophyta
Manihot esculenta
0.03mTP 0.96cTP 0.02ER 0.04other
chlo: 13.5, chlo_mito: 7.5
0.972cTP 0.116mTP 0.025SP 0.009other RC=1
YES
0.00 0.01 0.99 0.00 0.00
MeSLPa
cassava4.1_008887m|PACid:17972799
Streptophyta
Manihot esculenta
0.01mTP 0.00cTP 0.02ER 0.97other
cyto: 7.0, cysk: 3.0, chlo: 2.0, nucl: 2.0
0.104cTP 0.128mTP 0.114SP 0.818other RC=2
NO
0.01 0.05 0.00 0.93 0.00
0.066881 0 0.412251 other
nucleus or cytosol / mito
No
No
No
MgSLPa
mgv1a007295m|PACid:17681918
Streptophyta
Mimulus guttatus
0.01mTP 0.92cTP 0.01ER 0.08other
chlo: 12.0, cyto: 1.0
0.592cTP 0.188mTP 0.004SP 0.058other RC=3
YES
0.01 0.26 0.63 0.09 0.00
0.859418 0.000801 0.208313
nucleus or cytosol/ chloro
mTP
cTP
mTP / cTP
Chloro
MgSLPb
mgv1a007297m|PACid:17681917
Streptophyta
Mimulus guttatus
0.01mTP 0.92cTP 0.01ER 0.08other
chlo: 12.0, cyto: 1.0
0.592cTP 0.188mTP 0.004SP 0.058other RC=3
YES
0.01 0.26 0.63 0.09 0.00
0.859418 0.000801 0.208313
nucleus or cytosol/ chloro
mTP
cTP
mTP / cTP
Chloro
mgv1a007897m|PACid:17678561
Streptophyta
NO
MgSLPc
Mimulus guttatus
0.01mTP 0.00cTP 0.01ER 0.99other
0.07 0.11 0.01 0.80 0.00
0.622945 0 0.613167
SP / chloro
Micromonas pusilla
0.01mTP 0.00cTP 0.05ER 0.95other
cyto: 11.0, pero: 2.0
0.039cTP 0.085mTP 0.126SP 0.928other RC=1
NO
0.02 0.09 0.00 0.89 0.00
0.000101 0.002354 0.428438other
chloro / nucleus or cytosol
jgi|MicpuC3|187449|gm1.4570_g
Chlorophyta
Micromonas pusilla
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 10.0, chlo: 2.0, pero: 2.0
0.039cTP 0.085mTP 0.126SP 0.928other RC=1
NO
0.01 0.05 0.00 0.93 0.00
0 0.015426 0.024612 other
nucleus or cytosol / SP
No
No
No
Cyto
MpSLPc
jgi|MicpuC3|60158|MicpuC2.EuGene.0000080352
Chlorophyta
Micromonas pusilla
0.91mTP 0.04cTP 0.02ER 0.08other
chlo: 12.0, mito: 2.0
0.555cTP 0.735mTP 0.001SP 0.015other RC=5
YES
0.00 0.98 0.02 0.00 0.94
0.002625 0.999423 0.000124
chloro / mito
mTP
cTP
mTP / cTP
Chloro/Mito
MpSLPd
jgi|MicpuN3|62511|EuGene.1100010297
Chlorophyta
Micromonas pusilla
0.92mTP 0.04cTP 0.05ER 0.07other
chlo: 7.0, mito: 7.0
0.099cTP 0.959mTP 0.013SP 0.006other
YES
0.00 0.99 0.00 0.00 0.26
0.001919 0.99919 0.000532
mito / chloro
mTP
cTP
mTP / cTP
Mito/Chloro
MpSLPe
jgi|MicpuC3|152093|e_gw1.3.1360.1
jgi|MicpuN3|69358|gw2.05.320.1
Chlorophyta
Chlorophyta
Micromonas pusilla
**partial sequence
0.130cTP 0.028mTP 0.275SP 0.642other RC=4
xxx
xxx
xxx
xxx
xxx
xxx
No
No
xxx
No
No
xxx
No
Cyto
MpSLPb
MpSLPa
cyto: 8.0, chlo: 3.0, nucl: 3.0
0.999827 0.00018 0.000406
No
xxx
Cyto
Cyto
xxx
MpSLPf
jgi|MicpuN3|69434|gw2.13.202.1
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
MtSLPb
Medtr5g081100.1|PACid:17467021
Streptophyta
Medicago truncatula
0.01mTP 0.96cTP 0.01ER 0.04other
chlo: 13.5, chlo_mito: 7.5
0.955cTP 0.065mTP 0.016SP 0.035other RC=1
YES
0.00 0.00 0.99 0.00 0.00
0.999925 0.000181 0.000159
chloro / nucleus or cytosol
mTP
cTP
mTP / cTP
Chloro
MtSLPa
Medtr7g005770.1|PACid:17472861
Streptophyta
Medicago truncatula
0.01mTP 0.01cTP 0.14ER 0.84other
cyto:
y
9.0,, nucl: 2.0,, chlo: 1.0,, cysk:
y
1.0
0.195cTP 0.044mTP 0.062SP 0.503other RC=4
YES
0.03 0.12 0.02 0.82 0.00
0.610576 0 0.66658
chloro / nucleus or cytosol
No
cTP
No
y / Chloro
Cyto
OlSLPc
jgi|Ost9901_3|3839|gwEuk.4.274.1
Chlorophyta
Ostreococcus lucimarinus
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
jgi|Ost9901_3|38815|e_gwEuk.5.237.1
Chlorophyta
Micromonas pusilla
**partial sequence
xxx
Chlorophyta
Ostreococcus lucimarinus
0.03mTP 0.00cTP 0.01ER 0.96other
cyto: 7.0, pero: 4.0, mito: 2.0
0.185cTP 0.055mTP 0.074SP 0.509other RC=4
NO
0.03 0.31 0.01 0.65 0.00
0.000565 0.60464 0.001904
nucleus or cytosol/ chloro
No
OlSLPa
jgi|Ost9901_3|89674|ost_20_004_020
Chlorophyta
Ostreococcus lucimarinus
0.23mTP 0.35cTP 0.04ER 0.48other
chlo: 9.5, chlo_mito: 7.5, mito: 4.5
0.740cTP 0.621mTP 0.013SP 0.009other RC=5
YES
0.01 0.36 0.62 0.01 0.00
0.988133 0.009078 0.012316
mito / chloro
mTP
cTP
cTP
Chloro/Mito
OrcSLPa
jgi|OstRCC809_2|31427|e_gw1.4.298.1
Chlorophyta
Ostreococcus RCC809
0.20mTP 0.28cTP 0.02ER 0.56other
chlo: 9.5, chlo_mito: 6.0, pero: 3.0
0.792cTP 0.569mTP 0.007SP 0.016other RC=4
YES
0.00 0.17 0.82 0.00 0.00
0.003046 0.99932 0.000136
mito / chloro
mTP
cTP
mTP / cTP
Chloro/Mito
Chlorophyta
Ostreococcus RCC809
0.43mTP 0.21cTP 0.05ER 0.43other
0.764cTP 0.514mTP 0.005SP 0.008other RC=4
YES
0.00 0.28 0.71 0.00 0.00
0.068505 0.906441 0.005721
Chlorophyta
Ostreococcus RCC809
0.34mTP 0.00cTP 0.01ER 0.65other
OlSLPb
OrcSLPb
jgi|OstRCC809_2|43148|e_gw1.20.557.1
OrcSLPc
jgi|OstRCC809_2|57133|fgenesh1_pm.chr_5_#_202
chlo: 9.0, mito: 5.0
chlo: 9.0, mito: 3.0, plas: 1.0
0.214cTP 0.140mTP 0.008SP 0.796other RC=3
mito / chloro
mTP
No
cTP
No
xxx
cTP
Cyto
Chloro/Mito
No
0.02 0.89 0.00 0.09 0.00
0.012245 0.996921 0
nucleus or cytosol / SP
No
cTP
No
OsSLPa
Os10g25430.1
Streptophyta
Oryza sativa
0.33mTP 0.49cTP 0.03ER 0.33other
chlo: 14.0
0.831cTP 0.142mTP 0.010SP 0.021other RC=2
YES
0.01 0.11 0.85 0.03 0.00
0.603789 0.069789 0.002153
chloro / SP
mTP
cTP
mTP / cTP
Chloro
OsSLPb
Os11g15570.1
Streptophyta
Oryza sativa
0.01mTP 0.07cTP 0.00ER 0.92other
chlo: 7.0, cyto: 5.0, pero: 2.0
0.176cTP 0.039mTP 0.155SP 0.394other RC=4
YES
0.10 0.20 0.07 0.63 0.00
0.994859 0.000133 0.005704
chloro / nucleus or cytosol
No
cTP
No
Cyto/Chloro
OtSLPc
jgi|Ostta4|32574|0400010520
Chlorophyta
Ostreococcus tauri
0.01mTP 0.00cTP 0.00ER 0.99other
chlo: 6.0, nucl: 3.0, mito: 2.0, cyto: 1.0, pero: 1.0
0.513cTP 0.109mTP 0.005ER 0.482other RC=5
NO
0.03 0.24 0.11 0.62 0.65
0.005741 0.000825 0.007086other
chloro / nucleus or cytosol
No
No
No
Chloro/Cyto
Cyto/Mito
OtSLPb
jgi|Ostta4|21075|e_gw1.14.00.151.1
Chlorophyta
Ostreococcus tauri
0.02mTP 0.00cTP 0.04ER 0.94other
cyto: 6.0, chlo: 4.0, mito: 4.0
0.005cTP 0.626mTP 0.354SP 0.136other RC=4
NO
0.02 0.88 0.00 0.10 0.00
0 0.942125 0.045213
nucleus or cytosol / mito
mTP
No
No
Mito/Cyto
jgi|Ostta4|3633|gw1.05.00.226.1
Chlorophyta
Ostreococcus tauri
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
PvSLPa
Pavirv00009175m|PACid:23776527
Streptophyta
Panicum virgatum
0.40mTP 0.06cTP 0.24ER 0.43other
0.603cTP 0.522mTP 0.004SP 0.023other RC=5
YES
0.01 0.60 0.38 0.02 0.00
0.363239 0.88343 0.000139
mito / chloro
mTP
cTP
mTP / cTP
Chloro / Mito
PvSLPb
Pavirv00009176m|PACid:23776528
Streptophyta
Panicum virgatum
0.40mTP 0.06cTP 0.24ER 0.43other
chlo: 12.0, plas: 1.0
0.603cTP 0.522mTP 0.004SP 0.023other RC=5
YES
0.01 0.60 0.38 0.02 0.00
0.363239 0.88343 0.000139
mito / chloro
mTP
cTP
mTP / cTP
Chloro / Mito
PvSLPc
Pavirv00010440m|PACid:23782081
Streptophyta
Panicum virgatum
0.44mTP 0.23cTP 0.11ER 0.39other
chlo: 12.5, chlo_mito: 7.3
0.702cTP 0.525mTP 0.004SP 0.011other RC=5
YES
0.00 0.61 0.38 0.01 0.00
0.790952 0.525919 0.000136
mito / chloro
mTP
cTP
mTP
Chloro / Mito
Panicum virgatum
0.01mTP 0.40cTP 0.00ER 0.59other
cyto: 7.0, chlo: 3.0, pero: 2.0, plas: 1.0
Chloro / Cyto
OtSLPd
xxx
chlo: 12.0, plas: 1.0
xxx
PvSLPd
Pavirv00011040m|PACid:23782673
Streptophyta
0.479cTP 0.012mTP 0.114SP 0.189other RC=4
YES
0.11 0.21 0.40 0.29 0.00
0.563268 0.003873 0.184248
chloro / SP
No
cTP
No
PvulSLPb
Phvul.008G256500.1|PACid:27155250
Streptophyta
Phaseolus vulgaris
0.01mTP 0.86cTP 0.03ER 0.13other
chlo: 13.0
0.910cTP 0.123mTP 0.049SP 0.069other RC=2
YES
0.00 0.02 0.96 0.02 0.00
0.999922 0.000177 0.00017
chloro / SP
mTP
cTP
mTP / cTP
PvulSLPa
Phvul.005G085100.1|PACid:27150157
Streptophyta
Phaseolus vulgaris
0.01mTP 0.01cTP 0.06ER 0.93other
cyto: 5.0, chlo: 4.0, nucl: 4.0
0.388cTP 0.041mTP 0.033SP 0.765other RC=4
NO
0.03 0.10 0.01 0.87 0.00
0.477711 0 0.058615 other
nucleus or cytosol/ chloro
No
cTP
No
Cyto
PpaSLPa
Pp1s194_144V6.1|PACid:18058638
Streptophyta
Physcomitrella patens
0.01mTP 0.04cTP 0.00ER 0.95other
cyto: 7.0, E.R.: 3.5, E.R._plas: 2.5, chlo: 1.0
0.202cTP 0.211mTP 0.009SP 0.476other
YES
0.50 0.20 0.28 0.03 0.00
0.997764 0.002009 0
chloro / nucleus or cytosol
No
cTP
cTP
Chloro/Cyto
PpaSLPc
Pp1s412_38V6.1|PACid:18061202
Streptophyta
Physcomitrella patens
0.01mTP 0.04cTP 0.00ER 0.95other
cyto: 7.0, E.R.: 3.5, E.R._plas: 2.5, chlo: 1.0
0.202cTP 0.211mTP 0.009SP 0.476other
YES
0.50 0.20 0.28 0.03 0.00
0.997764 0.002009 0
chloro / nucleus or cytosol
No
cTP
cTP
Chloro/Cyto
Pp1s98 64V6 1|PACid:18070451
Pp1s98_64V6.1|PACid:18070451
Streptophyta
Physcomitrella patens
0 01mTP 0.00cTP
0.01mTP
0 00cTP 0.01ER
0 01ER 0.99other
0 99other
PpaSLPb
0 118cTP 0.153mTP
0.118cTP
0 153mTP 0.080SP
0 080SP 0.863other
0 863other RC=2
NO
0 02 0.08
0.02
0 08 0.00
0 00 0.90
0 90 0.00
0 00
0 0.060762
0 060762 0.458938
0 458938 other
ppa025609m|PACid:17645981
Streptophyta
Prunus persica
0.01mTP 0.94cTP 0.00ER 0.06other
chlo: 13.0
0.948cTP 0.053mTP 0.030SP 0.078other RC=1
YES
0.00 0.02 0.97 0.01 0.00
0.999922 0.000189 0.000164
chloro / SP
PperSLPa
ppa007078m|PACid:17667068
Streptophyta
Prunus persica
0.01mTP 0.01cTP 0.00ER 0.98other
cyto: 8.0, nucl: 2.0, cysk: 2.0, chlo: 1.0
0.394cTP 0.082mTP 0.108SP 0.795other RC=3
NO
0.01 0.06 0.00 0.92 0.00
0.009578 0 0.123285 other
nucleus or cytosol/ chloro
No
No
No
Cyto
PtSLPa
Potri.009G077900.1|PACid:26987030
Streptophyta
Populus trichocarpa
0.03mTP 0.81cTP 0.03ER 0.18other
chlo: 13.0
0.904cTP 0.158mTP 0.039SP 0.026other RC=2
YES
0.00 0.02 0.98 0.01 0.00
0.999738 0.000361 0.000527
chloro / mito
mTP
cTP
cTP
Chloro
PtSLPb
Potri.009G077900.2|PACid:26987029
Streptophyta
Populus trichocarpa
0.03mTP 0.81cTP 0.03ER 0.18other
chlo: 13.0
0.904cTP 0.158mTP 0.039SP 0.026other RC=2
YES
0.00 0.02 0.98 0.01 0.00
0.999738 0.000361 0.000527
chloro / mito
mTP
cTP
cTP
Chloro
PtSLPc
Potri.009G077900.3|PACid:26987029
Streptophyta
Populus trichocarpa
0.03mTP 0.81cTP 0.03ER 0.18other
chlo: 13.0
0.904cTP 0.158mTP 0.039SP 0.026other RC=2
YES
0.00 0.02 0.98 0.01 0.00
0.999738 0.000361 0.000527
chloro / mito
mTP
cTP
cTP
Chloro
PtSLPd
Potri.009G077900.4|PACid:26987031
mTP
PtSLPe
PperSLPb
E R : 4.5,
E.R.:
4 5 cyto: 4.0
4 0, E.R._vacu:
E R vacu: 3.5
35
nucleus or cytosol / SP
No
Chloro
mTP
No
No
Cyto
cTP
mTP / cTP
Chloro
Streptophyta
Populus trichocarpa
0.03mTP 0.81cTP 0.03ER 0.18other
chlo: 13.0
0.904cTP 0.158mTP 0.039SP 0.026other RC=2
0.00 0.02 0.98 0.01 0.00
0.999738 0.000361 0.000527
mito / chloro
cTP
cTP
Potri.013G127500.1|PACid:26995499
Streptophyta
Populus trichocarpa
0.01mTP 0.00cTP 0.05ER 0.95other
cyto: 10.0, nucl: 2.0, golg: 1.0
0.125cTP 0.083mTP 0.115SP 0.702other RC=3
NO
0.01 0.06 0.00 0.92 0.00
0.00149 0.0004 0.018489 other
nucleus or cytosol / mito
No
No
No
Cyto
RcSLPb
30128.m008860|PACid:16818446
Streptophyta
Ricinus communis
0.01mTP 0.01cTP 0.99ER 0.01other
chlo: 11.0, nucl: 1.0, plas: 1.0
0.108cTP 0.008mTP 0.920SP 0.004other RC=1
YES
0.99 0.01 0.00 0.00 0.00
0.015698 0.0001 0.992733
SP / chloro
mTP
cTP
No
SP / Chloro
RcSLPa
29747.m001066|PACid:16807732
Streptophyta
Ricinus communis
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 9.0, nucl: 2.0, cysk: 2.0
0.051cTP 0.121mTP 0.302SP 0.794other RC=3
NO
0.01 0.06 0.00 0.93 0.00
0.011124 0 0.28284 other
nucleus or cytosol / SP
No
No
No
Cyto
SbSLPb
Sb05g006560.1|PACid:1969658
Streptophyta
Sorghum bicolor
0.01mTP 0.41cTP 0.07ER 0.54other
chlo: 7.0, cyto: 6.0
0.552cTP 0.018mTP 0.090SP 0.155other RC=4
YES
0.07 0.18 0.48 0.27 0.00
0.989283 0.000106 0.015257
chloro / nucleus or cytosol
No
cTP
No
Chloro
SbSLPa
Sb01g023200.1|PACid:1952078
Streptophyta
Sorghum bicolor
0.32mTP 0.02cTP 0.62ER 0.25other
chlo: 9.0, mito: 1.5, cyto_mito: 1.5, cyto: 1.0
0.515cTP 0.691mTP 0.009SP 0.020other RC=5
YES
0.02 0.73 0.24 0.02 0.00
0.14299 0.965856 0.000114
mito / chloro
mTP
cTP
mTP
Mito / Chloro
YES
Chloro
SiSLPb
Si026407m|PACid:19708378
Streptophyta
Setaria italica
0.01mTP 0.34cTP 0.02ER 0.64other
cyto: 6.0, chlo: 3.0, pero: 3.0, plas: 1.0
0.338cTP 0.007mTP 0.183SP 0.217other RC=5
YES
0.11 0.19 0.39 0.32 0.04
0.973411 0 0.070853
chloro / SP
No
Chloro
SiSLPa
Si036062m|PACid:19679889
Streptophyta
Setaria italica
0.33mTP 0.07cTP 0.28ER 0.45other
chlo: 9.0, mito: 2.5, cyto_mito: 2.0, plas: 1.0
0.436cTP 0.581mTP 0.005SP 0.027other RC=5
YES
0.04 0.68 0.25 0.02 0.00
0.101303 0.955894 0.000527
mito / chloro
mTP
cTP
mTP
Mito / Chloro
SmSLPa
123773|PACid:15403392
Streptophyta
Selaginella moellendorffii
0.01mTP 0.06cTP 0.26ER 0.69other
chlo: 14.0
0.102cTP 0.298mTP 0.036SP 0.085other RC=5
YES
0.05 0.77 0.13 0.05 0.00
0.892789 0.511599 0
mito / SP
mTP
cTP
mTP / cTP
Chloro/Mito
SmSLPb
65599|PACid:15406428
Streptophyta
Selaginella moellendorffii
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
ThalSLPb
Thhalv10009773m|PACid:20186509
Streptophyta
Thellungiella halophila
**partial sequence
xxx
ThalSLPa
Thhalv10007840m|PACid:20186790
Streptophyta
Thellungiella halophila
0.01mTP 0.00cTP 0.01ER 0.98other
chlo: 5.0, cyto: 5.0, nucl: 1.0, E.R.: 1.0, cysk: 1.0
0.091cTP 0.079mTP 0.418SP 0.469other RC=5
NO
0.04 0.10 0.01 0.86 0.00
VcSLPb
Vocar20007918m|PACid:23138954
Chlorophyta
Volvox carteri
0.01mTP 0.01cTP 0.97ER 0.03other
chlo: 12.0, mito: 2.0
0.404cTP 0.120mTP 0.319SP 0.004other RC=5
YES
0.28 0.46 0.08 0.18 0.00
Vocar20010810m|PACid:23129267
0.050cTP 0.233mTP 0.077SP 0.893other RC=2
GSVIVT01035133001|PACid 17840382
GSVIVT01035133001|PACid:17840382
VcSLPa
xxx
xxx
Chlorophyta
Volvox carteri
0.01mTP 0.00cTP 0.02ER 0.97other
cyto: 4.0, nucl: 3.0, chlo: 2.0, mito: 2.0
Streptophyta
Vitis vinifera
0.01mTP 0.04cTP 0.00ER 0.95other
mito: 6.5, cyto_mito: 4.0, chlo: 2.0, nucl: 2.0
VvSLPb
GSVIVT01024637001|PACid:17832792
Streptophyta
Vitis vinifera
0.09mTP 0.14cTP 0.07ER 0.72other
chlo: 14.0
0.702cTP 0.269mTP 0.010SP 0.030 RC=3
YES
ZmSLPa
GRMZM2G155058_T01|PACid:20861573
Streptophyta
Zea mays
0.01mTP 0.61cTP 0.01ER 0.38other
cyto: 9.0, chlo: 3.0, pero: 2.0
0.435cTP 0.009mTP 0.148SP 0.131other RC=4
YES
VvSLPa
0.201cTP 0.145mTP 0.017SP 0.608other RC=3
NO
NO
xxx
0.01 0.06 0.00 0.92 0.00
0.03 0.17 0.04 0.76 0.00
0.00 0.19 0.80 0.01 0.00
0.07 0.19 0.48 0.25 0.00
xxx
0.001589 0.000436 0.020343 other
0.043505 0.000249 0.980277
xxx
No
xxx
cTP
xxx
xxx
xxx
nucleus or cytosol/ chloro
No
No
No
Cyto
SP / mito
mTP
cTP
No
Chloro/Mito
0 0.127076 0.095335 other
nucleus or cytosol/ chloro
No
Cyto
0.945737 0 0.149592
chloro / nucleus or cytosol
No
No
No
Cyto / Chloro
0.132973 0.948294 0.000409
chloro / mito
mTP
cTP
mTP
Chloro / Mito
chloro / SP
No
cTP
No
Chloro
0.986554 0 0.03539
No
No
xxx
GRMZM2G125140_T01|PACid:20871059
Streptophyta
Zea mays
0.42mTP 0.18cTP 0.28ER 0.34other
chlo: 12.0, cyto: 1.0
0.752cTP 0.721mTP 0.004SP 0.011other RC=5
YES
0.01 0.38 0.61 0.01 0.00
0.355635 0.907809 0
mito / chloro
mTP
cTP
mTP
Chloro / Mito
ZmSLPc
GRMZM2G306348_T01|PACid:20872112
Streptophyta
Zea mays
0.19mTP 0.36cTP 0.01ER 0.51other
nucl: 6.0, chlo: 4.0, mito: 2.0, cysk: 1.0
0.169cTP 0.638mTP 0.002 0.135 RC=3
No
0.01 0.78 0.14 0.07 0.00
0 0.898969 0.144492
mito / nucleus or cytosol
mTP
cTP
mTP
Mito / Cyto
ZmSLPd
GRMZM2G459370_T01|PACid:20873602
Streptophyta
Zea mays
0.12mTP 0.02cTP 0.09ER 0.78other
chlo: 9.0, E.R.: 2.0, cyto: 1.0, mito: 1.0
0.051cTP 0.880mTP 0.023SP 0.213other RC=2
No
0.09 0.69 0.02 0.21 0.00
0 0.951541 0.223712
nucleus or cytosol / SP
mTP
No
mTP / cTP
ZmSLPe
GRMZM2G364863_T01|PACid:20852560
Streptophyta
Zea mays
0.01mTP 0.77cTP 0.00ER 0.23other
chlo: 12.0, mito: 1.0
0.969cTP 0.022mTP 0.016SP 0.059other RC=1
YES
0.00 0.01 0.99 0.00 0.00
0.999886 0.000198 0.000157
chloro / nucleus or cytosol
mTP
cTP
mTP / cTP
Chloro
ZmSLPf
GRMZM2G313253_T02|PACid:20822007
Streptophyta
Zea mays
0.26mTP 0.00cTP 0.02ER 0.73other
cyto: 6.0, chlo: 5.0, vacu: 2.0
0.056cTP 0.905mTP 0.018SP 0.232other RC=2
No
0.01 0.91 0.00 0.07 0.00
0.00028 0.999636 0.001091
mito / nucleus or cytosol
mTP
No
No
Mito/Cyto
ZmSLPg
GRMZM2G313253_T02|PACid:20822006
Streptophyta
Zea mays
0.26mTP 0.00cTP 0.02ER 0.73other
cyto: 6.0, chlo: 5.0, vacu: 2.0
0.056cTP 0.905mTP 0.018SP 0.232other RC=2
No
0.01 0.91 0.00 0.07 0.00
0.00028 0.999636 0.001091
mito / nucleus or cytosol
mTP
No
No
Mito/Cyto
Chlamydomonas reinhardtii
0.22mTP 0.01cTP 0.09ER 0.70other
ZmSLPb
Mito/Cyto
Green Algae
CrSLPc
Cre02.g144900.t1.3|PACid:26900638
Chlorophyta
chlo: 6.0, cyto: 6.0, nucl: 1.0
0.022cTP 0.507mTP 0.075SP 0.364other RC=5
0.000344 0.999784 0.000839
mito / SP
No
No
mTP
chlo: 6.0, plas: 5.0, E.R.: 2.0
0.005cTP 0.295mTP 0.006SP 0.866other RC=3
No
0.11 0.65 0.02 0.22 0.00
0 0.969472 0.138575
mito / nucleus or cytosol
mTP
No
mTP / cTP
Mito / Cyto
0.202cTP 0.891mTP 0.020SP 0.047other RC=2
YES
0.25 0.51 0.12 0.05 0.00
0.032756 0.765469 0.176738
mito / chloro
mTP
No
mTP
Mito / Chloro
0.001cTP 0.822mTP 0.446SP 0.005other RC=4
NO
No
0.91 0.08 0.00 0.00 0.00
0.033852 0.006602 0.913536
mito / SP
mTP
No
No
Mito / Cyto
NO
0.03 0.34 0.01 0.62 0.00
0.000303 0.999775 0.000972
mito / nucleus or cytosol
No
No
No
Cyto / Mito
NO
0.02 0.11 0.00 0.87 0.00
0.001143 0.742107 0.000557
nucleus or cytosol / mito
No
No
No
Cyto / Mito
NO
0.00 0.97 0.00 0.02 0.00
0.000354 0.999805 0.000814
mito / nucleus or cytosol
mTP
No
mTP
Mito
0.03 0.93 0.01 0.03 0.00
0.000401 0.999761 0.000983
chloro / mito
mTP
No
mTP
Mito/Chloro
No
mTP / cTP
VcSLPc
fgenesh4_pg.C_70252
Volvox carteri
0.32mTP 0.00cTP 0.04ER 0.66other
MpSLPg
jgi|MicpuN3|99756|fgenesh2_pg.C_Chr_03000537
Chlorophyta
Micromonas pusilla
0.08mTP 0.03cTP 0.10ER 0.81other
chlo: 10.0, mito: 3.0
MpSLPh
jgi|MicpuC3|54428|MicpuC2.EuGene.0000170274
Chlorophyta
Micromonas pusilla CCMP1545
0.22mTP 0.00cTP 0.99ER 0.01other
mito: 4.0, vacu: 4.0, chlo: 3.0, nucl: 1.0
OlSLPd
jgi|Ost9901_3|28347|eugene.1700010140
Chlorophyta
treococcus lucimarinus CCE99
0.14mTP 0.00cTP 0.02ER 0.84other
cyto: 7.0, chlo: 4.0, pero: 3.0
0.035cTP 0.831mTP 0.015SP 0.313other RC=3
OtSLPa
jgi|Ostta4|14383|fgenesh1_pg.C_Chr_18.0001000104
Chlorophyta
Ostreococcus tauri
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 11.0, chlo: 1.0, nucl: 1.0
0.255cTP 0.148mTP 0.008SP 0.791other RC=3
OrcSLPd
jgi|OstRCC809_2|60729|fgenesh1_pm.chr_17_#_106
Chlorophyta
Ostreococcus RCC809
0.32mTP 0.00cTP 0.17ER 0.56other
chlo: 10.0, mito: 4.0
0.055cTP 0.930mTP 0.012SP 0.107other RC=1
Stramenopiles
Aureococcus anophagefferens
0.22mTP 0.01cTP 0.44ER 0.43 other
12.0 chloro 2.0 mito
0.33cTP,0.912mTP,0.005SP 0.000other RC=3
YES
0.138cTP,0.257mTP,0.011SP,0.540other RC=4
Chlorophyta
0.02 0.87 0.01 0.10 0.00
Mito / Cyto
Red & Brown Algae
AaSLPa
jgi|Auran1|18391
CMJ015C
Rhodophyta
Cyanidioschyzon merolae
0.01mTP 0.28cTP 0.02ER 0.69other
cyto: 9.0, pero: 3.0, chlo: 2.0
NO
0.03 0.74 0.02 0.21 0.00
0.024699 0.964272 0
mito / chloro
No
CpSLP
Contig7282
Glaucocystophyceae
Cyanophora paradoxa
0.01mTP 0.00cTP 0.00ER 0.98other
cyto: 8.0, chlo: 3.0, mito: 3.0
0.045cTP 0.681mTP 0.015SP 0.575other RC=5
NO
0.02 0.79 0.03 0.16 0.00
0 0.392988 0.011258 other
mito / nucleus or cytosol
mTP
No
No
Mito/Cyto
EsSLPa
Esi0089_0101
Stramenopiles
Ectocarpus silicuosus
0.82mTP 0.01cTP 0.57ER 0.08 other
10.0 chloro, 4.0mito
0.188cTP,0.789mTP,0.010SP,0.006other RC=2
YES
0.01 0.98 0.01 0.00 0.00
0.003188 0.99818 0.000906
mito / chloro
mTP
cTP
mTP
Mito/Chloro
CmSLP
42
Mito/Cyto
EsSLPb
Esi0387_0013
Stramenopiles
Ectocarpus silicuosus
0.09mTP 0.00cTP 0.00ER 0.90 other
8.5cyto, 3.0 chloro, 2.0nuc
nucleus or cytosol / SP
No
No
EhSLPa
jgi|Emihu1|107003|fgeneshEH_pg.86__79
Stramenopiles
Emiliania huxleyi CCMP1516
0.04mTP 0.01cTP 0.99ER 0.01other
vacu: 4.0, chlo: 3.0, cyto: 3.0, E.R.: 3.0
0.006cTP,0.357mTP,0.688SP,0.207other RC=4
NO
0.99 0.00 0.00 0.01 0.00
0 0.993843 0.027233
SP / mito
mTP
No
No
SP/Mito
EhSLPb
jgi|Emihu1|220501|gm1.9100138
Stramenopiles
Emiliania huxleyi CCMP1517
0.19mTP 0.01cTP 0.24ER 0.61other
cyto: 11.0, chlo: 2.0
0.078cTP 0.165mTP 0.348SP 0.338other RC=5
NO
0.59 0.07 0.01 0.33 0.00
0 0.082223 0.979695
SP / mito
No
No
mTP / cTP
SP/Cyto
0.019cTP,0.122mTP,0.590SP,0.400other RC=5
NO
0.05 0.09 0.00 0.86 0.00
0.012091 0 0.536857 other
No
Cyto/SP
EhSLPc
jgi|Emihu1|199870|gm1.1100042
Stramenopiles
Emiliania huxleyi CCMP1518
0.01mTP 0.02cTP 0.01ER 0.96other
cyto: 5.0, extr: 4.0, chlo: 3.0, E.R.: 1.0
0.070cTP 0.150mTP 0.139SP 0.668other RC=3
NO
0.04 0.11 0.00 0.84 0.00
0 0.671407 0.745108
mito / nucleus or cytosol
No
No
No
Cyto
FcSLP
jgi|Fracy1|274756|estExt_fgenesh2_pm.C_40052
Stramenopiles
Fragilariopsis cylindrus
0.02mTP 0.00cTP 0.00ER 0.98other
pero: 7.0, mito: 4.0, nucl: 1.0, plas: 1.0
0.018cTP 0.957mTP 0.012SP 0.205other RC=2
NO
0.01 0.95 0.00 0.04 0.00
0 0.597124 0.83023
mito / nucleus or cytosol
No
No
mTP
Mito / Cyto
GtSLPa
jgi|Guith1|163734|estExt_fgenesh2_pg.C_440018
Stramenopiles
Guillardia theta
0.03mTP 0.00cTP 0.99ER 0.01other
chlo: 9.0, vacu: 2.0, E.R.: 2.0
0.412cTP 0.662mTP 0.068SP 0.003other RC=4
YES
0.70 0.29 0.01 0.01 0.00
0.023462 0.980096 0.001554
mito / chloro
mTP
cTP
mTP
Mito / Chloro
GtSLPb
jgi|Guith1|95409|estExt_Genewise1.C_460167
0.024cTP 0.445mTP 0.069SP 0.794other RC=4
NO
0.02 0.16 0.00 0.81 0.00
0 0.100823 0.036067 other
nucleus or cytosol / mito
GtSLPc
Stramenopiles
Guillardia theta
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 9.0, nucl: 2.0, mito: 1.0, plas: 1.0
No
mTP / cTP
jgi|Guith1|162103|estExt_fgenesh2_pg.C_170187
Stramenopiles
Guillardia theta
0.01mTP 0.00cTP 0.98ER 0.02other
chlo: 3.0, vacu: 3.0, E.R.: 2.0, nucl: 1.0, cyto: 1.0
0.082cTP 0.009mTP 0.741SP 0.042other RC=2
YES
0.98 0.01 0.00 0.01 0.00
0.003128 0 0.998129
mito / SP
mTP
No
No
SP/Mito
GtSLPd
jgi|Guith1|96181|estExt_Genewise1.C_630107
Stramenopiles
Guillardia theta
0.01mTP 0.02cTP 0.99ER 0.01other
chlo: 13.0
0.217cTP 0.336mTP 0.188SP 0.011other RC=5
YES
0.87 0.10 0.02 0.01 0.00
0.034939 0.000298 0.98338
chloro / SP
mTP
cTP
No
Chloro/SP
GtSLPe
jgi|Guith1|161633|estExt_fgenesh2_pg.C_120106
Stramenopiles
Guillardia theta
0.02mTP 0.00cTP 0.99ER 0.01other
cyto: 9.0, E.R.: 2.0, plas: 1.0, vacu: 1.0
0.066cTP 0.120mTP 0.662SP 0.176other RC=3
NO
0.99 0.00 0.00 0.01 0.00
0 0.004464 0.998495
mito / SP
No
No
No
SP/Cyto
0.08mTP 0.08cTP 0.00ER 0.84other
No
mTP
PiSLP
PITG_07305T0 | PITG_07305
Stramenopiles
Phytophthora infestans T30-4
PrSLP
jgi|Phyra1_1|94718|C_scaffold_22000047
Stramenopiles
Phytophthora ramorum
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, cyto: 2.0, mito: 1.0, plas: 1.0, extr:
0.007cTP 0.256mTP 0.776SP 0.018other RC=3
NO
PsSLP
jgi|Physo3|478619|e_gw1.2.4097.1
Stramenopiles
Phytophthora sojae
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 9.0, nucl: 1.0, plas: 1.0, vacu: 1.0, E.R.: 1.0
0.038cTP 0.616mTP 0.304SP 0.006other RC=4
NO
PuSLP
0.01mTP 0.01cTP 0.05ER 0.94other
cyto: 5.0, nucl: 4.0, chlo: 3.0, plas: 1.0
mito: 6.0, chlo: 5.0, nucl: 2.0
0.114cTP 0.539mTP 0.100SP 0.193other RC=4
NO
0.03 0.68 0.06 0.23 0.00
0.97 0.03 0.00 0.00 0.00
0.000475 0.985514 0.001242
mito / SP
No
mTP
Cyto
Mito
0 0.569835 0.81068
mito / SP
mTP
No
No
SP/Mito
0.49 0.47 0.01 0.03 0.00
0.000103 0.999244 0.003591
SP / mito
mTP
No
No
Mito/SP
0.03 0.26 0.00 0.70 0.00
0 0.337718 0.442579 other
Cyto
PYU1_T014025
Stramenopiles
Pythium ultimum
0.111cTP 0.212mTP 0.088SP 0.444other RC=4
NO
mito / nucleus or cytosol
No
No
No
TpSLP
649363167 XP_002296838 THAPSDRAFT_264391
Stramenopiles
Thalassiosira pseudonana
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
LmSLPa
LmjF.22.1490
Euglenozoa
Leishmania major
0.01mTP 0.00cTP 0.83ER 0.17other
vacu: 4.0, chlo: 3.0, E.R.: 3.0, nucl: 2.0, cyto: 1.0
0.000cTP 0.445mTP 0.966SP 0.055other RC=3
NO
1.00 0.00 0.00 0.00 0.00
0.000157 0 0.999557
SP / mito
No
No
No
SP
LmSLPb
LmjF.29.0440
Euglenozoa
Leishmania major
0.35mTP 0.00cTP 0.03ER 0.63other
plas: 8.5, cyto_plas: 5.0, chlo: 2.0, E.R.: 2.0
0.010cTP 0.494mTP 0.162SP 0.202other Rc=4
NO
0.54 0.33 0.01 0.12 0.00
0 0.023565 0.991472
mito / nucleus or cytosol
mTP
No
mTP
SP/Mito
LmSLPc
LmjF.30.3280
Euglenozoa
Leishmania major
0.28mTP 0.00cTP 0.99ER 0.01other
plas: 7.0, chlo: 2.0, E.R.: 2.0, extr: 1.0, vacu: 1.0
0.001cTP 0.134mTP 0.990SP 0.012other RC=1
NO
1.00 0.00 0.00 0.00 0.00
0 0.097454 0.982496
SP / nucleus or cytosol
No
No
No
SP
NgSLP
xxx
xxx
xxx
Euglenozoa
NW_003163286
Heterolobosea
Naegleria gruberi
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
TbSLPa
Tb427.06.750
Euglenozoa
Trypanosoma brucei TREU927
0.03mTP 0.00cTP 0.99ER 0.01other
vacu: 4.0, E.R.: 3.0, nucl: 2.0, chlo: 1.0, cyto: 1.0
0.003cTP 0.269mTP 0.874SP 0.052other RC=2
NO
0.99 0.00 0.00 0.00 0.00
0 0.000255 0.999184
SP / mito
mTP
No
No
SP
TbSLPb
Tb427.06.4630
Euglenozoa
Trypanosoma brucei TREU927
0.01mTP 0.01cTP 0.99ER 0.01other
cyto: 6.5, cyto_nucl: 4.0, chlo: 2.0, E.R.: 2.0
0.031cTP 0.069mTP 0.748SP 0.284ER RC=3
NO
0.87 0.02 0.00 0.11 0.00
0 0.006315 0.998321
nucleus or cytosol / SP
mTP
No
No
SP
mTP
No
No
SP/Mito
TcSLPb
Tc00.1047053511731.30
Euglenozoa
Trypanosoma cruzi
0.03mTP 0.00cTP 0.99ER 0.01other
plas: 8.0, E.R.: 3.0, golg: 2.0
0.003cTP 0.407mTP 0.881SP 0.004other RC=3
NO
0.99 0.01 0.00 0.00 0.00
0 0.977022 0.123631
SP / nucleus or cytosol
TcSLPc
T 00 1047053508323 190
Tc00.1047053508323.190
Euglenozoa
Trypanosoma cruzi
0.02mTP 0.00cTP 0.99ER 0.01other
extr: 5.0, chlo: 3.0, vacu: 3.0, cyto: 1.0, plas: 1.0
0.004cTP 0.046mTP 0.986SP 0.057other RC=1
NO
1.00 0.00 0.00 0.00 0.00
0.000147 0 0.999557
SP / mito
No
No
No
TcSLPa
Tc00.1047053508075.14
Euglenozoa
Trypanosoma cruzi
0.01mTP 0.00cTP 0.99ER 0.01other
plas: 9.5, cyto_plas: 5.5, E.R.: 3.0
0.005cTP 0.284mTP 0.928SP 0.025other RC=2
NO
1.00 0.00 0.00 0.00 0.00
0 0.532602 0.861938
SP / mito
No
No
No
SP
CparSLP
639606472 cgd8_290
Apicomplexa
Cryptosporidium parvum
0.08mTP 0.00cTP 0.97ER 0.03other
vacu: 5.0, extr: 3.0, chlo: 2.0, plas: 2.0, E.R.: 1.0
0.003cTP 0.074mTP 0.973SP 0.029other RC=1
NO
1.00 0.00 0.00 0.00 0.00
0 0.067682 0.987443
mito / SP
No
No
No
SP
PmSLPa
649397013 XP_002778164 Pmar_PMAR018604
Alveolata
Perkinsus marinus ATCC 5098
0.02mTP 0.00cTP 0.99ER 0.01other
chlo: 8.0, mito: 2.0, vacu: 2.0, nucl: 1.0
0.227cTP 0.079mTP 0.824SP 0.003other RC=3
NO
0.95 0.02 0.00 0.03 0.00
0.008994 0.007277 0.951841
chloro / mito
mTP
No
No
SP/Mito
PmSLPb
649408011 XP_002787064 Pmar_PMAR006484
Alveolata
Perkinsus marinus ATCC 5098
0.02mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, mito: 3.0, E.R.: 3.0, vacu: 2.0
0.095cTP 0.145mTP 0.732SP 0.004other RC=3
0.98 0.01 0.00 0.01 0.00
0.000174 0.382049 0.844572
mito / chloro
mTP
No
SP/Mito
SP
Apicomplexa
NO
No
PbSLPa
PBANKA_133240
0.04mTP 0.00cTP 0.99ER 0.01other
extr: 7.0, vacu: 4.0, cyto: 1.0, mito: 1.0
0.095cTP 0.145mTP 0.732SP 0.004other RC=4
NO
0.99 0.00 0.00 0.01 0.00
0 0.041936 0.988473
SP / mito
No
No
No
PbSLPb
PBANKA_060470
Apicomplexa
Plasmodium berghei ANKA
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, extr: 5.0, cyto: 2.0
0.005cTP 0.209mTP 0.928SP 0.028other RC=2
NO
0.99 0.01 0.00 0.00 0.00
0 0.513375 0.811642
SP / mito
No
No
mTP
PchaSLPa
PCHAS_060650
Apicomplexa
lasmodium_chabaudi_chabau
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, extr: 5.0, cyto: 2.1
0.005cTP 0.209mTP 0.928SP 0.028other RC=2
NO
0.99 0.01 0.00 0.00 0.01
0 0.513375 0.811642
SP / mito
No
No
mTP
SP
PchaSLPb
PCHAS_133700
Apicomplexa
lasmodium_chabaudi_chabau
0.05mTP 0.00 cTP 0.99ER 0.01other
NO
0.98 0.00 0.00 0.02 0.00
0 0.060306 0.957058
SP / nucleus or cytosol
No
No
No
SP
PvxSLPa
PVX_117005
Apicomplexa
SP / mito
mTP
No
mTP
SP/Mito
PvxSLPb
PVX_084335
Apicomplexa
Plasmodium_vivax_SaI-1
0.07mTP 0.00cTP 0.99ER 0.01other
cyto: 5.0, chlo: 4.0, extr: 2.0, nucl: 1.0, mito: 1.0
NO
0.98 0.00 0.00 0.01 0.00
0 0.004935 0.99743
SP / nucleus or cytosol
mTP
No
No
PySLPa
PY00915
Apicomplexa
lasmodium_yoelii_yoelii_17XN
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, extr: 5.0, cyto: 2.0
0.005cTP 0.112mTP 0.971SP 0.023other
NO
0.57 0.01 0.00 0.42 0.00
0 0.258925 0.652454
SP / nucleus or cytosol
No
No
No
PySLPb
PY03645
Apicomplexa
lasmodium_yoelii_yoelii_17XN
0.04mTP 0.00cTP 0.99ER 0.01other
vacu: 4.0, extr: 3.0, cyto: 2.0, golg: 2.0
0.021cTP 0.087mTP 0.909SP 0.128other
NO
0.97 0.00 0.00 0.02 0.00
0 0.081172 0.979407
SP / mito
No
No
No
SP
TgSLP
TGGT1_004320
Apicomplexa
Toxoplasma gondii GT1
0.18mTP 0.14cTP 0.02ER 0.70other
chlo: 6.0, nucl: 2.0, vacu: 2.0, cyto: 1.0, plas: 1.0
0.399cTP 0.297mTP 0.005SP 0.060other RC=5
NO
0.36 0.40 0.20 0.04 0.00
0.000349 0.999578 0.00084
chloro / mito
No
cTP
mTP / cTP
Chloro/Mito
SP, mTP, other, PTS1
mTP, SP, Other
MgloSLP
A8Q735_MALGO
Fungi
Malassezia globosa
0.00mTP 0.01ER 0.99other
mito: 8.0, cysk: 8.0, cyto: 5.5, cyto_pero: 4.0
0.116mTP 0.063SP 0.873other RC=2
xxx
0.01 0.03 0.96 0.00
0.004395 0.050324 other
Mito/Cyto
PgraSLP
E3JUL7_PUCGT
Fungi
Puccinia graminis
0.31mTP 0.46ER 0.37other
mito: 11.0, golg: 5.0, cyto: 2.0, plas: 2.0, extr: 2.0
0.555mTP 0.643SP 0.014other RC=5
xxx
0.67 0.11 0.22 0.00
0.989244 0.007792
Apicomplexa
Plasmodium berghei ANKA
Plasmodium_vivax_SaI-1
Fungi
0.41mTP 0.00cTP 0.99ER 0.00other
0.000cTP 0.025mTP 0.916SP 0.096other
extr: 4.0, vacu: 3.0, cyto: 2.0, mito: 2.0
0.000 cTP 0.092mTP 0.970SP 0.014other
cyto: 6.0, mito: 3.0, nucl: 2.0, plas: 2.0
0.005cTP 0.097mTP 0.948SP 0.067other
mTP, ER, Other
NO
mTP, SP, Other
0.67 0.04 0.00 0.29 0.00
0 0.847531 0.421309
xxx
No
mTP
xxx
mTP
BdenSLP
F4NYP9_BATDJ
Fungi
Batrachochytrium dendrobatidis
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
F4RN30_MELLP
Fungi
u g
Melampsora
e a pso a larici-populina
a c popu a
0.06mTP
0
06
0.90ER
0
90
0.09other
0
09ot e
mito:
to 20.0,
0 0, nucl:
uc 4.5,
5, cyto_
cyto nucl:
uc 4.0
0
0.363mTP
0
363
0.589SP
0
589S 0
0.023other
0 3ot e RC=4
C
xxx
0.43
0
30
0.18
80
0.39
39 0
0.00
00
0.978622
0
9 86 0
mito
to / nucleus
uc eus o
or cytoso
cytosol
mTP
xxx
No
o
Mito/SP
to/S
RgluSLP
G0T0W9_RHOG2
Fungi
Rhodotorula glutinis
0.16mTP 0.99ER 0.01other
extr: 19.0, E.R.: 4.0, mito: 1.0, plas: 1.0
0.332mTP 0.717SP 0.012other RC=4
xxx
0.98 0.00 0.02 0.00
0.085595 0.937863
mito / SP
mTP
xxx
No
SP/Mito
LbSLP
jgi|Lacbi1|189922|estExt_GeneWisePlus_worm.C_80340
0.022mTP 0.980SP 0.033other RC=1
extr: 21.0, plas: 2.0, golg: 2.0
extr: 13.0, E.R.: 6.0, golg: 3.0, mito: 2.0, cyto: 2.0
0.139952 0.933613
Fungi
Laccaria bicolor
xxx
0.95 0.00 0.05 0.00
YQJ7_SCHPO
Fungi
Schizosaccharomyces pombe
0.021mTP 0.943SP 0.060other RC=1
xxx
0.98 0.00 0.02 0.00
SP / mito
No
No
SP/ER
Q5KCT2_CRYNJ
Fungi
Cryptococcus neoformans
0.01mTP 0.99ER 0.01other
extr: 23.0, plas: 3.0
0.094mTP 0.930SP 0.011other RC=1
xxx
0.96 0.00 0.04 0.00
0.140072 0.933536
SP / mito
mTP
xxx
No
SP/Mito
UmSLP
jgi|Ustma1|3316|UM03316
Fungi
Ustilago maydis
0.03mTP 0.01ER 0.97other
extr: 12.0, mito: 7.0, cyto: 4.5, cyto_nucl: 4.0
0.416mTP 0.088SP 0.508other RC=5
xxx
0.01 0.03 0.96 0.00
0.098582 0.030083 other
nucleus or cytosol / mito
No
xxx
No
Cyto
Aureococcus anophagefferens
0.01mTP 0.00 cTP 0.34ER 0.65 other
3.0 chloro, 3.0vac, 3.0ER, 2.5cyto, 2.0 nuc
0.03cTP,0.064mTP,0.853SP, 0.290other RC=3
NO
0.96 0.01 0.00 0.03 0.00
0 0.004694 0.990785
SP / nucleus or cytosol
No
No
No
SP/Cyto
xxx
0.006074 0.960532
SP / mito
No
xxx
xxx
No
Mito/SP
CneoSLP
SpSLP
0.02mTP 0.99ER 0.01other
mito / SP
mTP
SP
SP
a pS
MlarpSLP
0.01mTP 0.99ER 0.01other
nucleus or cytosol / mito
SP
SP
SP
Chromalveolates
AaSLPb
gi|323451994
PcapSLP
jgi|Phyca11|66148|gw1.23.354.1
Stramenopiles
Phytophthora capsici
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
PiSLP
PITG_07305T0
Stramenopiles
Phytophthora infestans T30-4
0.08mTP 0.08cTP 0.00ER 0.84other
mito: 6.0, chlo: 5.0, nucl: 2.0
0.659mTP 0.058SP 0.224other RC=3
NO
0.03 0.68 0.06 0.23 0.00
0.000475 0.985514 0.001242
mito / SP
mTP
No
mTP
Mito/SP
PrSLP
jgi|Phyra1_1|94718|C_scaffold_22000047
Stramenopiles
Phytophthora ramorum
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 6.0, cyto: 2.0, mito: 1.0, plas: 1.0, extr: 1.0
0.099mTP 0.918SP 0.019other RC=1
NO
0.97 0.03 0.00 0.00 0.00
0 0.569835 0.81068
mito / SP
mTP
No
No
SP/Mito
Phytophthora sojae
0.01mTP 0.00cTP 0.99ER 0.01other
chlo: 9.0, nucl: 1.0, plas: 1.0, vacu: 1.0, E.R.: 1.0
0.124mTP 0.932SP 0.011other RC=1
mTP
Pythium ultimum
0.01mTP 0.01cTP 0.05ER 0.94other
cyto: 5.0, nucl: 4.0, chlo: 3.0, plas: 1.0
jgi|Physo3|478619|e_gw1.2.4097.1
Stramenopiles
xxx
xxx
xxx
NO
0.49 0.47 0.01 0.03 0.00
0.000103 0.999244 0.003591
mito / SP
No
No
PutSLP
PYU1_T014025
Stramenopiles
0.132mTP 0.088SP 0.757other RC=2
NO
0.03 0.26 0.00 0.70 0.00
0 0.337718 0.442579 Other
mito / nucleus or cytosol
No
No
mTP
TpSLP
649363167 XP_002296838 THAPSDRAFT_264391
Stramenopiles
Thalassiosira pseudonana
**partial sequence
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
xxx
TtSLP
AMSG_02057T0
Apusozoa
hecamonas trahens ATCC 500
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 4.0, chlo: 3.0, plas: 3.0, pero: 3.0
0.106mTP 0.070SP 0.878other RC=2
NO
0.01 0.06 0.00 0.93 0.99
0.024642 0 0.290571 other
nucleus or cytosol / SP
No
No
No
Cyto / Perox
PsSLP
Stramenopiles
Mito/SP
Cyto/Mito
have occurred during SLP sequence evolution. A clear example of this is provided by the group
of sequences from Apicomplexa. This group contains the only other SLP protein which has been
characterized in detail biochemically, the SHLP1 protein of Plasmodium berghei (the causative
agent of malaria in the mouse) (Patzewitz et al., 2013). This protein (corresponding to sequence
PbSLPa) has been shown to be localized in the ER membrane, which is consistent with the
prediction of a signal peptide (SP). Analysis indicates this is a conserved feature of most of the
SLP proteins from Apicomplexa. It is interesting in this regard that most of SLP sequences in the
dataset from the parasitic Euglenozoa also manifest a predicted signal peptide. Furthermore, it
should be noted that the non-pathogenic fungi Schizosaccharomyces pombe and Laccaria bicolor
also possess SLP proteins with predicted signal peptides. Indeed, previous findings indicate that
the Schizosaccharomyces pombe SLP phosphatase is also ER localized (Matsuyama et al., 2006).
43
2.3.2.2 RLPH phosphatases
Data uncovered regarding the distribution and interrelationships of the Rhizobiales-like
phosphatases (RLPHs) are also presented (Figure 2.4). Once again, we see that the species
representation is heavily weighted toward photosynthetic organisms, with the only exceptions
being the Heterolobosean Naegleria gruberi and the Choanoflagellate Salpingoeca rosetta.
Amongst photosynthetic organisms the RLPH distribution is dominated by land plants. Despite
intensive searching, RLPH sequences could only be detected in two different strains of a single
green algal species, Micromonas pusilla. None of the photosynthetic Chromalveolates contained
a RLPH sequence, with the sole remaining eukaryotic organism being the photosynthetic
Rhizarian Bigelowiella natans. Intensive searching by TBLASTN also failed to reveal any
additional RLPHs amongst previously unannotated sequences from other species of Green
Algae, or any other eukaryotic group. At the base of the RLPH distribution is a closely related
set of sequences from Planctomycetes bacteria.
Closely associated with the RLPH sequence distribution is a set of sequences from αProteobacteria. Other, more distantly related bacterial sequences include representatives from a
variety of groups including Cyanobacteria, δ-Proteobacteria, Bacteroidetes and Thermotogae. No
RLPH sequences were detected from Archaea, either by HMM searching of protein databases
derived from completely sequenced archaeal genomes, BLASTP searching of archaeal protein
databases, or TBLASTN searching amongst archaeal nucleotide databases.
The RLPHs have a distinctive predicted subcellular localization not shared by the SLPs,
with most sequences maintaining a predicted cytoplasmic / nuclear localization (Table 2.2). This
is true not only of the land plants, but also the Naegleria sequence, the most deeply
44
Figure 2.4: Orthogonal phylogenetic tree depicting RLPH phosphatase distribution and
interrelationships across both Eukaryotes and Prokaryotes.
Phylogenetic tree inference was performed as outlined in the Materials and Methods. The
most crucial nodes are labelled. Branch support values with the four inference methods
(PhyML [aBayes]; RAxML [RBS]; MrBayes [PP]; PhyloBayes_MPI [PP]) are as follows
(see Materials and Methods for details). Node A: (0.999, 99, 0.98, 1.00); Node B: (0.575, 80,
0.95, 0.86); Node C: (0.999, 90, 0.93, 1.00); Node D: (1.00, 100, 0.98, 1.00); Node E: (0.999,
16, 0.83, 0.95); Node F: (0.999, 12, 0.90, 0.91). Branch support values for all trees are
summarized as Appendix B. Predicted in silico subcellular localizations are represented by
Mt (mitochondria), Cy (cytosol), Nu (Nuclear). Sequences used in tree generation are listed
in Appendix A.2 and in silico subcellular localization data are listed in Table 2.2.
45
Table 2.2: In silico subcellular prediction data for eukaryotic RLPH phosphatases used
in phylogenetic tree and alignment construction.
Prediction consensus is highlighted in yellow. Tree labels, gene identifiers, organism group
and organism information are also provided.
RLPH Phosphatases
Prediction Program
Tree
Designation
Gene Identifier
AlRLPHa
Predotar
Organism
Group
Organism
478324|PACid:16048263
Streptophyta
Arabidopsis lyrata
0.01mTP 0.00cTP 0.00ER 0.99other
Wolf pSORT
TargetP
ChloroP
Protein Prowler
PredSL
cTP, mTP, SP, other
Yes, No
SP, mTP, cTP, other,
PTS1
cTP, mTP, SP, Other
nucl: 9.0, cyto: 3.0, mito: 1.0
0.046cTP 0.252mTP 0.213SP 0.536other RC=4
No
0.03 0.12 0.00 0.85 0.00
0 0.003997 0.638358
nucleus or cytosol / SP
mTP, cTP, ER, Other
SLP-Local
MitoProt
PCLR
iPSORT
mTP, No
cTP, No
mTP, cTP,
No
No
No
mTP
cyto/nuc
Consensus
AlRLPHc
478323|PACid:16040833
Streptophyta
Arabidopsis lyrata
0.01mTP 0.00cTP 0.00ER 0.99other
nuc l: 6.0, cyto: 5.0, mito: 1.0, pero: 1.0
0.037cTP 0.254mTP 0.227SP 0.680other RC=3
No
0.02 0.08 0.00 0.91 0.00
0 0.024833 0.099683 other
nucleus or cytosol / SP
No
No
No
cyto/nuc
AcRLPHa
Aquca_027_00420.1|PACid:22045939
Streptophyta
Aquilegia coerulea
0.01mTP 0.00cTP 0.00ER0.99other
cyto: 8.0, nucl: 3.0, cysk: 2.0
0.075cTP 0.062mTP 0.137SP 0.895other RC=2
No
0.02 0.06 0.00 0.93 0.00
0 0.000509 0.975568
nucleus or cytosol / SP
No
No
No
cyto/nuc
AtRLPHa
AT3G09960
Streptophyta
Arabidopsis thaliana
0.02mTP 0.00cTP 0.01ER 0.98other
nucl: 9.0, cyto: 3.0, mito: 1.0
0.075cTP 0.199mTP 0.101SP 0.756other RC=3
No
0.02 0.08 0.00 0.90 0.00
0.000119 0.000834 0.619835
nucleus or cytosol / SP
No
No
No
cyto/nuc
AtRLPHb
AT3G09970
Streptophyta
Arabidopsis thaliana
0.01mTP 0.00cTP 0.00ER 0.99other
nucl: 10.0, cyto: 2.0, mito: 1.0
0.051cTP 0.175mTP 0.223SP 0.628other RC=3
No
0.02 0.09 0.00 0.89 0.00
0 0.001327 0.512618 other
nucleus or cytosol / SP
No
No
No
BdRLPH
Bradi5g09180.1|PACid:21826565
Streptophyta
Brachypod ium distachyon
0.01mTP 0.03cTP 0.13ER0.85other
cyto: 6.0, chlo: 5.0, plas: 2.0
0.160cTP 0.158mTP 0.090SP 0.619other RC=3
No
0.06 0.13 0.02 0.79 0.00
0 0.009879 0.997885
nucleus or cytosol / chloro
No
No
mTP/cTP
cyto/nuc
BnRLPH
jgi|Bigna1|87196|estExt_fgenesh1_pg.C_170184
Rhizaria
Bigelowiella natans CCMP2755
0.36mTP 0.10cTP 0.01ER 0.57other
chlo: 7.0, mito: 3.0, nucl: 2.0, E.R.: 2.0
0.224cTP 0.706mTP 0.006SP 0.099other RC=3
No
0.01 0.95 0.02 0.03 0.00
0.000318 0.99905 0.001265
mito / nucleus or cytosol
mTP
No
No
mito/cyto
nuc l: 7.0, cyto: 6.0
nucleus or cytosol / mito
No
BrRLPH
Bra029809|PACid:22685889
Streptophyta
Brassica rapa
0.047cTP 0.210mTP 0.185SP 0.591other RC=4
No
0.03 0.01 0.96 0.00
0 0.037468 0.983744
CruRLPHa
Carubv10014233m|PACid:20900199
Streptophyta
Capsella rubella
0.01mTP 0.00cTP 0.00ER 0.99other
nucl: 9.0, cyto: 3.0, mito: 1.0
0.048cTP 0.183mTP 0.281SP 0.561other RC=4
No
0.02 0.02 0.97 0.00
0.00019 0.001662 0.090186 other
nucleus or cytosol / SP
No
No
No
cyto/nuc
CruRLPHb
Carubv10014229m|PACid:20899239
Streptophyta
Capsella rubella
0.01mTP 0.00cTP 0.02ER 0.97other
nucl: 9.0, cyto: 3.0, mito: 1.0
0.091cTP 0.192mTP 0.141SP 0.609other RC=3
No
0.04 0.01 0.95 0.00
0 0.00413 0.945158
nucleus or cytosol / mito
No
No
No
cyto/nuc
CpaRLPH
Carica papaya
0.01cTP 0.00mTP 0.00ER 0.99other
No
No
cyto/nuc
cyto/nuc
evm.model.supercontig_5.61|PACid:16421512
Streptophyta
0.01mTP 0.00cTP 0.07ER 0.92other
cyto: 11.0, chlo: 2.0
0.055cTP 0.173mTP 0.094SP 0.927other RC=2
No
0.01 0.06 0.00 0.93 0.00
0 0.014847 0.134998 other
nucleus or cytosol / mito
No
No
mTP
cyto/nuc
CcRLPHa
clementine0.9_016692m|PACid:19266957
Streptophyta
Citrus clemntina
0.03mTP 0.00cTP 0.01ER 0.97other
cyto: 11.0, chlo: 2.0
0.060cTP 0.440mTP 0.052SP 0.554other RC=5
No
0.03 0.29 0.00 0.68 0.00
0 0.921123 0.353943
nucleus or cytosol / mito
No
No
mTP/cTP
cyto/nuc
CcRLPHb
clementine0.9_019398m|PACid:19266958
Streptophyta
Citrus clemntina
0.03mTP 0.00cTP 0.01ER 0.97other
cyto: 11.0, chlo: 2.0
0.060cTP 0.440mTP 0.052SP 0.554other RC=5
No
0.03 0.29 0.00 0.68 0.00
0 0.921123 0.353943
nucleus or cytosol / mito
No
No
mTP/cTP
cyto/nuc
CsRLPH
orange1.1g025290m|PACid:18101020
Streptophyta
Citrus sinensis
0.03mTP 0.00cTP 0.01ER 0.97other
cyto: 10.0, mito: 2.0, chlo: 1.0
0.060cTP 0.440mTP 0.052SP 0.554other RC=5
No
0.03 0.29 0.00 0.68 0.00
0 0.921123 0.353943
nucleus or cytosol / mito
No
No
mTP/cTP
CsatRLPHa
Cucsa.378400Cucsa.378400.1|PACid:16982048
Streptophyta
Cucumis sativus
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 7.0, nucl: 3.0, chlo: 2.0, plas: 1.0
0.064cTP 0.152mTP 0.158SP 0.784other RC=2
No
0.02 0.07 0.00 0.91 0.00
0 0.001349 0.705375
nucleus or cytosol / SP
No
No
mTP
cyto/nuc
CsatRLPHb
Cucsa .338320.1|PACid:16978737
Streptophyta
Cucumis sativus
0.01cTP 0.00mTP 0.01ER 0.98other
cyto: 10.5, cyto_E.R.: 6.0, nucl: 1.0, cysk: 1.0
0.092cTP 0.122mTP 0.108SP 0.830other RC=2
No
0.02 0.02 0.96 0.00
0 0.003026 0.741126
nucleus or cytosol / SP
No
No
No
cyto/nuc
0.827 0 0.036403
nucleus or cytosol / chloro
No
cyto/chloro/mito
EgRLPH
Eucgr.A01531.1|PACid:23563318
Streptophyta
Eucalyptus grandis
0.01mTP 0.01cTP 0.00ER 0.99other
cTP
No
GmRLPHb
Glyma10g07800.1|PACid:26346430
Streptophyta
Glycine max
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 11.0, nucl: 1.0, E.R.: 1.0
0.085cTP 0.047mTP 0.384SP 0.671other RC=4
No
0.02 0.06 0.00 0.92 0.00
0 0.010259 0.048305 other
nucleus or cytosol / mito
No
No
mTP/cTP
cyto/nuc
GmRLPHa
Glyma13g21640.1|PACid:26315536
Streptophyta
Glycine max
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 9.0, chlo: 2.0, nucl: 1.0, plas: 1.0
0.072cTP 0.096mTP 0.140SP 0.797other RC=2
No
0.01 0.07 0.00 0.91 0.00
0 0.027197 0.090347 other
nucleus or cytosol / chloro
No
No
No
cyto/nuc
GrRLPHa
Gorai.009G406100.1|PACid:26769011
Streptophyta
Gossyp ium raimondii
0.01mTP 0.00cTP 0.04ER 0.96other
cyto: 10.0, chlo: 2.0, nucl: 1.0
0.071cTP 0.181mTP 0.176SP 0.782other RC=2
No
0.01 0.06 0.00 0.93 0.00
0.000229 0.000929 0.196542 other
nucleus or cytosol / chloro
No
No
No
cyto/nuc
GrRLPHb
Gorai.009G405800.1|PACid:26764307
cyto: 11.0, nucl: 1.0, cysk: 1.0
0.061cTP 0.170mTP 0.194SP 0.786other RC=3
cysk: 10.0, cyto: 2.0, plas: 1.0
0.877cTP 0.018mTP 0.075SP 0.477other RC=4
No
cyto/nuc
0.02 0.09 0.01 0.89 0.00
Streptophyta
Gossyp ium raimondii
0.01mTP 0.00cTP 0.06ER 0.93other
No
0.02 0.07 0.00 0.91 0.00
nucleus or cytosol / SP
No
No
cyto/nuc
LuRLPH
Lus10014383|PACid:23143669
Streptophyta
Linum usitatissimum
0.01mTP 0.00cTP 0.02ER 0.98other
cyto: 10.5, cyto_E.R.: 6.0, nucl: 2.0
0.047cTP 0.108mTP 0.300SP 0.661other RC=3
No
0.02 0.07 0.00 0.90 0.00
0 0.017295 0.530941 other
nucleus or cytosol / mito
No
No
No
cyto/nuc
MdpRLPHa
MDP0000218397|PACid:22671674
Streptophyta
Malus domestica
0.01mTP 0.65cTP 0.00ER 0.34other
chlo: 6.0, cyto: 3.0, mito: 2.0, pero: 2.0
0.824cTP 0.036mTP 0.060SP 0.256other RC=2
YES
0.00 0.01 0.98 0.01 0.00
0.999909 0.000194 0.000199
chloro / nucleus or cytosol
No
cTP
mTP/cTP
MdpRLPHb
MDP0000131572|PACid:22643486
Streptophyta
Malus domestica
0.01mTP 0.88cTP 0.00ER 0.12other
chlo: 11.0, mito: 2.0
0.968cTP 0.024mTP 0.001ER 0.083other
YES
0.999845 0.000134 0.00026
chloro / nucleus or cytosol
mTP
cTp
cTP
chloro
MeRLPH
cassava4 .1_012125m.g
Streptophyta
Manihot esculenta
0.01mTP 0.00cTP 0.10ER 0.90other
cyto: 11.0, chlo: 2.0
0.019cTP 0.181mTP 0.260SP 0.770other RC=3
No
0.02 0.07 0.00 0.91 0.00
0 0.012238 0.16485 other
nucleus or cytosol / chloro
No
No
No
cyto/nuc
MpRLPHa
jgi|MicpuC3|19876|MicpuC2.e_gw1.9.342.1
Chlorophyta
Micromonas pusilla CCMP 1545
0.04mTP 0.01cTP 0.02ER 0.94other
chlo: 5.0, mito: 5.0, cyto: 2.0, nucl: 1.0
0.098cTP 0.621mTP 0.020 0.195 RC=3
No
0.02 0.82 0.02 0.14 0.00
0.000126 0.996704 0.003362
nucleus or cytosol / chloro
mTP
No
No
mito/cyto
xxx
xxx
xxx
xxx
MpRLPHb
xxx
0.00 0.01 0.99 0.00 0.00
0 0.004408 0.056452 other
No
xxx
chloro
jgi|MicpuN3|82680|e_gw2.06.480.1
Chlorophyta
Micromonas pusilla CCMP1545
**Partial Sequence Only
xxx
xxx
xxx
xxx
MgRLPH
mgv1a008117m|PACid:17676001
Streptophyta
Mimulus guttatus
0.01mTP 0.40cTP 0.00ER 0.60other
cyto: 7.0, nuc l: 2.0, pero: 2.0, chlo: 1.0
0.195cTP 0.072mTP 0.119SP 0.593other RC=4
No
0.03 0.11 0.01 0.85 0.00
0.237309 0 0.467375 other
nucleus or cytosol / chloro
No
No
No
cyto/nuc
OsRLPH
LOC_Os04g33470.1|PACid:21897059
Streptophyta
Oryza sativa
0.01mTP 0.00cTP 0.02ER 0.98other
chlo: 4.0, cyto: 3.0, nucl: 2.0, pero: 2.0
0.239cTP 0.188mTP 0.051SP 0.632other RC=4
No
0.02 0.09 0.00 0.89 0.00
0 0.005448 0.998691
nucleus or cytosol / chloro
No
No
mTP/cTP
cyto/nuc
No
mTP/cTP
cyto/mito
y
PvRLPH
Pavirv00063697 m|PACid:23762733
|
Streptophyta
p p y
0.264cTP 0.158mTP 0.046SP 0.711other RC=3
No
0.04 0.15 0.03 0.78 0.00
0 0.023443 0.993304
y
/ chloro
nucleus or cytosol
No
PvuRLPH
Phvul.007G222200.1|PACid:27159562
Streptophyta
Phaseolus vulgaris
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 7.0, nucl: 5.0, chlo: 2.0
0.117cTP 0.120mTP 0.112SP 0.776other RC=2
No
0.01 0.07 0.00 0.92 0.00
0.000102 0.002787 0.090987 other
nucleus or cytosol / chloro
No
No
No
PpaRLPH
Pp1s118_177 V6.1|PACid:18069084
Streptophyta
Physco mitrella patens
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 7.5, cyto_E.R.: 4.5, pero: 2.0, chlo: 1.0
0.093cTP 0.153mTP 0.168SP 0.794other RC=2
No
0.01 0.08 0.00 0.91 0.00
0 0.039665 0.090002 other
nucleus or cytosol / mito
No
No
No
cyto/nuc
PperRLPH
ppa015436m|PACid:17668853
Streptophyta
Prunus persica
0.01mTP 0.00cTP 0.00ER 0.99other
cyto: 12.0, chlo: 1.0
0.050cTP 0.235mTP 0.112SP 0.847other RC=2
No
0.000109 0.001869 0.180797 other
nucleus or cytosol / mito
No
No
mTP
cyto/nuc
PtRLPHa
Potri.002G156200.1|PACid:27024057
Streptophyta
Populus trichocarpa
0.02mTP 0.00cTP 0.00ER0.98other
cyto: 10.0, pero: 2.0, mito: 1.0
0.369cTP 0.069mTP 0.118SP 0.795other RC=3
No
PtRLPHc
Potri.002G156500.1|PACid:27023072
Streptophyta
Populus trichocarpa
0.02mTP 0.00cTP 0.00ER0.97other
cyto: 9.0, pero: 2.0, nucl: 1.0, mito: 1.0
0.397cTP 0.060mTP 0.139SP 0.800other RC=3
No
RcRLPH
27496.m000096|PACid:16798510
Streptophyta
Ricinus communis
0.01mTP 0.02cTP 0.01ER0.97other
chlo: 6.0, mito: 4.0, cyto: 3.0
0.061cTP 0.169mTP 0.165SP 0.902other RC=2
No
0.02 0.09 0.01 0.88 0.00
0.347824 0 0.43965 other
SbRLPH
Sb06g015410.1|PACid:1972275
Streptophyta
Sorghum bicolor
0.01mTP 0.00cTP 0.07ER 0.92other
cyto: 7.0, chlo: 4.0, plas: 2.0
0.142cTP 0.192mTP 0.180SP 0.658other RC=3
No
0.05 0.11 0.01 0.82 0.00
SiRLPH
Si010641m|PACid:19695892
Streptophyta
Setaria italica
0.01mTP 0.00cTP 0.08ER 0.91other
cyto: 8.0, chlo: 3.0, plas: 2.0
0.165cTP 0.213mTP 0.094SP 0.670other RC=3
No
SmRLPH
130493|PACid:15421901
Streptophyta
Selaginella moellendorfii
0.03mTP 0.00cTP 0.01ER 0.95other
chlo: 5.0, mito: 4.0, vacu : 2.0, E.R.: 2.0
0.022cTP 0.771mTP 0.016SP 0.600other RC=5
ThalRLPH
Thhalv10021191 m|PACid:20183521
Streptophyta
Thellungiella halophila
0.01mTP 0.01cTP 0.00ER 0.98other
cyto: 7.0, cysk: 5.0, nucl: 2.0
0.197cTP 0.115mTP 0.092SP 0.718other RC=3
Panicum virgatum
g
0.01mTP 0.00cTP 0.01ER 0.98other
chlo: 10.0,, mito: 2.0,, cyto:
y
1.0
0.01 0.07 0.00 0.92 0.00
0.03 0.13 0.02 0.82 0.00
cyto/nuc
0.001104 0.000528 0.029679 other
nucleus or cytosol / mito
No
No
No
cyto/nuc
0.003919 0.000115 0.0854 other
nucleus or cytosol / mito
No
No
No
cyto/nuc
nucleus or cytosol / mito
No
No
mTP/cTP
cyto/nuc
0 0.007303 0.963105
nucleus or cytosol / SP
No
No
mTP/cTP
0.04 0.13 0.01 0.82 0.00
0 0.031257 0.992595
nucleus or cytosol / chloro
No
No
mTP/cTP
cyto/nuc
No
0.03 0.71 0.01 0.25 0.00
0 0.998186 0.008288
nucleus or cytosol / mito
mTP
No
mTP
mito/cyto
No
0.02 0.08 0.00 0.90 0.00
0.000527 0.000324 0.293576 other
nucleus or cytosol / SP
No
No
No
cyto/nuc
cyto/mito
0.03 0.15 0.05 0.76 0.00
cyto/nuc
VvRLPH
GSVIVT01033803001 |PACid:17839446
Streptophyta
Vitis vinifera
0.06mTP 0.00cTP 0.00ER 0.94other
cyto: 4.0, chlo_mito: 4.0, chlo: 3.5, mito: 3.5
0.010cTP 0.883mTP 0.045SP 0.260other
No
0.01 0.87 0.00 0.12 0.00
0 0.995098 0.009557
mito / nucleus or cytosol
No
No
No
ZmRLPH
GRMZM2G128399_T01|PACid:20872846
Streptophyta
Zea mays
0.01mTP 0.00cTP 0.10ER 0.89other
cyto: 5.0, chlo: 4.0, mito: 2.0, plas: 2.0
0.122cTP 0.236mTP 0.076SP 0.745other RC=3
No
0.05 0.13 0.01 0.81 0.00
0 0.017542 0.994634
nucleus or cytosol / chloro
No
No
mTP/cTP
cyto/nuc
NgRL PH
649365958 XP_002669792 NAEGRDR AFT_59846
Heterolobosea
Naegleria gruberi
0.01mTP 0.02cTP 0.02ER0.96other
nucl: 11.0, chlo: 1.0, cyto: 1.0
0.086cTP 0.064mTP 0.038SP 0.840other RC= 2
No
0.50 0.08 0.00 0.42 0.00
0.6341 0.000658 0.000369
nucleus or cytosol / SP
No
No
No
cyto/nuc
SrRLPH
PTSG_01069T0 | PTSG_01069
Choanoflagellida
Salpingoeca rosetta
0.01mTP 0.03cTP 0.01ER 0.95other
mito: 13.0, nucl: 9.5, cyto_nucl: 8.5, cyto: 6.5
0.141mTP 0.154SP 0.699other RC=3
nucleus or cytosol / SP
No
xxx
No
cyto/mito
Excavate
Choanozoa
mTP, SP, Other
xxx
SP, mTP, other, PTS1
mTP, SP, Other
0.03 0.01 0.96 0.00
0.004088 0.050907 other
diverging RLPH sequence in the tree, which suggests that a distinctive targeting of RLPH class
sequences may have occurred early in eukaryotic evolution.
2.3.3 Sequence motif identification
Upon the classification of SLP and RLPH phosphatases, a novel C-terminal sequence
motif I/L/V-D-S/T-G (labeled Motif 2 here) was revealed (Andreeva and Kutuzov, 2004). Data
confirm the conservation of sequence Motif 2 across all eukaryotic bacterial-like phosphatases
(Figure 2.1-2.2, 2.5), in addition to revealing a second C-terminal motif (Motif 1) (M/I/V)(I/L/V)-(V/S/F)-G-H-(T/H/D) upstream of Motif 2 (Figure 2.1-2.2, 2.6). Within both of these
sequence motifs, each eukaryotic bacterial-like phosphatase subclass was found to maintain
46
Figure 2.5: Compiled bacterial-like phosphatase Motif 2 from SLP and RLPH
phosphatases.
Amino acid positional probability consensus within the bacterial-like motif 2 of (A) SLP and
(B) RLPH phosphatases from eukaryotic organisms outlined in each respective phylogenetic
tree and listed in Figures 2.3 and 2.4 as well as Appendix A.1 and A.2. Amino acid positional
probability consensus within bacterial-like motif 2 of photosynthetic Eukaryote SLP (C) and
RLPH (D) phosphatases only. Largest diversity was observed in motif position 3 where
threonine (T), conserved amongst prokaryotic and eukaryotic SLP and RLPH phosphatases
alike, was replaced with valine (V) and glutamic acid (E) in photosynthetic Eukaryote SLP
and RLPH phosphatases, respectively. Amino acid colors represent polar (Green), Neutral
(Purple), Basic (Blue), Acidic (Red) and Hydrophobic (Black) amino acids. Each amino acid
positional probability consensus was constructed using MAFFT aligned sequences submitted
to WebLogo 3 (http://weblogo. threeplusone.com/).
47
Figure 2.6: Compiled bacterial-like phosphatase Motif 1 from SLP and RLPH
phosphatases.
Amino acid positional probability consensus within the bacterial-like motif 1 of (A) SLP and
(B) RLPH phosphatases from eukaryotic organisms outlined in each respective phylogenetic
tree and listed in Figures 2.3 and 2.4, respectively, as well as Appendix A.1 and A.2. (C-D)
Amino acid positional probability consensus within bacterial-like motif 1 of photosynthetic
Eukaryote SLP and RLPH phosphatases only. Bacterial-like motif 1 exhibited largest
diversity in motif positions 1 through 3, where predominantly a mixed variety of
hydrophobic amino acids were observed. Similar to position 3 of motif 2, position 6 of motif
1 also exhibited conserved bacterial-like class diversity, with photosynthetic Eukaryote SLP
and RLPH phosphatases predominantly maintaining threonine (T) and histidine (H) residues,
respectively. Amino acid colors represent polar (Green), Neutral (Purple), Basic (Blue),
Acidic (Red) and Hydrophobic (Black). Each amino acid positional probability consensus
was constructed using MAFFT aligned sequences submitted to WebLogo 3
(http://weblogo.threeplusone.com/).
48
distinct diversity at specific motif positions which parallels their classification (Figure 2.5-2.6).
This was most pronounced when examining these motifs from photosynthetic Eukaryotes
(Figure 2.5-2.6).
2.3.4 In silico gene structure analysis of bacterial-like PPP phosphatases from photosynthetic
Eukaryotes
Using the online resource Phytozome v7.0 (www.phytozome.com), in silico annotated
gene models of SLP and RLPH phosphatases ranging from green algae through to land plants
were extracted and manually analyzed (Figure 2.7). Distinct differences in SLP1 and 2
phosphatase gene architecture was observed over evolutionary time between green algae and
land plants. SLP1 and SLP2 phosphatases from the representative green alga Chlamydomonas
reinhardtii maintained a similar number of introns, while in land plants, intron number increased
in SLP1 phosphatases and decreased to being virtually intronless in SLP2 phosphatases (Figure
2.7A). This trend in SLP2 phosphatase gene architecture was conserved across all sequenced
land plants (Table 2.3). Over a similar evolutionary time frame, RLPH phosphatases only lost a
single intron (Figure 2.7B) and showed clear signs of a recent gene duplication in dicotyledonous
plant species (e.g. Arabidopsis thaliana). Gene duplication was not observed in any
monocotyledonous plant species (e.g. Oryza sativa). SLP3 phosphatases were not subjected to
this assessment due to their restrictive evolutionary distribution and absence in land plants.
2.4 Discussion
For each type of eukaryotic bacterial-like PPP phosphatase investigated here, a well
supported group of sequences from α-Proteobacteria lies in close phylogenetic association. The
49
Figure 2.7: Gene structure of eukaryotic SLP and RLPH phosphatases.
(A) SLP1 and 2 phosphatases maintain similar gene structures in lower Eukaryotes indicative
of likely gene duplication. Exon to intron ratio increased in SLP1 phosphatases versus
complete intron loss in SLP2 phosphatases. Representative SLP1 and 2 phosphatases are
shown in green and blue, respectively. (B) RLPH phosphatases demonstrated only a slight
reduction in their exon to intron ratio in higher plants. Some higher plant dicotyledonous
species indicated likely RLPH phosphatase gene duplication (e.g. A. thaliana).
Monocotyledonous plants completely lacked any indication of RLPH phosphatase gene
duplication. Representative RLPH phosphatases are shown in yellow, while higher plant
RLPH1 and RLPH2 phosphatases are depicted in yellow (original gene) and red (putative
duplicated gene).
50
Table 2.3: Intron quantity for photosynthetic Eukaryote SLP phosphatases.
Highlighted are Green Algae (grey), Selaginella moellendorffii (Lycophyte) and
Physcomitrella patens (Moss) (green) and A. thaliana (blue) SLP1 and 2 phosphatases.
Additional information includes their corresponding ‘SLP1’ and ‘SLP2’ tree identifiers, as
well as organismal and gene identifier information. Gene models were obtained from
Phytozome v7.0 (www.phytozome.net).
most straightforward interpretation of this observation is that these bacterial-like PPP genes
entered Eukaryotes very early in their history, with the advent of mitochondria. This is consistent
with the broad eukaryotic distribution of these sequences. Current concepts of the origin of
mitochondria and early Eukaryotes differ somewhat, with endosymbiosis occurring through an
α-Proteobacterium within an amitochondriate eukaryotic host, or symbiogenesis combining an αProteobacterium with another Prokaryote (usually deemed to be an Archaeon) (Koonin, 2010).
These concepts are embodied in competing and as yet unresolved models of early eukaryotic
evolution (Embley and Martin, 2006; Poole and Penny, 2007). However, all are agreed that the
advent of mitochondrial formation, with its large-scale genetic transfer to the eukaryotic nucleus,
51
together with intracellular retargeting of translated proteins, was a major driver of eukaryotic
evolution. Classically, the donor α-proteobacterium was held to be an ancient Rickettsia-like
organism (Gray, 1998; Lang et al., 1999). The data here fail to support this hypothesis. None of
the deeply placed α-proteobacterial sequences found in any of the bacterial-like PPP phosphatase
phylogenetic trees are from the order Rickettsiales. These findings are consistent with a recent
review (Gray, 2012) which emphasized that the true identity of the ancestral α-proteobacterium
has yet to be definitively established.
Superimposed on a basic pattern of α-proteobacterial ancestry is a more complex picture
of bacterial-like PPP phosphatase origins (Figure 2.8 and 2.9). In both subclasses studied here,
there is a group of bacterial sequences which cluster closely with the radiation of each sequence
type in Eukaryotes. In the case of the SLPs, these are from the δ-Proteobacteria (Myxobacteria),
while in the case of the RLPHs, these are from the Planctomycetes. As befits their positioning in
the phylogenetic trees, these sequences are much more closely related to their respective
eukaryotic sequence group than are those of the presumably ancestral α-Proteobacteria. It is
possible to interpret these results in different ways. One possibility might be HGT. A hallmark of
this process is a "discordant" clustering of sequences from distant organismal sources in the same
gene tree (Keeling and Palmer, 2008; Boto, 2010). Alternatively, given the likelihood of the αproteobacterial ancestry detailed above, a more attractive possibility becomes apparent. In this
interpretation, the particular bacterial-like PPP sequence radiation observed in Eukaryotes (e.g.
the SLPs) would be viewed as the "sister-group" of the closely related bacterial sequence cluster
(e.g. the "Outer Myxobacteria" sequences). Both would derive from the same α-proteobacterial
source (Figure 2.8 and 2.9). If this hypothesis is true, what is striking is the high degree of
52
Figure 2.8: Proposed model of RLPH phosphatase molecular evolution.
The RLPH phosphatase molecular evolution model was derived from phylogenetic tree
inference methodologies combined with consensus in silico subcellular localization data.
Dashed arrows represent the potential HGT event delivering a Planctomycetes RLPH
phosphatase to the nucleus of an ancestral non-photosynthetic Eukaryote and its subsequent
targeting to the cytosol/nucleus. Through photosynthetic Eukaryote evolution the predominant
predicted localization of RLPH phosphatases remained cytosolic/nuclear; however, the few
identified RLPH phosphatases of Green Algae and S. mollendorffii seemed to also adopt a
predicted mitochondrial localization. Solid black arrows depict primary endosymbiosis and
the corresponding gene transfer events to the eukaryotic nucleus. Light grey arrows denote
movement through evolutionary time, while dark grey arrows depict likely secondary gene
loss events. The ‘OR’ notation represents an intersection in the model where two possibilities
exist (HGT or mitochondrial endosymbiosis) for how RLPH phosphatase genes integrated into
modern Eukaryotes.
53
Figure 2.9: Proposed model of SLP phosphatase molecular evolution.
The SLP phosphatase molecular evolution model was derived from phylogenetic tree inference
methodologies combined with consensus in silico subcellular localization data. Dashed arrows
(coarse) represent the HGT-A event delivering the "outer" myxobacterial SLP3 phosphatase to
the nucleus of an ancestral non-photosynthetic Eukaryote and its subsequent targeting to the
mitochondria/cytosol. Dashed arrows (fine) represent the independent HGT-B event delivering
the "inner" myxobacterial SLP phosphatase to the nucleus of an ancestral photosynthetic
Eukaryote (i.e. glaucophyte) and its subsequent targeting to the mitochondria/cytosol. Dashed
arrows (fine) also depict SLP phosphatase gene duplication in Green Algae which lead to
distinct SLP1 (chloroplast targeted) and SLP2 (cytosolic targeted) subpopulations. Through
photosynthetic Eukaryote evolution the respective chloroplast and cytosolic localizations of
SLP1 and SLP2 phosphatases were conserved. Solid black arrows depict primary
endosymbiosis and the corresponding gene transfer events to the eukaryotic nucleus. Light grey
arrows denote movement through evolutionary time, while dark grey arrows depict likely
secondary gene loss events. The ‘OR’ notation represents an intersection in the model where
two possibilities exist (HGT or mitochondrial endosymbiosis) for how SLP phosphatase genes
integrated into modern Eukaryotes.
54
conservation between the bacterial and the eukaryotic sequences, such that a strong relationship
is apparent.
The two mechanisms outlined above are sufficient to explain the structure of the RLPH
tree, which is the simplest of the two (Figure 2.8 and 2.9). In the SLP tree, there is a further
complication in that there is a second group of "Inner Myxobacteria" sequences nested within the
overall eukaryotic radiation (Figure 2.3). This could be explained by a second application of the
"sister-group" argument, where a more basal eukaryotic SLP sequence ancestor gave rise to both
a further, more derived eukaryotic SLP radiation, and also a second side cluster of Myxobacteria
sequences. However, given that the origin of the sequences would be eukaryotic and the
destination bacterial, these would qualify as instances of HGT.
Other hallmarks of HGT besides phylogenetically "discordant" clustering patterns are socalled "patchy distributions", where there is non-uniform sequence representation amongst a
broad organismal phylogenetic group (Snel et al., 2002). Instances must be judged as individual
situations, and sometimes it still remains difficult or impossible to establish an unambiguous
mechanism. In the case of the "Inner Myxobacteria" sequences within the eukaryotic SLP
sequence distributions, HGT is favoured, as it would be difficult to conceptualize this as a case
of differential gene transmission and loss (Figure 2.9). This would require, for example, that
SLPs would be widely distributed ancestrally within both Bacteria and Eukarya (perhaps by a
universal common ancestor), then preferentially lost in such a way that lineages coincidentally
wound up closely related in a segment of Eukaryotes and Myxobacteria. Given the apparent
entry of SLP genes into Eukaryotes through α-proteobacterial endosymbiosis, this scenario
seems to be very unlikely.
55
It is worth considering the nature of the two bacterial groups (Myxobacteria and
Planctomycetes) whose sequences are most closely related to the eukaryotic bacterial-like PPP
phosphatases. It is entirely possible that the ancestral α-Proteobacteria transmitted these
sequences to a variety of bacterial offspring groups by vertical transmission, or perhaps to other
groups by HGT. Why are they now found in these particular bacterial groups with such high
degree of similarity to the eukaryotic sequences? Interestingly, both the Myxobacteria and
Planctomycetes have been noted as being "eukaryotic-like" in terms of possessing features
unusual for Bacteria. Myxobacteria possess a complex life cycle and social behaviour heavily
dependent on intercellular signaling (Goldman et al., 2006; Perez et al., 2008), while
Planctomycetes have shown signs of intracellular compartmentation (Fuerst and Sagulenko,
2011). It is possible that in each case the emerging adoption of unusually complex aspects of
bacterial cellular physiology pre-adapted this organismal group to retain and profit from the
acquisition of additional signaling protein phosphatases. Alternatively, it might be that the
acquisition of these sequences was itself important in allowing such unusual cellular adaptations.
It would seem that further research into the role of the SLPs in Myxobacteria, and RLPHs in
Planctomycetes is warranted.
The distribution of the bacterial-like PPP phosphatases with respect to Eukaryotes is truly
remarkable. SLPs are present across the entire range of photosynthetic organisms (and greatly
expanded in land plants), while being completely absent in Animals, while RLPHs are present in
only a few non-photosynthetic organisms and one species of green alga, but are widely
represented in land plants. In all cases the actual functions of the bacterial-like PPP phosphatases
in photosynthetic organisms remain largely unknown and given the differential biases in their
56
distribution, it remains impossible to formulate a general hypothesis that would encompass them
all.
Interestingly, the SLPs underwent a large gene expansion in Green Algae, with the initial
acquisition of an ancestral SLP3 phosphatase and subsequently an SLP form that gave rise to the
SLP1 and SLP2 forms later inherited by land plants. This suggests that each related gene product
might serve distinct cellular functions (Kutuzov and Andreeva, 2012). Supporting this inference
are the distinct localizations described here by in silico subcellular localization prediction data.
Finally, the RLPH gene lineage has become nearly extinct in living Green Algae, while being
ubiquitous in land plants. This might suggest again co-option of gene function in algae. It is
noteworthy that the RLPHs of land plants show a predicted cytoplasmic / nuclear localization,
which is unique within the eukaryotic bacterial-like PPP phosphatases, suggesting a marked
change in cellular function, which deserves further research exploration.
It is ironic that at present the most is known about the function of an SLP protein from a
non-photosynthetic organism. In Plasmodium berghei (the causative organism of malaria in
mice), the SHLP1 protein has been shown to be necessary for a critical life cycle stage transition,
and for development of ultrastructural features important for host cell infection (Patzewitz et al.,
2013). It is well established that Plasmodium (as all Alveolates) were ancestrally photosynthetic,
retaining an altered chloroplast remnant, the apicoplast (Kalanon and McFadden, 2010). This
indicates that in this organism the SHLP1 gene, freed from possible previous functional
constraints in a photosynthetic ancestor, evolved a novel role important to the pathogenic
lifestyle. Since both the mouse and human hosts of malaria parasites lack any evidence of SLP
genes and proteins, SHLP1 represents an attractive target for therapeutic drug development.
57
It is interesting that in the SLP phylogenetic tree there are several groups of sequences in
the deep eukaryotic portion of the tree which have long branches (indicating probable rapid
sequence evolution), and which are encoded by parasitic organisms. Most sequences in the
Apicomplexa group (including several species of Plasmodium) have a predicted signal peptide,
confirming previous findings (Kutuzov and Andreeva, 2008). This correlates with the discovery
that plasmodial SHLP1 is localized to the ER membrane. There is a single sequence from the
genus Perkinsus (a marine shellfish pathogen), a group from the Euglenozoa (including the
genera Leishmania and Trypanosoma) and a group including Oomycete plant pathogens from the
genera Phytophthora and Pythium. In the case of the Euglenozoa and Perkinsus, there is also a
marked tendency toward possession of a signal peptide. The predicted localizations are more
mixed for the Oomycete sequences, which may be due to the fact that they are the most divergent
SLP sequences in the dataset and/or the amino termini may have been mis-annotated.
In
contrast, amongst SLP sequences from photosynthetic organisms, predicted signal peptides
outside of mitochondrial- and chloroplast-targeting peptides were rare. Taken together, these
observations suggest that the SLP sequences of pathogens of both plants and animals may have
taken alternative evolutionary trajectories from those in currently photosynthetic organisms.
These genes and proteins may thus represent attractive targets of further research efforts.
The catalytic subunits of eukaryotic phosphoprotein phosphatases such as PP1 and PP2A
are well known to combine with a variety of regulatory subunits to form holoenzymes, which
provides for substrate specificity, subcellular localization, and enzymatic regulation (Virshup and
Shenolikar, 2009). In PP1, for example, these interactions are mediated by small canonical
motifs such as the RVxF motif (Templeton et al., 2011). It has been recently suggested that SLP
phosphatases might also interact with a diverse set of regulatory proteins (Kutuzov and
58
Andreeva, 2012). The data on carboxy-terminal motifs presented here demonstrates that they
maintain conserved class specific alterations, which are most pronounced in photosynthetic
Eukaryotes. Amino acid substitutions in position 6 and 3 of Motif 1 and 2, respectively, would
be expected to alter motif charge, polarity, and hydrophobicity. This could alter protein binding
specificity without an overall change in phosphatase conformation, suggesting that a regulatory
protein binding strategy might be a general feature of the bacterial-like PPP phosphatases.
Exploration of this possibility represents an attractive option for future research.
Another interesting characteristic of the bacterial-like SLP and RLPH phosphatases
examined was their gene architecture. Of particular note was a complete loss of introns in the
SLP2 phosphatase lineage over time from green algae to land plants. In A. thaliana,
Schizosaccharomyces pombe, Mice and Teleostei (fish) genes involved in processes requiring
rapid and abundant transcriptional expression have been found to possess a lower intron
abundance (Jeffares et al., 2008; Kawaguchi et al., 2010). These include genes involved in stress
response (Jeffares et al., 2008) and development (Kawaguchi et al., 2010), where transcriptional
splicing may impede the required response time for gene expression in addition to incurring
added energy costs associated with longer transcript production and splicing (Jeffares et al.,
2008). Contrary to SLP phosphatases, the RLPH phosphatase lineage underwent no notable gene
architecture changes; however, likely gene duplication in land plants was observed.
2.5 Conclusion
Here, the evolutionary trajectories of two recently resolved eukaryotic bacterial-like PPP
phosphatases was examined. SLP and RLPH phosphatase evolution was found to be unique, in
that it possessed elements of classic endosymbiotic origins as well as HGT. Emphasizing the
59
likelihood of HGT are the SLP1 and 2 phosphatases of photosynthetic Eukaryotes, which were
found to maintain closely related homologs in the distantly related δ-proteobacteria
Myxobacteria, with which they phylogenetically cluster. Further intrigue stemmed from the
revelation that SLP1 and SLP2 phosphatases are conserved across photosynthetic Eukaryotes
and predicted to be located in completely different subcellular compartments. As well, they were
found to maintain completely different gene architectures, which in conjunction with their
predicted contrast in subcellular localization, likely indicates independent cellular functions.
Similarly, the RLPH phosphatases were also predicted to possess a subcellular location differing
from the SLP phosphatases and to have recently undergone a gene duplication event in
dicotyledonous plants; however, no rationale for why this occurred was uncovered. Collectively,
the comprehensive compilation of SLP and RLPH phosphatases revealed here provides insight
into their potential application in biomedical and/or agricultural biotechnology. This is
highlighted by the lack of SLP phosphatases in humans, but their presence in the parasitic
protozoa Plasmodium.
60
Chapter Three: Bacterial-like SLP protein phosphatases from Arabidopsis
thaliana are highly conserved plant proteins that possess unique properties
3.1 Introduction
Since their discovery, protein phosphatases have emerged as key components of cellular
regulation with roles in essentially all aspects of biology. Numerous phosphatases exist in nature,
ranging from phosphate-scavenging acid phosphatases (Bozzo et al., 2002), to highly specific
protein phosphatases, which remove covalently attached phosphate groups from amino acids
phosphorylated by a specific protein kinase (DeLong, 2006; Moorhead et al., 2007). Reversible
protein phosphorylation has been documented to occur on five amino acids in Eukaryotes, with
most phosphorylation events occurring on serine (Ser), threonine (Thr) and tyrosine (Tyr)
residues (Olsen et al., 2006; Sugiyama et al., 2008). Mass spectrometry-based phosphoproteomic
studies have estimated that 70% of all proteins are regulated by reversible phosphorylation,
emphasizing the prevalence of regulatory phosphorylation events in biological systems (Olsen et
al., 2010).
Despite having similar catalytic mechanisms and targeting the same phosphorylated
residues, the PPP and PPM phosphatases differ significantly. Unlike the PPP enzymes, PPMs are
Mg2+/Mn2+-dependent, generally lack associated regulatory subunits, and are insensitive to small
molecule inhibitors, such as okadaic acid (OA) and microcystin (MCLR) (Shi, 2009). Most PPP
protein phosphatase catalytic subunits lack accessory domains and associate with additional
proteins (regulatory subunits) to direct their cellular function (Moorhead et al., 2009). In A.
thaliana and other plants, the PPP family consists of PP1, PP2A (PP2), PP4, PP5, PP6 and PP7
mammalian protein homologs, while lacking PP2B (also known as PP3)-related phosphatases
(Moorhead et al., 2009). In addition to these highly conserved PPP subclasses, Arabidopsis
61
thaliana also possess eight conserved PPP protein phosphatases that are absent in mammals
(Chapter 2; (Kerk et al., 2008; Moorhead et al., 2009)). Four of these phosphatases are Kelchrepeat PPKL protein phosphatases shown to be involved in brassinosteroid signaling (MoraGarcia et al., 2004) and another two are uncharacterized Rhizobiales-like phosphatases. The last
2 phosphatases are distantly-related to several bacterial phosphatases from Shewanella and, as
such, were termed Shewanella-like phosphatases, or SLPs (Andreeva and Kutuzov, 2004). Due
to their ancient prokaryotic origins and horizontal gene transfer from Myxobacteria to early
photosynthetic Eukaryotes (Chapter 2), as well as a number of unique structural elements within
their primary amino acid sequence, characterization of the SLP protein phosphatases from
Arabidopsis thaliana was undertaken to elucidate their biochemical properties.
3.2 Materials and Methods
3.2.1 Bioinformatics
The online search engine Genevestigator (www.genevestigator.com), which contains
pooled ATH1: 22k microarray data, and NCBI Gene Expression Omnibus (GEO) Profiles
(http://www.ncbi.nlm.nih.gov/geoprofiles) were used to assess the transcriptional expression of
At1g07010 (AtSLP1) and At1g18480 (AtSLP2) in various tissue types and across plant
development.
3.2.2 Molecular cloning
Fluorescent construct creation utilized full-length clones obtained from TAIR
(http://www.arabidopsis.org/). Propagated by PCR with Gateway compatible primers, AtSLP1
and AtSLP2 were inserted into pDONR201 via BP ligation reactions (Invitrogen). Each
62
construct
was
further
sub-cloned
into
the
plant
expression
vector
pB7RWG2
(http://gateway.psb.ugent.be/) via LR ligation reactions (Invitrogen) to create C-terminal RFP
(cRFP) fusion constructs. All cloning done in conjunction with fluorescent construct creation
was accomplished using DH5α E. coli. Agrobacterium tumefaciens strain GV3101
(Agrobacterium) was transformed with each fluorescent construct for wild-type A. thaliana cell
culture transfection purposes. Heterologous protein expression employed full-length AtSLP1
(minus the predicted chloroplast transit peptide, amino acids 1 - 53) and AtSLP2 cloned
containing EcoRI and NotI restriction sites. Each construct was initially TA cloned into pGEM-T
(Promega) and subsequently sub-cloned into pET47b(+) for antibody production and pET48b(+)
for purification and enzymatic characterization (EMD-Chemicals).
3.2.3 Cell culture transfection and protoplast creation
Wild-type A. thaliana cell culture transfection was modeled from previous studies
(Forreiter et al., 1997). Empirical testing of the cell culture transfection process found 3 d old A.
thaliana liquid cell culture grown under 24 h light in 1 x Murashige Skoog (MS) medium along
with 3% w/v sucrose, 0.5 mg/mL 1-naphthaleneacetic acid (NAA), 0.05 mg/mL kinetin (1x
Growth Media) combined with transformed Agrobacterium grown to an OD600 = 0.5 at 28oC,
150 rpm re-suspended in 10 mL of 1 x growth media as ideal for successful A. thaliana cell
transfection. Incubation of Agrobacterium and A. thaliana liquid cell culture was performed for 2
d at room temperature under 24 h light shaking at 150 rpm. The Agrobacterium-A. thaliana cell
culture mixture was then pelleted at 300 x g for 1 min and re-suspended in 1 x growth media
containing 100 µg/mL ampicillin (repeated 3 x). Cells were then spread on 0.8% w/v agar plates
containing 1 x growth media and 10 µg/mL Basta and grown under 24 h light for 2 weeks to
63
select for positively transfected A. thaliana cells. Positive transfectants grown as callus cells
were visually identified (green papules) and re-plated on 1x selective media then sub-cultured to
liquid 1 x growth media and grown as above under 24 h light. Protoplasts were subsequently
derived from 10 mL of positively transfected Arabidopsis thaliana cell culture as previously
described (Lingard et al., 2008). Protein expression was confirmed by Western blotting with antiRFP IgG (Chromotek).
3.2.4 Transient AtSLP expression in Vicia faba epidermal leaf cells
Transient co-expression of AtSLP1-RFP and AtSLP2-RFP with fluorescent marker
constructs was conducted using equimolar amounts of gold coupled with DNA and particle
bombardment. Plasmid DNA (10 µg) was coated onto 1 mg of gold microcarriers, washed,
spotted on a macrocarrier and accelerated at the epidermal layer of Vicia faba leaves using a
PDS-1000/He System (BioRad) as per (Russell et al., 1992). AtSLP1-RFP was co-bombarded
with GFP constructs specifically designed to target the endoplasmic reticulum (ER), Golgi and
peroxisome (Nelson et al., 2007), while AtSLP2-RFP was co-bombarded with established
mitochondria, ER, Golgi (Nelson et al., 2007) and cytosolic/nuclear targeted GFP (vector
p2FGW7; GFP with no targeting motif). AtSLP2-RFP was also co-bombarded with GFP-tagged
nucleoporin protein 50a to illuminate the nucleus (Tamura et al., 2010).
3.2.5 Microscopy
All imaging was conducted using a Leica DMIRE2 spectral confocal and multiphoton
microscope with a Leica TCS SP2 acoustic optical beam splitter (AOBS) (Leica Microsystem,
Richmond Hill, ON, Canada), with all cells and leaves visualized using the 63x water immersion
64
lens. The excitation / emission wavelengths (nm) respectively employed: GFP 488/505-515, RFP
594/610-650 and chlorophyll autofluorescence 488 & 594/685-715. Subsequent image
processing
was
performed
using
the
freely
accessible
MacBiophotonics
ImageJ
(http://www.macbiophotonics.ca/downloads.htm).
3.2.6 Heterologous protein expression and antibody production
AtSLP1- and AtSLP2-HIS6 fusion proteins were expressed in BL21 (DE3) Codon(+)RIL E.coli grown in LB containing 1 mM MnCl2 and 1 % glucosamine at 8oC, 200 rpm by
induction with 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 24 h. Bacteria were
pelleted at 4000 x g for 15 min and re-suspended in 1 x extraction buffer (50 mM Hepes-NaOH
pH 7.5, 150 mM NaCl, 5 % (v/v) glycerol and 10 mM imidazole) followed by snap freezing and
storage at -80oC prior to use. Day of purification saw the addition of 1 % (v/v) Tween-20, 20
mM imidazole, 1 mM phenylmethanesulfonylfluoride (PMSF), 1 mM benzamidine, 2 µg/mL
leupeptin, 5 µg/mL pepstatin upon thawing. Extraction involved mechanical lysis via French
press at 3 x 16000 PSI (Sim-Aminco Spectronic Instruments). Crude lysates were clarified at
40000 x g for 30 min at 4oC. Supernatants were removed and 1 mM PMSF and 1 mM
benzamidine added prior to incubation with NiNTA agarose matrix (Qiagen) end-over-end
mixing at 4oC for 1 h. Matrix was poured into a column and washed with 500 column volumes
(cv) of wash buffer A (1 x extraction buffer with 1 M NaCl, 1% (v/v) Tween-20 and 20 mM
imidazole, minus protease inhibitors) by gravity followed by 100 cv of wash B (1x extraction
buffer minus protease inhibitors). NiNTA-bound proteins were eluted using 1 x extraction buffer
containing 500 mM imidazole pH 7.5. Protein eluates were concentrated using a 30000 MWCO
Amicon concentrator (Millipore).
65
Purification of AtSLP1 to homogeneity involved four steps: 1) NiNTA purification, 2)
size exclusion chromatography, 3) cleavage of the affinity tag by HRV3c protease (Novagen),
and 4) anion exchange chromatography. Heterologous HIS6-AtSLP1 expression and subsequent
purification by NiNTA was conducted as outlined above from 4 x 1 L of bacterial cell culture.
The NiNTA HIS6-AtSLP1 eluate was concentrated to 500 µL and subjected to size exclusion
chromatography using a HiLoad 16/60 Superdex 200 (Amersham) column. The sample was
chromatographed at 0.5 mL/min, collecting 1 mL fractions in buffer A (50 mM Hepes-NaOH
pH 7.5, 50 mM NaCl, and 5% (v/v) glycerol). Column fraction proteins were resolved by SDSPAGE (12%) and visualized by Colloidal blue. Peak fractions were pooled and concentrated to
500 µL in a 30000 MWCO Amicon concentrator. Cleavage of the HIS6-tag was performed on
500-700 µg of total protein from the concentrated size exclusion chromatography eluate using 10
U of Novagen HRV 3C protease (EMD Chemicals) in the presence of 1 mM dithiothreitol (DTT)
overnight at 4oC rotating gently end-over-end. Anion exchange chromatography was performed
on a Mono-Q 5/50 GL (Amersham) column employing Buffer A (as above) and Buffer B
consisting of 50 mM Hepes-NaOH pH7.5, 1 M NaCl, 5% (v/v) glycerol and 1 mM DTT. The
concentrated Superdex 200 eluate was loaded at 0.5 mL/min, the column washed at 1 mL/min
for 10 cv and protein eluted using a gradient of 50 - 400 mM NaCl over 35 cv collecting 1 mL
fractions. Peak fractions were run on SDS-PAGE, pooled, concentrated as above, aliquoted, snap
frozen in liquid nitrogen and stored at -80oC for subsequent use.
AtSLP1- and AtSLP2-pET47b were expressed in BL21 (DE3) Codon(+)-RIL E. coli at
37oC and induced with 0.5 mM IPTG for 4 h and purified from inclusion bodies using NiNTA
Agarose as per the manufacturer's instructions (Qiagen). Purified protein was dialyzed against
water, freeze dried and used for polyclonal antibody production in a New Zealand White rabbit
66
as described (Tran et al., 2004). Both the AtSLP1 and AtSLP2 antibodies were affinity purified
using nitrocellulose membrane as previously described in the absence of BSA (Plaxton, 1989).
3.2.7 Enzymatic analysis
Enzymatic assessment of AtSLP1 and AtSLP2 was conducted using the small molecule
phosphatase substrate para-nitrophenyl phosphate (pNPP) (Sigma). Enzymatic assays comparing
AtSLP1 and AtSLP2 were conducted using day-of purified and concentrated NiNTA eluates. All
assays were done in parallel with NiNTA eluates generated from uninduced BL21 (DE3)
Codon(+)-RIL E. coli to account for potential background pNPP cleavage from non-specific copurifying proteins (NSCP). HIS6-AtSLP1 and HIS6-AtSLP2 percent activities were calculated
relative to assays conducted with 0.5 mM Mn2+ or 5 mM EDTA, respectively. Due to its
stability, pure, untagged AtSLP1 was used to perform more thorough enzymatic analyses in
conjunction with completely purified AtPP1/TOPP2 (At5g59160) and partially purified (~50%)
human PP1 gamma (HsPP1γ; NP_002701) as assay benchmarks. Both TOPP2 and HsPP1γ were
cloned, expressed and purified from E. coli as previously described (Douglas et al., 2001;
Templeton et al., 2011). Enzymatic assay strategy was adapted from the SensoLyte pNPP Protein
Phosphatase Assay Kit (http://www.anaspec.com). Base Buffer consisted of 100 mM HEPES pH
7.5 and 150 mM NaCl. Dilution buffer(s) consisted of base buffer plus 4 mM DTT and 0.2 mM
EDTA with either 0.5 mM metal cation (metal-dependent activity) or 5 mM EDTA (metal
independent activity). Assay buffer consisted of dilution buffer plus a final concentration of 5
mM pNPP. All protein samples were pre-mixed under their experimental condition, brought to
20 µL with the respective dilution buffer and pre-incubated at 30oC for 10 min prior to
enzymatic analysis. Enzyme assays were initiated by the addition of 180 µL of assay buffer and
67
incubation at 30oC for 30 min (NiNTA AtSLP eluates and controls) or 20 min (pure AtSLP1 and
controls). For the NiNTA AtSLP and control eluates 750 ng of total protein was used per assay.
Assays involving pure AtSLP1 employed 100 ng AtSLP1, 200 ng TOPP2 and 400 ng total
Human (Hs) PP1γ protein (200 ng pure HsPP1γ). Assays were quenched with 200 µL of 0.5 M
EDTA and pNPP cleavage assessed using an Ultrospec 2000 spectrophotometer set to 405 nm.
3.3 Results
3.3.1 AtSLP1 and AtSLP2 localize to different cellular compartments
Full-length AtSLP1 and AtSLP2 were fused to a C-terminal RFP and transfected into A.
thaliana cell culture to create protoplasts constitutively expressing either AtSLP1-RFP or
AtSLP2-RFP for live-cell imaging. Stably transfected cell culture was extracted, resolved by
SDS-PAGE and probed with anti-RFP to verify expression of the fusion protein (Figure 3.1).
Positive AtSLP1-RFP cell culture demonstrated chloroplastic localization (Figure 3.2A), while
positive AtSLP2-RFP cell culture maintained a non-chloroplastic localization (Figure 3.2B).
Transient co-expression of RFP-tagged AtSLPs in Vicia faba epidermal leaf cells with
various fluorescence marker proteins was also performed to further resolve the specific
subcellular localization of AtSLP1 and AtSLP2. AtSLP1-RFP, co-expressed with GFP
specifically targeted to the endoplasmic reticulum (ER) or peroxisome, confirmed the exclusive
chloroplast subcellular localization of AtSLP1 by the co-localization of AtSLP1-RFP with
chlorophyll autofluorescence and not with either GFP construct (Figure 3.3). The localization of
AtSLP2 was resolved through transient co-expression of AtSLP2-RFP with non-targeted GFP
(GFP alone resides in both the cytosol and nucleus; Figure 3.4) and GFP targeted specifically to
either the ER or mitochondria (Figure 3.4). AtSLP2-RFP demonstrated specific
68
Figure 3.1: Development of AtSLP1 and 2 stably-transfected A. thaliana cell culture.
(A) Schematic of fluorescent constructs used in AtSLP1 and AtSLP2 subcellular localization.
(B) Schematic depicting the use of Agrobacterium to transfect A. thaliana cell culture. Cell
culture was grown under 24 h light on selective 1 x MS-Agar as a primary screen for positive
transfectants. Modified from http://www.accessscience.com. (C) Western blot verification of
stably-transfected cell culture constitutively expressing AtSLP1-RFP or AtSLP2-RFP. Each
lane contains 30 µg of crude cell culture lysate probed with 1:100 Anti-RFP IgG (Chromotek).
69
Figure 3.2: In vivo subcellular localization of AtSLP1 and 2 using stably-transfected A.
thaliana cell culture.
(A) and (C) Fluorescence images of protoplasts derived from stably transfected A. thaliana
cell culture expressing AtSLP1-RFP or AtSLP2-RFP (red), respectively. Chlorophyll
autofluorescence is also shown (green) and images merged to reveal localization. All images
are single slices obtained using a confocal laser scanning microscope (Leica). Scale bars = 10
µm. (B) and (D) depict merged stacks of protoplast image slices derived from stably
transfected A. thaliana cell culture stably expressing AtSLP1-RFP and AtSLP2-RFP (red)
with chlorophyll fluorescence (green). Overlapping RFP and GFP signals are yellow. The X
and Y axis define the 2D sides of the merged image (i.e. top view), while the Z-axes depict a
view through the stacked slices (i.e. side view) giving a 3D rendering of fluorescence overlap
through the X and Y axes.
70
Figure 3.3: Co-localization of AtSLP1 with fluorescent marker constructs in Vicia faba
epidermal leaf cells.
(A) and (B) are representative images of guard cells co-bombarded with AtSLP1-RFP (red)
and either endoplasmic reticulum (ER)- or peroxisomal-targeted GFP (green), respectively.
Chlorophyll fluorescence (blue) is shown along with the image merge (right). The specific
organelle targeting of GFP was achieved though fusion with conserved targeting motifs
(Nelson et al., 2007). All images are single slices obtained using a confocal laser scanning
microscope (Leica). Scale bars = 10 µm.
71
Figure 3.4: Co-localization of AtSLP2 with fluorescent marker constructs in Vicia faba
epidermal leaf cells.
(A) and (B) represent images of pavement cells co-bombarded with AtSLP2-RFP and either
mitochondrial- or endoplasmic reticulum (ER)-targeted GFP, respectively. The specific
organelle targeting of GFP was achieved as described in Figure 3.3. (C) AtSLP2-RFP cobombarded with GFP-tagged nucleoporin protein 50a (Nup50a-GFP) (Tamura et al., 2010).
(D) AtSLP2 co-localized with GFP-only (no targeting motif). GFP-only localizes to both the
nuclear and cytosolic compartments (Seibel et al., 2007) but only co-localizes with AtSLP2RFP in the cytosolic fraction. All images are single slices obtained using a confocal laser
scanning microscope (Leica). Scale bars = 10 µm.
72
co-localization with only the non-targeted (cytosolic) GFP indicating a cytosolic subcellular
localization (Figure 3.4). Oddly, the cytosolic AtSLP2-RFP signal was punctate in nature.
AtSLP2-RFP was also co-expressed with GFP-tagged nucleoporin protein 50a (Nup50a), a
known nuclear protein (Tamura et al., 2010), to account for the dual subcellular localization of
non-targeted GFP to the cytosol and nucleus (Seibel et al., 2007). AtSLP2-RFP did not colocalize with Nup50a-GFP (Figure 3.4). Overall, these studies suggest that SLP1 phosphatases
reside in the chloroplast and SLP2 phosphatases are present in the cytosol.
3.3.2 Temporal and spatial expression of AtSLP1 and AtSLP2
Initial characterization of endogenous AtSLP phosphatases consisted of transcriptional
expression analysis using Genevestigator (www.genevestigator.com). The Genevestigator search
engine houses a number of A. thaliana microarray experiments based on the commercially
available A. thaliana ATH1: 22k array (http://www.affymetrix.com) to explore gene expression
under various experimental conditions. This screen indicated AtSLP1 and AtSLP2 phosphatases
are differentially expressed among A. thaliana plant tissues and throughout plant development
(Figure 3.5A). AtSLP1 had highest expression in cauline leaves, sepals and in photosynthetic
tissues but was absent in roots and other non-photosynthetic tissues (i.e. seeds), while AtSLP2
demonstrated elevated transcriptional expression in protoplasts, roots and the endosperm of seeds
(Figure 3.5A). Both AtSLP1 and AtSLP2 also exhibited different transcriptional expression
patterns across plant development (Figure 3.5B) and in response to diurnal cycling. AtSLP1
transcript expression demonstrated a sharp increase upon germination followed by a subsequent
decrease towards plant maturity (Figure 3.5B) as well as a clear diurnal fluctuation in expression
pattern (Figure 3.6A). AtSLP2 however, did not demonstrate any major transcriptional
73
Figure 3.5: AtSLP transcript expression data.
AtSLP transcript expression from (A) different tissue types and (B) across plant development.
Transcript expression is indicated by a gradient of white (low expression) to blue (high
expression) as a percentage of the top 1% genes expressed in each tissue. The numbers beside
(A) and above (B) describe the number of total arrays used in determining the displayed level
of relative gene expression for AtSLP1 and AtSLP2. Data obtained from Genevestigator
(www.genevestigator.com).
74
fluctuations over the course of plant development or during the diurnal cycle (Figure 3.6B)
To examine if AtSLP gene expression was paralleled at the protein level, antibodies were
raised using heterologously expressed HIS6-AtSLP1 and HIS6-AtSLP2 protein purified by
NiNTA (Figure 3.7). Affinity purification (AP) of each antibody was performed to enhance the
specific detection of each phosphatase (Figure 3.8). The AP anti-AtSLP1 IgG provided monospecific detection of AtSLP1, while the AP of anti-AtSLP2 IgG resulted in an antibody that
detects both AtSLP1 and AtSLP2, but with greater affinity for AtSLP2 (Figure 3.8). The
documented transcript expression profile of AtSLP1 and AtSLP2 uncovered using Genevestigator
was largely paralleled at the protein level (Figure 3.9A) with AtSLP1 protein observed in all
photosynthetic tissues with the exception of siliques. Conversely, AtSLP2 was only definitively
detectable in roots and intact siliques (containing seeds). Immunoblotting was less conclusive for
other tissues due to the cross-reactivity of the AP anti-AtSLP2 IgG with AtSLP1 and a small 2
kDa mass difference between endogenous AtSLP1 (~41 kDa) and AtSLP2 (~43 kDa).
Interestingly, AtSLP2 was barely detectable in intact siliques despite comparatively high levels
of transcript in seeds relative to other plant tissues.
AtSLP1 protein expression was also examined over a light / dark photoperiod consisting
of 12 h light and 12 h dark. Initial examination of online accessible transcript expression data
from NCBI Gene Expression Omnibus (GEO) Profiles (www.ncbi.nlm.nih.gov/geoprofiles)
demonstrated a diurnal cycling of AtSLP1 transcript, but no such pattern for AtSLP2 expression
(Figure 3.6). Despite the noted changes in AtSLP1 transcript levels, AtSLP1 protein was basally
detectable over the entire diurnal cycle, with protein expression beginning to increase at 4 h light
and peaking at the 8 h light time point before decreasing back to basal expression levels (Figure
3.9B).
75
Figure 3.6: NCBI Gene Expression Omnibus (GEO) profile of diurnal AtSLP1 and
AtSLP2 transcript cycling (www.ncbi.nlm.nih.gov/geoprofiles).
(A) AtSLP1 and (B) AtSLP2 transcript expression over the course of a 12 h light and 12 h
dark photoperiod. The red columns each represent the expression measurement from an
original GEO profile submitter-supplied sample record. The grey boxes located below each
red column represent the original sample accession. The blue squares on top of each column
represent where the expression of that gene falls with respect to all other genes measured on
that array.
76
Figure 3.7: Colloidal blue stained 12 % SDS-PAGE of the HIS6-AtSLP1 and HIS6AtSLP2 NiNTA eluates employed in producing anti-AtSLP1 and anti-AtSLP2
polyclonal antibodies.
NiNTA purifications shown in panels (A) and (B) were performed as per the manufacturer's
instructions (Qiagen) using purified inclusion bodies. Each lane contains 5 µg of total
protein. W/C and S/N represent the whole cell lysate and supernatant, respectively.
77
Figure 3.8: Western blot assessment of affinity-purified (AP) anti-AtSLP1 and antiAtSLP2 IgG detection limits and specificity.
Both NiNTA-purified antigen (HIS6-AtSLP1 & HIS6-AtSLP2) as well as AtSLP-RFP fusion
proteins stably expressed in light-grown A. thaliana cell culture were used to test the detection
limits and cross reactivity of each set of antibodies. In (A) 0.01 to 10 ng of recombinant
AtSLP1 and AtSLP2 was blotted with either 1.5 or 1.2 µg/mL AP anti-AtSLP1 or antiAtSLP2 IgG, respectively. Anti-AtSLP1 IgG demonstrated mono-specific detection of
AtSLP1, while AP anti-AtSLP2 IgG demonstrated detection of AtSLP2 in addition to cross
reacting with AtSLP1. In (B) 0.1 to 30 µg of extracted AtSLP1-RFP and AtSLP2-RFP
expressing cell culture was blotted as above. Anti-AtSLP1 IgG again demonstrated
monospecific detection of AtSLP1 (AtSLP1-RFP), while Anti-AtSLP2 IgG exhibited the same
non-specific detection of AtSLP1 (AtSLP1-RFP) as in (A) in addition to clear detection of
AtSLP2 (AtSLP2-RFP).
78
Figure 3.9: Spatial and temporal western blot analysis of AtSLP1 and AtSLP2 protein
expression.
(A) Tissue-specific expression of AtSLP1 and AtSLP2 from different A. thaliana Col-0 tissues.
Each lane contains 30 µg of clarified cell lysate. (B) Time-course analysis of AtSLP1 protein
expression over 12 h light / 12 h dark photoperiod. Each lane contains 30 µg of clarified lysate
from rosette leaf tissue. All tissues were harvested from 21 d old plants. Lower panels of both
(A) and (B) represent Ponceau S (0.1 % w/v)-stained membranes demonstrating equal protein
loading prior to incubation with either affinity-purified (AP) anti-AtSLP1 or -2 IgG.
79
3.3.3 AtSLP phosphatase primary sequence and enzymatic properties
Alignment of the AtSLPs with the other A. thaliana PPP protein phosphatases and
representative human PPP protein phosphatases uncovered many amino acid substitutions and
unique regions within each full-length AtSLP amino acid sequence. An alignment containing
representative PPP protein phosphatases is shown along with AtSLP1 and AtSLP2 (Figure 3.10).
Human PP1γ (HsPP1γ) and an A. thaliana PP1 (TOPP2) were found to be 76 % identical to each
other, but only possessed ~10 % identity to either of the AtSLP phosphatases. Regions of
conserved identity between HsPP1γ, TOPP2 and the AtSLP phosphatases were found to largely
reside in the key amino acid clusters comprising the active site of PPP protein phosphatases
(Figure 3.10; Shi 2009). AtSLP1 and AtSLP2 however, share only 31% identity to each other
and possess many overlapping regions of identity beyond those comprising the PPP protein
phosphatases active site (Figure 3.10). SLP phosphatases were also found to lack 7 of the 8 key
amino acids involved in the docking of mammalian and yeast PP1 regulatory subunits through
the RVXF sequence motif found in essentially all PP1 interactors (Egloff et al., 1997; Moorhead
et al., 2007; Dancheck et al., 2011). Key amino acid stretches shown to be involved in the
formation of PP2A trimeric complex were also absent (Xu et al., 2006). Of the many differences
revealed at the amino acid level between the SLPs and the eukaryotic PPP protein phosphatases,
the absence of the canonical SAPNYC motif was of particular interest (Figure 3.10). Although
not directly involved in catalysis or metal ion coordination, the cysteine within this motif forms a
covalent bond with the potent PPP phosphatase inhibitor microcystin-LR (MCLR) (MacKintosh
et al., 1995). SLP phosphatases were also found to lack other conserved amino acids within the
80
Figure 3.10: Alignment of AtSLP1 and AtSLP2 full-length protein sequences with
representative PP1 sequences from Arabidopsis thaliana (TOPP2; At5g59160), AtPP2A-1
(At1g59830) and Homo sapiens (HsPP1γ; NP_002701).
Alignment was performed using ClustalX followed by import into GeneDoc for image assembly.
Solid black line represents highly conserved motifs comprising the active site of canonical
Ser/Thr phosphatases. Dashed line represents a highly conserved region in PPP phosphatases
which conveys sensitivity to microcystin-LR (MCLR) through its covalent attachment to Cys273
(HsPP1γ) and equivalent residue in a plant PP1, Cys279 (TOPP2) (§). Black circles denote
amino acids specifically important to MCLR binding, while black squares denote amino acids
specific to OA binding, but are also key in coordinating MCLR. The arrows denote amino acids
found to play a role in metal ion coordination, while black triangles denote amino acids found to
dock the PP1 inhibitor I2 protein. Amino acids marked with an open square have been implicated
in the binding of MCLR, OA and I2.
81
SAPNYC motif and throughout their full-length sequence which coordinate okadaic acid (OA)
(Maynes et al., 2001) and the PP1 specific inhibitor I2 (Figure 3.10; (Hurley et al., 2007)). This
would suggest that the SLP phosphatases are resistant to the toxins and proteins that have
evolved to inhibit the PPP protein phosphatases by docking these regions. To explore this idea,
enzyme assays were performed using a selection of phosphatase inhibitors.
To assess the enzymatic properties of the AtSLP phosphatases each protein was
expressed as a HIS6-fusion protein in E. coli and purified to near homogeneity by NiNTA
(Figure 3.11). Both HIS6-AtSLP1 and HIS6-AtSLP2 NiNTA eluates had additional non-specific
co-purifying (NSCP) proteins, rendering a control NiNTA eluate from the same E. coli
expression strain necessary to control for potential NSCP phosphatase activity during enzymatic
analyses of each AtSLP (Figure 3.11). Enzymatic assays were conducted using the artificial
phosphatase substrate pNPP to assess the activity of each AtSLP phosphatase. HIS6-AtSLP1 was
most active in the presence of Mn2+, but could also use Fe3+ to generate 65% of its observed
Mn2+ activity (Figure 3.12A). Contrary to HIS6-AtSLP1, HIS6-AtSLP2 was seemingly most
active in the absence of additional metal ions (i.e. 5 mM EDTA). Application of a Student's Ttest found no significant difference between the activity achieved with 5 mM EDTA, and that
achieved with either 0.5 mM Mg2+ or 0.5 mM Zn2+. A notable inhibitory effect was observed in
the presence of Fe3+, which reduced HIS6-AtSLP2 activity by 70% (Figure 3.12A).
Along with examining metal cation dependency the AtSLP phosphatases were tested for
sensitivity to classic protein phosphatase inhibitors (Figure 3.12B). Both HIS6-AtSLP1 and
HIS6-AtSLP2 demonstrated a complete lack of inhibition in the presence of the PPP phosphatase
inhibitors OA (150 nM) and MCLR (10 nM). Surprisingly, HIS6-AtSLP1 phosphatase activity
82
Figure 3.11: 12 % SDS-PAGE of NiNTA-purified HIS6-AtSLP1, HIS6-AtSLP2 and
uninduced bacteria cell line control eluates stained with Colloidal blue.
HIS6-AtSLP1 and HIS6-AtSLP2 were partially purified by NiNTA along with non-specific copurifying (NSCP) NiNTA-binding proteins isolated in parallel from untransformed, uninduced
BL21 (DE3) CP-RIL grown under identical conditions. The BL21 (DE3) CP-RIL NiNTA
eluate controlled for non-specific cleavage of the phosphatase substrate pNPP by NSCP
proteins during enzymatic analysis of NiNTA-purified HIS6-AtSLP1 and HIS6-AtSLP2. The
NSCP protein glucosamine-fructose-6-phosphate aminotransferase (GFAT, *) was identified by
MALDI-TOF peptide mass fingerprinting, while (**) represents another NSCP protein. Each
lane contains 5 µg of concentrated NiNTA eluate.
83
Figure 3.12: Enzymatic analysis of HIS6-AtSLP1 and HIS6-AtSLP2.
(A) Metal dependency assessment of each SLP phosphatase was performed using a variety of
metal cations as well as 5 mM EDTA. (B) Analysis of known Ser/Thr phosphatase inhibitors
employed at concentrations known to fully inhibit bacterially-expressed and purified Homo
sapiens and Arabidopsis thaliana PP1 protein phosphatases. Dark grey bars represent HIS6AtSLP1 while the light grey bars represent HIS6-AtSLP2. Inhibition assays were conducted as
outlined in Materials and Methods and were performed using OA, MCLR, AtI2 (At5g52200),
pyrophosphate (PPi), phosphate (Pi) and sodium fluoride (NaF). 100% enzyme activity in (A)
and (B) is defined by the presence of 0.5 mM Mn2+ (AtSLP1 assays) or 5 mM EDTA (AtSLP2
assays). Error bars denote ± standard error; n = 3.
84
was activated in the presence of OA (~145%) and MCLR (~140%), while these exhibited no
overall effect on HIS6-AtSLP2 activity (Figure 3.12B). HIS6-AtSLP1 also showed enhanced
inhibition by 5 mM pyrophosphate (PPi) and 50 mM phosphate (Pi) relative to HIS6-AtSLP2,
while both HIS6-AtSLP1 and HIS6-AtSLP2 showed similar inhibition by 100 mM sodium
fluoride (NaF) (Figure 3.12B). Both AtSLPs were also tested for their sensitivity to the wellcharacterized and specific PP1 protein inhibitor I2 from A. thaliana (AtI2) (Templeton et al.,
2011). Both AtSLP1 and AtSLP2 exhibited minimal sensitivity to AtI2 being inhibited ~40 %
and ~20 %, respectively, at a 1 µM concentration (Figure 3.12B).
3.3.4 Characterization of purified untagged AtSLP1
Due to the inability to identify conditions permitting the stable purification and storage of
AtSLP2 past initial NiNTA elution, only AtSLP1 was purified to homogeneity. Initial HIS6AtSLP1 NiNTA eluate was concentrated and subjected to Superdex 200 size exclusion
chromatography (Figure 3.13A) followed by enzymatic affinity tag removal and subsequent
Mono-Q anion exchange separation as described in the Materials and Methods (Figure 3.13B).
Pooled and concentrated Mono-Q peak fractions were used for enzymatic analyses of untagged
AtSLP1 (Figure 3.14). Some degradation of full-length AtSLP1 was observed as a 20 kDa
fragment was confirmed as AtSLP1 by mass spectrometry (Table 3.1) and immunoblot analysis
(Figure 3.14).
85
Figure 3.13: Size exclusion and anion exchange chromatography steps of AtSLP1
purification.
(A) AtSLP1 was purified on NiNTA followed by concentration for further purification on
HiLoad 16/60 Superdex 200. Inset, aliquots (25 µL each) from various fractions were subjected
to SDS-PAGE and Colloidal blue staining. (B) Peak fractions from Superdex 200
chromatography were pooled, digested with HRV3c protease (Novagen) to release the HIS6tag (affinity tag), then run on Mono-Q 5/50 GL. Inset, aliquots (25 µL each) from various
fractions were also subjected to SDS-PAGE followed by protein staining with Colloidal blue.
The right hand inset represents the Mono-Q pool fraction used for enzymatic analysis of
AtSLP1. AtSLP1 polypeptide identifications were confirmed by immunoblot analysis (Figure
3.14).
86
Figure 3.14: Analysis of the major end-point purification steps employed to completely
purify AtSLP1.
(A) Represents the NiNTA (panel 1), Superdex 200 / proteolytic HIS6-tag cleavage (panel 2)
and Mono-Q (panel 3) purification steps resolved by 12 % SDS-PAGE and visualized by
Colloidal blue. (*) & (**) represent the NSCP proteins previously described (Figure 3.11). Each
lane contains 5 µg of total protein with the exception of the Mono-Q pool which contained 2 µg
of total protein. (B) Western blot of the Mono-Q purified AtSLP1 (25 ng) shown in (A)
performed using anti-AtSLP1 IgG. This purified AtSLP1 was subsequently used in enzymatic
analysis. S/N and F/T represent the supernatant and flow through, respectively.
87
Table 3.1: MALDI-TOF mass spectrometry identification of lower molecular weight
polypeptides in the Superdex 200 pool post-digest.
Peptides were searched against the NCBInr database (http://www.ncbi.nlm.nih.gov) using
MASCOT (http://www.matrixscience.com).
Enzymatic characterization of pure, HIS6-tag free AtSLP1 was conducted to elucidate the
impact of the HIS6 affinity tag on both small molecule and protein inhibitor sensitivity as well as
its affinity for a protein substrate. For comparison, control assays containing the same inhibitors
at the same experimental concentrations were performed in parallel using purified HIS6-TOPP2
and HsPP1γ (Figure 3.15). As observed with HIS6-AtSLP1, untagged AtSLP1 was again
activated by both OA and MCLR (Figure 3.16). Activation by OA was moderately reduced in
the absence of the HIS6-tag, while MCLR elicited the same 40 % increase in AtSLP1
phosphatase activity. PPi, Pi and NaF all had inhibitory effects identical to those observed with
HIS6-AtSLP1 (Figure 3.16).
3.4 Discussion
3.4.1 Subcellular targeting of the AtSLP phosphatases
Previous bioinformatic and proteomic studies indicated SLP phosphatases occupy a
number of subcellular locations (Chapter 2). In particular, AtSLP1 was speculated to be
peroxisome- (Fukao et al., 2002) and ER-targeted, while AtSLP2 was suggested to reside solely
in
88
Figure 3.15: Colloidal blue stained 12 % SDS-PAGE of purified HsPP1γ and HIS6-TOPP2.
Each protein was purified as previously described: TOPP2 (Templeton et al., 2011) and HsPP1γ
(Douglas et al., 2001). Each lane contains 5 µg of total protein.
89
Figure 3.16: Assessment of purified, untagged AtSLP1 sensitivity to small molecule
inhibitors.
(A) and (B) Inhibition curves comparing the phosphatase inhibitor sensitivity of AtSLP1 to
TOPP2 and HsPP1γ using the PPP protein phosphatase inhibitors OA and MCLR. (C - E)
Inhibition curves comparing the PPi, Pi and NaF sensitivity of AtSLP1 to TOPP2. All
phosphatase assays were conducted using the small molecule substrate pNPP as outlined in the
Materials and Methods. AtSLP1 (Black inverted triangle), TOPP2 (open circle) and HsPP1γ
(solid circle) are represented, respectively.
90
the ER (Kutuzov and Andreeva, 2008). Using a combination of fluorescent fusion constructs
stably transfected into cell culture, and transiently co-expressed Vicia faba leaves, a subcellular
localization was elucidated for AtSLP1 (chloroplast) and AtSLP2 (cytosol).
These AtSLP
findings substantiated the in silico subcellular localization prediction consensus for SLP1 and
SLP2 phosphatases (Chapter 2), emphasizing the likelihood of a conserved chloroplastic and
cytosolic location for the respective enzymes in all plants.
Previous application of prediction algorithm-assisted inspection of AtSLP1 and other
SLP1 phosphatase amino acid sequences using the peroxisomal targeting sequence (PTS1)
predictor (http://mendel.imp.ac.at /mendeljsp/sat/pts1/PTS1predictor.jsp), as well as Predotar and
Wolf pSORT, found no evidence of a canonical C-terminal PTS1 signaling motif (Chapter 2).
The possibility of a N-terminal PTS2 signal or a non-canonical PTS signaling motif was also
considered (Lazarow, 2006; Girzalsky et al., 2009), but with the C-terminal AtSLP1-RFP fusion
protein lacking observable peroxisome localization in either the stably-transfected cell culture or
transiently-expressed condition, it is unlikely that AtSLP1 resides in the peroxisome. Previous
accounts of AtSLP1 peroxisome targeting were based on the proteomic analysis of isolated plant
peroxisomes (Fukao et al., 2002), rendering chloroplast contamination during their purification a
possibility. Previous bioinformatic analysis of the SLP phosphatases suggested AtSLP1 was also
ER-targeted (Kutuzov and Andreeva, 2008). These predictions were based on the identification
of a putative C-terminal ER retention motif (KDEL or RDEL) and an in silico subcellular
prediction result from the online resource Predotar (Kutuzov and Andreeva, 2008). Despite these
findings, the consensus of 10 subcellular localization prediction algorithms employed previously
(Chapter 2), combined with experimentally verified subcellular localization results of AtSLP1
demonstrated here, do not support ER localization for SLP1 phosphatases. One of the 10 in silico
91
targeting programs employed in the subcellular localization prediction analysis was Predotar, and
as in the previous study, it predicted several SLP1 phosphatases to be ER targeted (Chapter 2).
ER localization, however, was not suggested by any other program, with the majority of
programs predicting the same SLP phosphatases to be chloroplast targeted (Chapter 2). Given the
overall predicted chloroplast localization consensus of SLP1 phosphatases, the experimentally
verified chloroplast localization of RFP-tagged AtSLP1 and high level of conserved identity
(~50-70%) across SLP1 phosphatase homologs, SLP1 phosphatases likely reside solely in the
chloroplast.
Previous bioinformatic work also predicted AtSLP2 to be an ER-localized protein
(Kutuzov and Andreeva, 2008). More comprehensive efforts alternatively identified a cytosolic
localization consensus for SLP2 phosphatases, with none of the in silico targeting programs
predicting the SLP2 phosphatases to possess any transit peptides (Chapter 2). Moreover, AtSLP2
expressed as an RFP-fusion protein in both stably-transfected cell culture and transiently
expressed in Vicia faba leaves lacked detectable ER localization, but maintains what looks to be
a consistent punctate cytosolic localization under both experimental conditions. Compared to
SLP1 phosphatases, SLP2 phosphatases exhibit an even higher level of sequence identity (~5080%) making it unlikely that an alternative localization in other plants would be observed.
3.4.2 Temporal and spatial differences in AtSLP phosphatase expression
Both AtSLP1 and AtSLP2 were found to be expressed in different A. thaliana plant
tissues indicating they may differ in biological function. Combined with differing subcellular
locations, AtSLP1 protein expression was only detected in photosynthetic tissues with the
exception of intact developing siliques, while maintaining complete absence from roots.
92
Conversely, AtSLP2 demonstrated clear expression in roots, with marginally detectable
expression in intact siliques. When compared, AtSLP1 and AtSLP2 tissue-specific protein
expression largely correlated with transcript expression. However, presence of AtSLP2 protein in
photosynthetic tissues cannot be discounted, as data suggest at least some AtSLP2 transcriptional
expression is occurring in rosettes, shoots and flowers. Unfortunately, the clear detection of
AtSLP2 protein in these tissues via immunoblotting was hindered by the cross-reactivity of AP
anti-AtSLP2 IgG with AtSLP1, coupled with the small size differences between AtSLP1 and
AtSLP2.
Interestingly, parallel temporal expression of AtSLP1 phosphatase transcripts and proteins
did not hold true within A. thaliana leaves. AtSLP1 presented a clear diurnal cycle in transcript
abundance, which did not completely translate to parallel AtSLP1 protein expression. AtSLP1
transcript abundance was shown to increase in the dark, peaking at the 24 h dark to 0 h light
interface, followed by a reduction in transcriptional abundance as the light period progressed.
Comparatively, AtSLP1 protein abundance seemed to peak at 8 h light followed by a reduction
back to a basal lower level of abundance for the remainder of the 24 h light / dark cycle. This
finding may indicate that AtSLP1 is not only transcriptionally regulated, but also regulated posttranslationally and is reminiscent of other chloroplast proteins involved in starch metabolism,
which demonstrate diurnal gene expression while maintaining constant protein levels (Lu et al.,
2005).
Collectively, AtSLP1 expression findings indicate two things: 1) AtSLP1 transcript levels
seem to peak prior to AtSLP1 protein production, analogous to other chloroplast-targeted
proteins which exhibit a pre-emptive transcript accumulation prior to translation as part of a
diurnal cycle anticipating the metabolic and cellular changes accompanying illumination
93
transition (Smith et al., 2004; Lu et al., 2005). 2) AtSLP1 transcripts and AtSLP1 protein lack
complete correlative expression, indicating AtSLP1 may possess more than just a light-regulated
role in the chloroplast and/or a complex cellular regulation mechanism involving a combination
of transcriptional and post-translational control factors. AtSLP2, on the other hand, was not
investigated for a diurnal fluctuation in protein expression since it was shown to be likely
cytosolic, expressed mainly in non-photosynthetic root tissue and exhibited no pre-existing
indication of diurnally regulated transcript fluctuations.
3.4.3 Conservation of essential PPP protein phosphatase motifs in AtSLP1 and AtSLP2
All SLP phosphatases possess the core signature motifs constituting the active site of PPP
protein phosphatases (Chapter 1 and 2; (Egloff et al., 1995; Xu et al., 2006)) and lack any of the
defining motifs for other protein phosphatase families (Kerk et al., 2008). As PPP protein
phosphatases, maintenance of these motifs also served as an indicator that the SLP phosphatases
are Ser/Thr protein phosphatases. As shown in Figure 3.9, the SLP phosphatases have all the
canonical amino acids involved in coordinating active site metal ions required for catalysis in
PPP protein phosphatases (Egloff et al., 1995; Zhang et al., 1996; Xu et al., 2006). These six
amino acids include D64 (D54), H66 (H56), D98 (D88), N124 (N114), H173 (H164) and H248
(H238) of the mammalian HsPP1γ and PP2A (in brackets), respectively.
Also important are the regulatory protein interactor coordination motifs of PP1 and PP2A
catalytic subunits. Our alignments revealed AtSLP phosphatases possess almost none of the
amino acids involved in coordinating the canonical RVxF motif of PP1 regulatory subunits
(Egloff et al., 1997). Nonetheless, AtI2, an ancient PP1 interactor with an RVxF motif, did
weakly inhibit the SLP phosphatases. With human I2 possessing multiple PP1 contact points
94
outside of the RVxF motif (Hurley et al., 2007), contribution from several interaction sites likely
allowed a weak association in vitro. This is consistent with data showing that human PP2A can
be inhibited by I2 at high concentrations (Brautigan et al., 1986). Since both AtSLP phosphatases
possess a slightly higher sequence identity to PP2Ac (10-14%) relative to PP1 (9-13%), the
presence of amino acid stretches involved in mediating PP2A-regulatory subunit interactions
were also examined. Neither AtSLP phosphatase was found to possess regions of amino acids
resembling these stretches (Xu et al., 2006). Together, this suggests that SLP phosphatases either
have unique targeting subunits or no targeting subunits at all.
3.4.4 Metal cation preferences of AtSLP phosphatases
Atomic structures of PP1 and PP2A revealed the presence of Mn2+/Fe2+ and Mn2+/Mn2+
di-metal cation arrangements in their active sites, respectively (Egloff et al., 1995; Cho and Xu,
2007). These metal cations function to coordinate negatively charged phosphate in the binding
pocket, while water initiates nucleophilic attack on a protein-coupled phosphate group and
mediates its release (Egloff et al., 1995; Xu et al., 2006; Cho and Xu, 2007). Our results revealed
PP1-like metal-dependent phosphatase activity for AtSLP1 using Mn2+ and also Fe3+. Zn2+ and
Mg2+ were also examined, as both these cations have documented importance in the catalytic
mechanisms of non-protein phosphatases (Kim and Wyckoff, 1991) and PP2C protein
phosphatases, respectively (Ingebritsen and Cohen, 1983; Cohen, 1997; Klumpp et al., 2006).
Neither Zn2+ nor Mg2+ could recover the activity of AtSLP1 beyond the basal activity observed
in the presence of 5 mM EDTA chelating agent.
Conversely, AtSLP2 displayed phosphatase activity whether additional metal ions were
present (Mg2+ or Zn2+) or not (EDTA), and was found to be inhibited by Fe3+. The lack of
95
discernible metal-dependent activity suggests AtSLP2 may maintain a conformation that renders
its bound metal ions unable to be quenched by 5 mM EDTA; however, AtSLP2 activity was
easily quenched by 200 mM EDTA indicating that active site metal ions are catalytically
important but not easily displaced in vitro.
3.4.5 Inhibition by classic PPP protein phosphatase inhibitors
As AtSLPs have the greatest identity to eukaryotic PPP protein phosphatases PP1 and
PP2A, each AtSLP phosphatase was examined for sensitivity to known small molecule and
protein inhibitors of PPP protein phosphatases. Both PP1 and PP2A type phosphatases have well
documented sensitivity to MCLR and OA, with PP1 possessing greater sensitivity to MCLR (0.3
- 1 nM) and PP2A to OA (0.1 - 0.3 nM) (Bialojan and Takai, 1988; MacKintosh et al., 1995).
These compounds have also been shown to inhibit the closely-related PPP protein phosphatases
PP4, PP5 and PP6 (Heidari et al., 2011). Neither AtSLP1 nor AtSLP2 were sensitive to MCLR
or OA. In fact, AtSLP1 demonstrated an unexpected activation by both MCLR and OA. Other
Ser/Thr protein phosphatases have been documented to lack sensitivity to these compounds
including AtPP7 (Kutuzov et al., 1998), TOPP6 (Templeton et al 2011) and PP2C protein
phosphatases (Bialojan and Takai, 1988), while no PPP protein phosphatase has demonstrated
activation by either MCLR or OA. This enzymatic activation phenomenon was exhibited by both
tagged and untagged AtSLP1 protein, eliminating the possibility of HIS6-tag influence over
AtSLP1 conformation and sensitivity to inhibitors. Lack of MCLR and OA inhibition was likely
attributed to the absence of a canonical inhibitor binding motif (SAPNYC) which comprises a
hydrophobic binding pocket that both covalently (MCLR) and non-covalently (OA)
accommodates these inhibitors (Zhang et al., 1996; Maynes et al., 2001). Furthermore, site96
directed mutagenesis of amino acid residues R221 and F276 of HsPP1γ, found outside the
SAPNYC motif, conveyed substantive decreases in OA inhibition (Maynes et al., 2001). AtSLP
phosphatases also lack these residues (R221 and F276) at equivalent positions, supporting the
lack of observable SLP phosphatase inhibition.
Additional PPP phosphatase inhibitors tested here included pyrophosphate (PPi),
phosphate (Pi) and sodium fluoride (NaF), which have all been shown to inhibit Arabidopsis
thaliana PP1 phosphatases (Stubbs et al., 2001). Previously, 6 of the 9 AtPP1 (TOPP)
phosphatase isoforms (TOPP1 - 6) were affinity purified from A. thaliana cell culture using a
MCLR conjugated Sepharose matrix (Stubbs et al., 2001). TOPP2 was amongst these six
phosphatases and was used here in its bacterially-expressed and purified form as an enzyme
assay control. Stubbs and colleagues (2001) found that the affinity-purified TOPPs were almost
completely inhibited by 100, 1 and 10 mM Pi, PPi and NaF, respectively. Comparatively, the
AtSLP phosphatases demonstrated a different pattern of inhibition by these compounds, possibly
emphasizing differences in their cellular roles relative to the TOPP phosphatases (Takemiya et
al., 2009). Both AtSLP1 and AtSLP2 were relatively insensitive to PPi inhibition, while AtSLP1
exhibited enhanced sensitivity to Pi with complete inhibition at a 50 mM concentration. An
inability to completely inhibit either AtSLP phosphatase at 100 mM NaF signifies a notable
enzymatic difference to the affinity-purified TOPPs, which were almost entirely inhibited at 20
mM NaF (Stubbs et al., 2001).
The sensitivity of AtSLP1 to Pi inhibition (IC50 ~1.5 mM) coupled to its chloroplast
localization may indicate an energy sensing function as the chloroplast stroma has been
documented to maintain Pi levels of ≥ 12 mM to support production of ATP upon the onset of
light (Bligny et al., 1990). Intriguingly, a light-induced decrease in stromal Pi levels to produce
97
ATP would parallel the corresponding increase in detectable AtSLP1 in A. thaliana rosette
leaves, suggesting that decreased stromal Pi levels could activate AtSLP1 to dephosphorylate its
substrates. Conversely, the relative insensitivity of AtSLP2 to Pi, coupled with a 50 % lower Pi
concentration in the cytosol (6 mM), would seem to indicate a function unrelated to cellular
energy levels (Bligny et al., 1990).
3.5 Conclusion
This work has characterized for the first time two novel, highly conserved plant PPP
protein phosphatases, the SLP phosphatases, from A. thaliana. Unique subcellular localizations
for each AtSLP were resolved, with AtSLP1 and AtSLP2 found to be chloroplastic and cytosolic,
respectively. Enzymatic analyses presented here coincide with bioinformatics, which suggested
that SLP phosphatases may be insensitive to MCLR and OA, while also highlighting AtSLP1
biochemistry as indicative of a potential role in regulating chloroplast energy- related processes.
Future endeavors will need to employ additional cell biological and biochemical approaches to
identify possible regulatory subunits as well as the substrates of these unique enzymes.
98
Chapter Four: Protein interactome analysis of SLP phosphatases 1 and 2 from
A. thaliana
4.1 Introduction
In A. thaliana, and all other plants, there exists a unique subclass of bacterial-like PPP
protein phosphatases called the Shewanella-like protein (SLP) phosphatases (Chapter 2,
(Andreeva and Kutuzov, 2004)). Biochemical characterization of this PPP phosphatase subclass
has found it further divided into two distinct groups called 'SLP1' and 'SLP2' phosphatases,
which differ in both subcellular localization and spatial expression across the plant (Chapter 3).
Specific characterization of SLP phosphatases from A. thaliana (At) found that AtSLP1 is
chloroplast targeted and expressed exclusively in photosynthetic tissues, while AtSLP2 displays
a punctate cytosolic localization and is expressed in non-photosynthetic tissues (Chapter 3).
AtSLP phosphatases were also found to maintain a unique insensitivity to classic PPP protein
phosphatase inhibitors MCLR and OA, which relates to their lack of key inhibitor binding
residues including the C-terminal SAPNYC motif (Chapter 3, (Andreeva and Kutuzov, 2004)).
Furthermore, SLP phosphatases are absent in humans, but found in a number of parasitic
protozoa in addition to plants (Chapter 2, (Andreeva and Kutuzov, 2004; Patzewitz et al., 2013)),
and have recently been proposed as a target of drug development against malaria (Patzewitz et
al., 2013).
The chloroplast is a major resource generator for photosynthetic Eukaryotes, assimilating
carbon into starch during the day to produce carbon skeletons for amino acid and fatty acid
biosynthesis during the night (Rolland et al., 2012). This ability of chloroplasts centers around
their energy generating capabilities. Light harvesting machinery embedded in the thylakoid
membrane establishes an electrochemical proton gradient across the thylakoid membrane, to
99
drive ATP production during the day through chloroplast (C) ATP synthase (Bunney et al.,
2001). Chloroplast ATP synthase structure includes 2 major protein complexes designated F0
and F1 (McCarty, 1992). The F0 domain is embedded in the chloroplast thylakoid membrane and
consists of a, b, c and b'-ring subunits (x14) (McCarty, 1992; Krah et al., 2010). The CF1 protein
complex; however, is membrane associated on the stromal side of the thylakoid membrane and is
comprised of alpha (α), beta (β), gamma (γ), delta (δ) and epsilon (ε) subunits (McCarty, 1992;
Groth and Pohl, 2001). Interestingly, all CF1 ATP synthase subunits are chloroplast encoded,
with the exception of the γ and δ subunits. Phosphoproteomics has revealed a number of
phosphorylation sites present on the CF1 ATP synthase β subunits from barley and A. thaliana
(del Riego et al., 2006; Reiland et al., 2009), with these β subunit phosphorylation sites being
implicated in the regulation of ATP synthase activity through the phosphorylation-dependent
interaction of 14-3-3 proteins (Moorhead et al., 1999; Bunney et al., 2001). Interestingly, casein
kinase II has been shown to phosphorylate CF1 ATP synthase β subunits; however, no opposing
protein phosphatases have yet been identified (Bunney et al., 2001; Reiland et al., 2009).
With 95% of all mitochondrial proteins being nuclear encoded and in need of import
from the cytosol, there has evolved a diversity of protein import mechanisms across Eukaryotes
(Carrie et al., 2010; Fraga and Ventura, 2013). Of recent interest is an unique mitochondrial
targeting peptide (mTP)-independent protein import mechanism responsible for the import and
assembly of mitochondrial intermembrane space (IMS) proteins. This import machinery consists
of two proteins, Essential for Respiration and Vegetative Growth 1 (ERV1; ALR) and
Mitochondrial Import and Assembly 40 (MIA40; TIM40) (Chatzi and Tokatlidis, 2012;
Herrmann and Riemer, 2012). These two proteins form a redox relay with cytochrome C through
their oxidoreductase activity (Chatzi and Tokatlidis, 2012; Herrmann and Riemer, 2012), with
100
MIA40 being responsible for catalyzing disulphide bridge formation on target proteins, trapping
them in the IMS (Banci et al., 2009; Sideris et al., 2009; Kawamata and Manfredi, 2010). In nonphotosynthetic Eukaryotes, such as yeast and humans, loss of this system has been found to be
lethal (Chacinska et al., 2008; Hell, 2008; Banci et al., 2009); however, in A. thaliana MIA40
was shown to be non-essential under standard growth conditions (Carrie et al., 2010). In all
photosynthetic and non-photosynthetic Eukaryotes alike, MIA40 was found to be necessary for
the import of the chaperone superoxide dismutase (Ccs1) and copper/zinc superoxide dismutase
(CSD1/SOD1) into the mitochondrial IMS (Carrie et al., 2010; Kawamata and Manfredi, 2010).
In addition to a mitochondrial IMS subcellular location, plant MIA40s are also targeted to the
peroxisome through a conserved PTS1 domain. In A. thaliana, MIA40 was found to import
CSD3 to the peroxisome (Carrie et al., 2010), indicating that plant MIA40 oxidoreductase may
be involved in regulating diverse cellular processes.
In addition to the cytosol, phosphorylated proteins have been annotated in organelles,
including the mitochondria (Ito et al., 2009) and the chloroplast (Reiland et al., 2009), indicating
protein kinases and phosphatases are operating within these compartments (Schliebner et al.,
2008; Bayer et al., 2012). Utilizing a proven tandem affinity purification (TAP) approach, the
protein interactome of AtSLP1 and AtSLP2 was characterized from both A. thaliana cell culture
and plant tissues. Findings presented here reveal that AtSLP1 and AtSLP2 are involved in
regulating different cellular processes located in different organelles, further emphasizing their
potential utility in agricultural biotechnology.
101
4.2 Materials and Methods
4.2.1 Plant growth conditions
Arabidopsis seeds were surface-sterilized in 0.525 % (v/v) NaOCl (Clorox® Bleach) and
0.2 % (w/v) sodium dodecyl sulfate (SDS), rinsed in deionized water, and spotted onto 0.5 x
Murashige-Skoog (MS) plates [0.5 x MS (2.1 g/L) pH 5.7 (PhytoTechnology Laboratories) and
0.8 % (w/v) agar]. Seeds were imbibed at 4oC in the dark for 2 d, followed by germination under
24 h light at 22oC at a light level of 125 µmol m-2s-1. Developed seedlings were transplanted to
soil pre-soaked with 1 g/L 20:20:20 fertilizer and covered with translucent plastic lids (i.e.
humidity control). Plants were grown in an Econoaire GC-50-BH growth chamber (Enconoaire
Systems Ltd) with a 12 h light / 12 h dark photoperiod at a temperature of 22°C and a light level
of 125 µmol m-2s-1, fertilized weekly with 0.5 g/L 20:20:20 fertilizer. Plastic lids were removed
after 1 week once rooting was established. Plant roots were isolated by germinating A. thaliana
seedlings grown as above in Magenta boxes for 21 d. Magenta box growth media consisted of
0.5 x MS media supplemented with 1 mM potassium phosphate. Media was changed every 10 d.
A. thaliana rosette tissue was germinated and grown as above under a 12 h light / 12 h dark
photoperiod for 21 d prior to harvesting. All tissues were harvested, snap frozen in liquid N2 and
stored at -80oC prior to extraction.
4.2.2 Molecular cloning and expression
Fluorescent and tandem affinity tag (TAP) constructs were created using full-length
At1g18480 (AtSLP2), At1g07010 (AtSLP1) and At5g23395 (AtMIA40) clones obtained from
The Arabidopsis Information Resource (TAIR; http://www.arabidopsis.org/). PCR amplification
of each respective cDNA was performed using gateway compatible primers and inserted into
102
pDONR221 (Invitrogen). Fluorescent constructs were subsequently created using the binary
vectors pB7RWG2 (cRFP) and pK7RWG2 (cGFP), respectively (http://gateway.psb.ugent.be/),
while TAP clones were created using pYL436 (Rubio et al., 2005). Transformation of each
construct into Agrobacterium tumefaciens strain GV3101 (Agrobacterium) was performed to
facilitate stable integration of each construct into both A. thaliana plants and cell culture.
At5g23395 was also cloned into pDEST15 (N-terminal GST-tag) and pDEST17 (N-terminal
HIS6-tag) for heterologous expression in and purification from E. coli, while AtSLP1 and 2 were
cloned into pET-48b(+) (Novagen) and purified via NiNTA as previously described (Chapter 3).
GST-only (pGEX4T-1) (Qiagen) was also expressed and purified for use as a control in enzyme
and in vitro interaction analyses.
4.2.3 A. thaliana cell culture transfection and TAP pull-downs
Wild-type, light-grown A. thaliana cell culture and plants were individually transfected
by Agrobacterium carrying AtSLP2-, AtSLP1-, AtMIA40- and GFP-cTAP constructs (Figure
4.1A) as previously described (Chapter 3). Positive transfectants were screened via
immunoblotting with anti-Myc IgG. A. thaliana cell culture was subsequently transitioned from
light to dark over a two week period, with subculturing performed every 7 d. TAP pull-downs
from either light or dark cell culture were performed as previously described (Figure 4.1B;
(Templeton et al., 2011)) using extracts derived from 50 g of cells per pull-down, while pulldowns from rosette and roots were performed using 10 and 5 g of tissue extract, respectively.
TAP expressing cell culture was mechanically separated and thawed in TAP buffer
consisting of 50 mM Hepes-NaOH (pH 7.5), 150 mM NaCl, 10 % (v/v glycerol) and 0.1 %
Nonidet P-40 plus protease inhibitors added day of use: 1 mM PMSF, 1 % PVP and 1 µg/mL
103
ProteCEASE-100. Cells were lysed by a single pass through a French Pressure Cell (CimAminco) at 16000 psi, followed centrifugation for 35 mins at 40000 x g. Root and rosette tissue
was ground in a pre-cooled mortor and pestle in the aforementioned buffer prior to centrifugation
as above. Supernatants were filtered through one layer of mirracloth prior to incubation with 100
µL of IgG-Sepharose (GE Healthcare) end-over-end for 1.5 h at 4oC. TAP-protein coupled IgGSepahrose matrix was collected in a 10 mL gravity column and washed with 30 mL of TAP
buffer containing 1 mM DTT without protease inhibitors. Coupled matrix was removed and
digested with Precision Protease (GE Healthcare) in a 2 mL eppendorf tube end-over-end at 4oC
for 1 h. Eluate was collected by gravity and incubated with 300 µL of settled NiNTA agarose
end-over-end for 30 min at 4oC. Coupled NiNTA agarose matrix was washed with 30 mL of
TAP buffer containing 5 mM imidazole. TAP pull-downs subjected to SDS-PAGE and / or
immunoblot analysis were eluted from NiNTA using a minimal volume of 1x SDS-PAGE
sample buffer and boiling for 5 min, while samples subjected to whole eluate analysis were
subsequently washed while bound to NiNTA agarose with 10 mL of triethylammonium
bicarbonate (pH 8.5) prior to on-bead, overnight (18 h) proteolysis using 750 ng of trypsin
(Promega) at 30oC in a water bath. Peptides were eluted using a 50 % acetonitrile : 2.5 % formic
acid solution and dried prior to analysis by mass spectrometry.
4.2.4 Mass spectrometry
Digested samples were re-suspended in 5 % (v/v) formic acid. After centrifugation at 4oC
(10 min, 12000 x g), the supernatant was directly loaded onto capillary columns packed in-house
with Magic 5 μM, 100 Å (1 Å=0.1 nm), C18AQ. The data acquisition protocol was modified
104
Figure 4.1: Schematic depiction of tandem affinity purification (TAP) protein isolation
methodology.
(A) Cartoon of protein construct stably expressed in, and TAP isolated from, A. thaliana plant
tissues. Stable plant transfection was performed as outlined in the Materials and Methods and
Chapter 3. (B) Workflow depiction of the TAP isolation protocol employed to isolate specific
AtSLP interactors. Alternatively, stages 3 & 4 would be combined by employing on-bead
trypsinization for subsequent whole eluate mass spectrometric analysis. Dashed line in (A) and
'3C' in (B) depict the human rhinovirus 3c (HRV3c) protease cleavage site.
105
from (Breitkreutz et al., 2010). MS/MS results were acquired in data-dependent mode (over a 2 h
period in an acetonitrile 2–40 % gradient) on a ThermoFinnigan LTQ equipped with a Proxeon
NanoSource and an Agilent 1100 capillary pump. Acquired RAW files were converted to mgf
(Mascot generic format) format, and searched with the Mascot search engine (Matrix Sciences)
against
the
A.
thaliana
complement
of
the
RefSeq
database
(http://www.ncbi.nlm.nih.gov/RefSeq/; release 38) with a precursor-ion-mass tolerance window
of 3.0 Da and a fragment-ion-mass tolerance of 0.6 Da. Methionine oxidation was allowed as a
variable modification, and trypsin specificity (with one missed cleavage allowed) was selected.
The search results were parsed into a relational database developed in-house (ProHits; (Liu et al.,
2010)). The initial filtering of the results was performed by removing the hits with a Mascot
score < 60 and only one unique peptide. In addition to GFP-cTAP pull-downs controlling for
experimentation involving cell culture, AtSLP1 offered a control for AtSLP2 pull-downs from
root tissue, as AtSLP1 is not expressed in 'non-green' tissues and is the closest ortholog of
AtSLP2 (Chapter 3). Bonafide binding partners were only considered if they were not found in
the majority of negative controls: 6 × GFP–cTAP and 7 × AtSLP1-cTAP for SLP2, 6 × GFP–
cTAP and 7 × AtSLP2-cTAP for SLP1 (Table 4.1).
4.2.5 Transient tobacco BY2 cell expression and imaging
Transient expression of AtSLP2-cRFP and AtMIA40-cGFP was performed using darkgrown tobacco BY2 suspension cell culture. Prior to bombardment, 3 mL of settled BY2 cell
culture was rinsed with wash solution (1.0 x MS, 3 % (w/v) sucrose, 0.25 % (w/v) KH2PO4, 4.5
% (w/v) sorbitol, 4.5 % (w/v) mannitol pH 5.0). BY2 cells were pelleted at 500 x g for 5 min
106
prior to plating on wash solution-moistened Whatman paper placed inside a Petri dish. 5 µg of
AtSLP2-cRFP and AtMIA40cGFP plasmid DNA was coupled to 1 mg of gold micro-carriers,
washed, spotted on a macro-carrier and accelerated at BY2 cell culture as previously described
(Chapter 3). Mitochondrial-targeted GFP plasmid DNA (10 µg) was separately accelerated at
BY-2 cells to assess the specificity of Far-Red MitoTracker staining (Invitrogen).
Microscopy was performed 18 h post-ballistics treatment. Prior to imaging, bombarded
cell culture was treated with 2 µM Far-Red MitoTracker (Invitrogen) for 10 min to stain for
mitochondria. Imaging was conducted using a Leica DMIRE2 spectral confocal and multiphoton
microscope with a Leica TCS SP2 acoustic optimal beam splitter (Leica Microsystems).
Excitation / emission spectra (nm) respectively employed included: GFP 488/500-510, RFP
543/600-625 and MitoTracker 633/680-750. All image processing was performed using freely
accessible MacBiophotonics ImageJ (http://www.macbiophotonics.ca).
4.2.6 Isolation of A. thaliana cell culture mitochondria
Seven day old, dark-grown A. thaliana suspension culture (120 g) was filtered through 1
layer of miracloth to remove growth media and homogenized batchwise 30 g at a time in a precooled 4oC mortar and pestle. Grinding of each 30 g was performed in 100 mL homogenization
buffer consisting of 0.3 M mannitol, 50 mM Mops-KOH pH 7.8, 5 mM EDTA, 0.5 % (w/v)
bovine serum albumin (BSA), 1.0 % (w/v) polyvinlypyrrolidone (PVP) and 10 mM DTT added
just before use. Isolation of purified mitochondria and the corresponding cytosolic fraction was
obtained by Percoll (Sigma P-1644) density gradient centrifugation as previously described
(Eubel et al., 2007). Isolated mitochondria were lysed with 0.2 % (v/v) Triton X-100 in 50 mM
Mops-KOH pH 7.5, 150 mM NaCl.
107
4.2.7 Enzymatic analysis
Analysis of AtSLP2 phosphatase activity was examined using either the small molecule
phosphatase substrate pNPP (Sigma) or malachite green reagent (Sigma) to detect the catalyzed
release of phosphate. Both pNPP and malachite green assays were assessed using a Spectromax
spectrophotometer set to 405 nm and 630 nm, respectively.
Enzymatic assays utilizing pNPP were conducted with NiNTA-purified HIS6-AtSLP2
and glutathione Sepharose-purified GST-AtMIA40. All assays were done in parallel with
NiNTA-purified eluates generated from uninduced BL21 (DE3) Codon(+)-RIL E. coli to account
for potential background pNPP cleavage from NSCPs as in Chapter 3. Phosphatase activities
were calculated relative to assays conducted with AtSLP2 only under previously determined
conditions rendering highest activity (Chapter 3). All pNPP assays were conducted as previously
described (Chapter 3) using either 5 mM DTT, Tris(2-carboxyethyl)phosphine (TCEP) or
reduced glutathione (GSH) as a reductant. All assays were quenched with 2.0 M NaOH.
Expressed and purified GST only was used as an assay control.
Malachite Green assays were performed as previously described (Baykov et al., 1988)
alternatively employing a 10 % ammonium molybdate solution. Each assay employed 1 µg of
recombinant phosphatase protein incubated for 1 h at 30oC in 160 µL of 1 x dilution buffer
containing each respective experimental phospho-peptide substrate. Addition of 40 µL malachite
green solution quenched the reaction to reveal phosphatase-catalyzed increase in free phosphate
relative to control assays lacking protein phosphatase. Quenched assays were left at room
temperature for 10 min prior to spectrophotometric assessment at 630 nm. Amount of free
phosphate was calculated using a standard curve derived from known amounts of potassium
108
phosphate. Several of the phosphorylated peptide substrates were generously provided by Dr. D.
Alessi of the Protein Phosphorylation Unit (Dundee, Scotland).
Assays performed using TAP-purified AtSLP2 employed 65 µL of protein phosphatase
bound to NiNTA that was isolated from 20 g of A. thaliana rosette tissue as described above.
Each aliquot of coupled NiNTA matrix was re-suspended in 235 µL of 1 x dilution buffer
containing 500 µM of the respective phosphorylated peptide and 1 µM HIS6-AtMIA40. Each 1x
assay mixture was incubated for 1 h in a 30oC water bath and NiNTA matrix was pelleted. 160
µL of reaction mix was removed and quenched with 40 µL of malachite green solution.
Quenched assays were evaluated spectrophotometrically as outlined above.
4.2.8 Antibody production
HIS6-AtMIA40 was expressed in BL21 (DE3) Codon(+)-RIL E. coli at 37oC and induced
with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 4 h and purified from inclusion
bodies using NiNTA according to the manufacturer’s instructions (Qiagen). Purified protein was
dialyzed against water and used for polyclonal antibody production in a New Zealand White
rabbit as described (Tran et al., 2004).
4.2.9 Western immunoblotting
Affinity-purified polyclonal rabbit anti-AtSLP1 and -2 antibodies previously generated
(Chapter 3) were used at 1.5 and 1.2 µg/mL, respectively. Rabbit anti-AtMIA40 crude immune
serum was used at 1:1000 dilution. Rabbit anti-plant-type phosphoenolpyruvate carboxylase
(PTPC) and sucrose synthase (SuSy) antibodies were used at 1:1000 and 1:10000 dilutions,
respectively (Uhrig et al., 2008). Mouse anti-mitochondrial pyruvate dehydrogenase complex
109
E1α (PDCmt-E1α) antibodies were used at 1:100 dilution (Szurmak et al., 2003). Commercially
available rabbit anti-Myc antibodies were used at 0.2 µg/mL (ICL Inc). A. thaliana CF1 ATPase
antibodies were used at 1:10000 dilution (Roy and Barkan, 1998).
4.2.10 Native polyacrylamide gel electrophoresis (PAGE)
Native-PAGE (10 %) was performed as outlined previously (Gennidakis et al., 2007).
Size exclusion gel filtration standards (GE Healthcare) run in parallel to immunoblot analysis
facilitated an estimation of protein complex sizes. All gels were run at 4oC at 100V prior to
transfer to nitrocellulose membrane for immunoblot analysis.
4.3 Results
4.3.1 AtSLP1 specifically interacts with chloroplast F1 ATP synthase subunits β and γ
AtSLP1 was found to be exclusively expressed in photosynthetic tissues, while its
paralog AtSLP2 was found to be expressed in dark-grown A. thaliana cell culture, roots and
seeds (Chapter 3). Given this, tandem affinity purification (TAP) tagged AtSLP1 was used to
isolate the AtSLP1 protein interactome from light-grown A. thaliana cell culture and rosettes
(Figure 4.2 and 4.3). AtSLP1-TAP was stably expressed in both wild-type A. thaliana cell
culture and plants alongside controls; GFP-TAP (A. thaliana cell culture) and AtSLP2-TAP (A.
thaliana rosettes and cell culture) (Figure 4.3). AtSLP1-TAP and control TAP pull-downs were
performed in parallel, with each isolated TAP-tagged protein immunodetected using anti-Myc
IgG to verify their specific isolation from clarified cell extracts (Figure 4.3). No obviously
enriched bands were resolved via one dimensional SDS-PAGE, therefore a subtractive mass
110
Figure 4.2: Western blot analysis of dark-grown, wild-type A. thaliana cell culture.
1:1 (w/v) 1 x SDS-PAGE sample buffer-extracted cell culture was loaded in increasing volumes
of 5, 15 and 30 µg. Proteins were resolved on 12 % SDS-PAGE, transferred to nitrocellullose
and probed with AP anti-AtSLP1 and anti-AtSLP2 IgG used at 1.5 and 1.2 µg/mL, respectively.
111
Figure 4.3: AtSLP-TAP pull-downs isolating AtSLP1- and AtSLP2-specific protein
interactors.
Representative silver stained SDS-PAGE gel (A) and anti-Myc immunoblot (B) of TAP pulldowns from stably-transfected, light (AtSLP1)- and dark (AtSLP2)-grown, A. thaliana cell
culture. GFP-cTAP pull-downs offered a control for NSCPs resulting from protein overexpression, the TAP tag and applied biochemical purification process. TAP pull-downs were
also performed from root and rosette tissue (Table 4.1). All TAP pull-down experimentation was
performed in at least biological duplicate (Table 4.1) as described in the Materials and Methods.
Indicated bands in (A) are enriched compared to other lanes and correspond to the
immunoreactive bands in (B). Band marked AtMIA40 was determined by mass spectrometry of
the excised band. Lanes contain 30 µL (SDS-PAGE) and 5 µL (Immunoblot) of TAP eluate
isolated from 50 g of A. thaliana cell culture.
112
spectrometry approach involving the direct comparison of on-bead digested AtSLP1-TAP and
control TAP pull-downs was performed to identify lower abundance AtSLP1 protein interactors.
Here, a number of AtSLP1-specific protein interactors common to both light-grown A. thaliana
cell culture and rosette AtSLP1-TAP pull-downs were uncovered, in addition to rosette-specific
protein interactors. AtSLP1 protein interactors found in both light grown A. thaliana cell culture
and rosettes included chloroplast-specific chaperonin proteins At2g28000, At1g55490,
At3g13470, At5g56500, while rosette-specific AtSLP1 protein interactors included CF1 ATP
synthase subunits β and γ (Table 4.1).
Interestingly,
examination
of
compiled
online
available
microarray
data
(www.genevestigator.com) revealed that neither AtSLP1 nor CF1 ATP synthase γ subunit were
abundantly expressed in callus / cell culture tissue (Figure 4.4A). This was corroborated by
immunoblotting light and dark cell culture for both AtSLP1 and CF1 ATP synthase β. Here,
extremely low levels of AtSLP1 and CF1 ATP synthase β subunit protein were detected in lightgrown A. thaliana cell culture when compared to rosette tissue (Figure 4.4B).
4.3.2 AtSLP2 interacts with mitochondrial redox relay protein AtMIA40
As AtSLP2 was found to be specifically expressed in tissues opposite to AtSLP1 (darkgrown A. thaliana cell culture, roots and seeds), AtSLP1-TAP offered a control for AtSLP2-TAP
pull-downs from these tissues (Chapter 3). This facilitated the isolation of the AtSLP2 protein
interactome from dark-grown A. thaliana cell culture and roots (Figure 4.3A and Table 4.1).
Identical to AtSLP1-TAP, AtSLP2-TAP was stably expressed in both dark grown, wild-type A.
113
Table 4.1: AtSLP1- and AtSLP2-specific protein interactors.
Each interactor was uncovered via TAP pull-downs. Specificity of the interaction with either
AtSLP1 or 2 was determined relative to control pull-downs involving GFP-TAP or the other
SLP-TAP construct. Numbers represent total number of identified peptides obtained for each
experiment.
114
Figure 4.4: Transcript and protein expression of AtSLP1 and AtCF1 ATP synthase
subunits.
(A) Transcript expression of AtSLP1 (At1g07010) and AtCF1 ATP synthase γ subunit
(At4g04640) across A. thaliana tissue types. In silico expression data presented here were
derived from compiled microarray data (www.genevestigator.com). A scale of white (no
expression) to red (high expression) is shown, along with the number of microarrays each dataset
was derived from (right). (B) AtSLP1 and AtCF1 ATP synthase β subunit protein expression in
cell culture and rosette leaf tissue. Increasing amounts of clarified cell lysates are shown
depicting 5, 15 and 30 µg of total protein per lane. AP anti-AtSLP1 IgG (Chapter 3) and AntiCF1 ATP synthase β crude immune serum (Roy and Barkan, 1998) were used for immunoblot
analysis.
115
thaliana cell culture and plants alongside controls GFP-TAP (A. thaliana cell culture) (Figure
4.3A) and AtSLP1-TAP (A. thaliana roots and cell culture). All TAP pull-downs were performed
in parallel, with each isolated TAP-tagged protein detected by immunoblot using anti-Myc IgG
to verify their specific isolation from clarified cell extracts (Figure 4.3B). Unlike AtSLP1-TAP
pull-downs, AtSLP2-TAP isolated a highly enriched ~25 kDa polypeptide, which was excised
from an SDS-PAGE gel and identified by mass spectrometry as Cox19-like CHCH family
mitochondrial import and assembly 40 or AtMIA40. In parallel, a subtractive mass spectrometry
approach involving the direct comparison of on-bead digested AtSLP2-TAP and control TAP
pull-downs was performed to identify additional AtSLP2 protein interactors (Table 4.1). This
approach revealed a number of specific AtSLP2 protein interactors (Table 4.1). AtSLP2 protein
interactors found in both dark-grown A. thaliana cell culture and roots included Cox19-like
CHCH family mitochondrial import and assembly 40 (MIA40) protein and heat shock protein
60-3a, while root-specific AtSLP2 protein interactors included Initiation factor 4A (eIF-4A)
DEAD box RNA helicase and glutamine synthetase (Table 4.1).
4.3.3 Reciprocal interaction analysis supports a specific AtSLP2-AtMIA40 interaction
With MIA40 having been shown to function in Eukaryote mitochondrial intermembrane
space protein import and assembly (Herrmann and Riemer, 2012), verification of a specific
AtSLP2-AtMIA40 protein complex was pursued and subsequently supported through reciprocal
TAP pull-downs using AtMIA40-TAP stably expressed in dark-grown A. thaliana cell culture
(Figure 4.5). Immunoblot of AtMIA40- and GFP-TAP eluates from dark-grown A. thaliana cell
culture demonstrated that endogenous AtSLP2 interacts with AtMIA40-TAP just as AtSLP2TAP interacted with endogenous AtMIA40 (Figure 4.5). Furthermore, AtSLP1-TAP pull-downs
116
from the same tissue failed to pull-down AtMIA40, corroborating the specificity of the observed
AtSLP2-AtMIA40 interaction (Table 4.1).
Further support for a specific AtSLP2-AtMIA40 protein complex was observed using
non-denaturing gel electrophoresis (Native-PAGE) (Figure 4.6). Bacterially-expressed and
purified HIS6-AtSLP2 and GST-AtMIA40 (Figure 4.6A and B) were incubated together and
resolved via Native-PAGE (Figure 4.6). Immunoblots employing affinity-purified (AP) antiAtSLP2 IgG resolved a higher molecular weight complex specific only to AtSLP2-AtMIA40
(Figure 4.6C). No higher molecular weight complexes were observed between GST-MIA40 and
HIS6-AtSLP1, while GST-only failed to form a higher molecular weight complex with either
HIS6-AtSLP1 or HIS6-AtSLP2 (Figure 4.6C). Both these approaches provide validation of
AtSLP2-AtMIA40 protein complex formation.
4.3.4 AtSLP2 and AtMIA40 co-localize to the mitochondria
MIA40 is a mitochondrial-targeted intermembrane space (IMS) oxidoreductase (Carrie et
al., 2010). Utilizing fluorescent AtSLP2-RFP and AtMIA40-GFP fusion protein constructs,
transient co-localization analysis was performed via particle bombardment of BY2 cell culture in
conjunction with FarRed MitoTracker fluorescent staining to specifically visualize mitochondria.
This demonstrated AtSLP2-RFP to co-localize with AtMIA40-GFP in the mitochondria (Figure
4.7A). The specific mitochondria staining capabilities of FarRed MitoTracker was assessed using
BY2 cell culture transiently expressing mitochondrial-targeted GFP (Mito-GFP) as outlined
above (Figure 4.7B).
117
Figure 4.5: Reciprocal TAP pull-downs verify specific interaction between AtSLP2 and
AtMIA40.
AtMIA40-TAP was stably transfected into dark grown A. thaliana cell culture and used as bait
to pull-down native AtSLP2. AtSLP2 was absent from in-parallel GFP-TAP pull-downs.
Proteins were resolved on 12 % SDS-PAGE, transferred to nitrocellulose and immunoblotted
using 0.5 µg/mL and 1.2 µg/mL AP anti-Myc (ICL Inc.) and anti-AtSLP2 IgG, respectively.
118
Figure 4.6: Reciprocal non-denaturing PAGE verifies specific interaction between AtSLP2
and AtMIA40.
(A) Purified fractions of HIS6-tagged AtSLP1, AtSLP2 and non-specific co-purifying proteins
(NCSP) as well as GST-tagged AtMIA40 and GST-only. (B) Purified HIS6-tagged AtMIA40
along with clarified HIS6-AtMIA40 expressing bacterial cell supernatant (S/N) and NiNTA
flow-through (F/T). Each lane contains 5 µg of total protein. (C) Purified HIS6-AtSLP2, HIS6AtSLP1 and GST-AtMIA40 were incubated for 1 h at 22oC prior to resolution by non-denaturing
(NATIVE) PAGE, followed by transfer to nitrocellulose for immunoblot analysis. A specific
high molecular weight complex was only observed between HIS6-AtSLP2 and GST-AtMIA40.
Neither AtSLP2 nor AtSLP1 interacted with GST-only. Immunoblotting was performed using
1.5 and 1.2 µg/mL affinity-purified anti-AtSLP1 and anti-AtSLP2 IgG as described previously
(Chapter 3).
119
Subsequent biochemical isolation of mitochondria from dark-grown wild-type A. thaliana
cell culture was performed to confirm transient co-localization data (Figure 4.7C). Immunoblot
analysis of purified mitochondria and the cytosolic fraction using AtSLP2- (Chapter 3) and
AtMIA40-specific antibodies (Figure 4.8) demonstrated that the endogenous AtSLP2 and
AtMIA40 reside in the mitochondria (Figure 4.7C). Additional immunoblotting of both the
purified mitochondria and cytosolic fractions with antibodies to known mitochondrial and
cytosolic proteins confirmed the successful isolation of intact mitochondria and the localizations
of AtSLP2 and AtMIA40 (Figure 4.7C). Immunodetection of mitochondrial pyruvate
dehydrogenase complex E1α subunit (Mito-PDC E1α) provided a marker for the isolation of
intact mitochondria (Szurmak et al., 2003), while immunodetection of plant-type
phosphoenolpyruvate carboxylase (PTPC) and sucrose synthase (SuSy) offered markers for the
cytosolic fraction (Gennidakis et al., 2007; Uhrig et al., 2008) and also showed that no crosscontamination occurred during the preparation of each fraction. These localization findings
further support the isolation of a specific AtSLP2-AtMIA40 protein complex by demonstrating
that AtSLP2 and AtMIA40 are both mitochondrial-targeted proteins.
4.3.5 AtMIA40 activates the Ser/Thr phosphatase activity of AtSLP2
Phosphatase assays employing the substrate pNPP and HIS6-AtSLP2 exhibited a GSTAtMIA40 concentration-dependent increase in phosphatase activity (Figure 4.9A). HIS6-AtSLP2
activation by GST-AtMIA40 was also found to be dependent on the presence of reductant
(Figure 4.9A); however, neither reductant alone (Figure 4.10), nor GST-only plus reductant
could activate HIS6-AtSLP2 (Figure 4.9A). As well, GST-AtMIA40 did not activate the AtSLP2
120
Figure 4.7: AtSLP2 and AtMIA40 are mitochondrial-targeted proteins.
(A) Transient co-expression of RFP-tagged AtSLP2 (red) with GFP-tagged AtMIA40 (green) in
tobacco BY2 cells displayed co-localization with the FarRed MitoTracker illuminated
mitochondria (blue). (B) Transient expression of mitochondrial-targeted GFP (green) exhibited
co-localization with the FarRed MitoTracker illuminated mitochondria (blue). Excitation and
emission wavelengths are outlined in the Materials and Methods. (C) Wild-type mitochondria
were isolated from dark-grown A. thaliana cell culture. Immunoblotting of the cytosolic (cyto)
and purified mitochondrial (mito) fractions was performed using 1:100 anti-mitochondrial
pyruvate dehydrogenase complex E1α (anti-PDCmt E1α), 1:1000 anti-plant-type phosphoenolpyruvate carboxylase (anti-PTPC) and 1:10000 anti-sucrose synthase (anti-SuSy) antibodies in
conjunction with affinity-purified anti-AtSLP2 and anti-AtMIA40 antibodies used at 1.2 µg/mL
and a 1:1000 dilution of crude immune serum respectively. Immunoblotting corroborated the
observed mitochondrial targeting of AtSLP2 and AtMIA40. Each lane contains 15 µg of total
protein.
121
Figure 4.8: Anti-AtMIA40 IgG production and testing.
(A) Purification scheme for 6 M urea-extracted HIS6-tagged AtMIA40. Whole cell (W/C),
clarified supernatant (S/N), 6 M urea-extracted protein pellet loaded on NiNTA (I), NiNTA
flow-through (F/T) and concentrated NiNTA eluate ([E]) are depicted. (B) Dilution series of
purified, 6 M urea-extracted HIS6-AtMIA40 [E] subjected to immunoblot analysis using antiHIS6 IgG. Each lane of the Colloidal stained gel contains 5 µg of total protein. (C) Wild-type A.
thaliana Col-O (WT Col-O) and AtMIA40 insertional mutant (atmia40) roots were employed to
assess the detection of endogenous AtMIA40 by anti-AtMIA40 crude immune serum. The total
protein of each lane is shown.
122
paralog AtSLP1, indicating that the activating effect of AtMIA40 is specific to AtSLP2 (Figure
4.9A). Furthermore, phosphatase assays performed using HIS6-AtSLP2 purified in the
continuous presence of reductant (reduced) but lacking exogenous reductant in the assay,
exhibited some activity increase when assayed in the presence of oxidized HIS6-AtMIA40.
HIS6-AtSLP2 purified in the absence of reductant (oxidized), was highly activated by oxidized
HIS6-AtMIA40, but only in assays conducted with 5 mM GSH (Figure 4.9B), while assay of
oxidized AtSLP2 and AtMIA40 in the absence of reductant resulted in no increase in activity
(Figure 4.9B). As MIA40 proteins maintain oxidoreductase activity towards mitochondrial IMS
proteins containing dual Cx9C, Cx3C and other dual CxnC motifs to catalyze the formation of
disulphide bridges (Banci et al., 2009; Sideris et al., 2009), AtSLP2 orthologs from completely
sequenced plant genomes were aligned to identify equivalent motifs. Two potential CxnC motifs
were identified within the C-terminus of SLP2 phosphatases consisting of a CxC and Cx14C
sequence arrangement (Figure 4.11).
Bacterially-expressed and purified HIS6-AtSLP2 was also found to possess an AtMIA40dependent increase in activity towards pSer, pThr, pTyr and pThr / pTyr phosphorylated peptides
(Table 4.2; Figure 4.12), which was consistent with previous phosphatase assays involving pNPP
(Figure 4.9). Despite an almost 20-fold activation of HIS6-AtSLP2 activity, AtMIA40 did not
alter the specificity of the AtSLP2 toward any one substrate peptide (Figure 4.12A).
Phosphopeptide substrate preferences obtained using recombinant HIS6-AtSLP2 also suggested
that AtSLP2 was a potential dual specificity protein phosphatase; however, assays conducted
using AtSLP2-TAP isolated from dark-grown A. thaliana cell culture found that AtSLP2
maintained a clear preference for pThr phosphopeptide substrates over pTyr-containing peptides
123
Figure 4.9: AtMIA40 activates AtSLP2 under reducing conditions.
(A) HIS6-AtSLP2 (250 ng) was incubated with increasing amounts of GST-AtMIA40 in the
presence of either 5 mM DTT or TCEP to evaluate the effect of reductant on AtSLP2 activation.
Control assays involving HIS6-AtSLP2 + GST-only as well as HIS6-AtSLP1 + GST-AtMIA40
and AtSLP1 + GST-only in the presence of 5 mM DTT demonstrated a specific activation effect
of AtMIA40 on AtSLP2. (B) AtMIA40 has been shown to function as an oxidoreductase in
humans and yeast. Phosphatase assays involving pre-oxidized (black) or reduced (red) AtSLP2
or oxidized AtMIA40 ± reductant. All phosphatase activities were assessed using the artificial
phosphorylated substrate pNPP at 30oC for 1 h. All error bars represent ± standard error; n = 3.
124
Figure 4.10: Effect of reductant only on AtSLP1 and AtSLP2 activity.
Phosphatase activity was assessed as outlined in the Materials and Methods using the artificial
phosphorylated substrate pNPP with either DTT or GSH at 30oC for 1 h. All error bars represent
± standard error; n = 3.
125
126
Figure 4.11: Amino acid sequence alignment of photosynthetic Eukaryote SLP2 phosphatases against select SLP1
phosphatases.
Alignment was performed using ClustalX followed by import into GeneDoc for image assembly. Amino acid sequence gene
identifiers are located in Appendix A.1. Highlighted in blue are representative green algal, moss and land plant SLP1 paralogous
phosphatases. All cysteines are highlighted in yellow. The CxC and Cx14C motifs are underlined in yellow.
(Figure 4.12B). Collectively, these results indicate that AtMIA40 functions to enhance AtSLP2
activity, not alter its phosphoprotein specificity, through the likely formation of disulphide
bridges.
4.4 Discussion
4.4.1 AtSLP1 phosphatase interacts with chloroplast F1 ATP synthase subunits β and γ.
With the finding that AtSLP1 protein phosphatase interacts with CF1 ATP synthase
subunits β and γ, another component of a reversible phosphorylation regulatory mechanism
discovered almost 15 years ago may have been uncovered (Kanekatsu et al., 1998; Moorhead et
al., 1999; Bunney et al., 2001). Previous phosphoproteomic research has found that both CF1
ATP synthase subunits β and γ are phosphorylated proteins (del Riego et al., 2006; Reiland et al.,
2009), and that β subunit phosphorylation is mediated by chloroplast caesin kinase II (Kanekatsu
et al., 1998). As well, it was shown that 14-3-3 proteins associate with CF1 ATP synthase β
subunit in cauliflower (Moorhead et al., 1999) and that this association was phosphorylation
dependent when also examined in barley (Bunney et al., 2001). It was also demonstrated that the
association of 14-3-3 proteins inhibited CF1 ATP synthase activity, indicating this interaction is
regulatory in nature (Bunney et al., 2001). The association of AtSLP1 protein phosphatase with
CF1 ATP synthase subunits suggests that AtSLP1 may function to dephosphorylate and activate
the β subunit, by alleviating 14-3-3 protein inhibition.
CF1 ATP synthase is activated in the light and inhibited in the dark through a
combination of reversible phosphorylation (Moorhead et al., 1999; Bunney et al., 2001), redox
mechanisms (Kohzuma et al., 2013) and ADP/ATP binding (Malyan, 2010). Progression from
127
Figure 4.12: Substrate specificity of AtSLP2-AtMIA40 complex.
(A) A series of phosphorylated peptides were screened using 250 ng of purified HIS6-AtSLP2
with (grey bars) and without (black bars) the addition of 1000 nM HIS6-AtMIA40 to assess the
influence of AtMIA40 on the substrate specificity of AtSLP2. Phosphorylated peptides depicted
here represent pSer (Ki67, B56), pThr (RRP1B, BRCA1), pTyr (Rat SAPK3), pThr/pTyr (p38,
p38beta)-containing peptides. (B) TAP-purified AtSLP2-cTAP was assessed using BRCA1
(pThr) and rat SAPK3 (pTyr) peptides to assess differences in bacterially-produced and in planta
assembled AtSLP2. HIS6-AtMIA40 (1000 nM) was added to assays to maximize AtSLP2
activity. All assays were conducted at 30oC for 1 h, with enzymatic activity deduced by
malachite green as outlined in the Materials and Methods. Error bars represent ± standard error;
n=3.
128
Table 4.2: Phosphorylated peptides used in AtSLP2 enzyme assays.
Listed are the phosphorylated peptide sequences and the corresponding protein frrom which they
were derived.
129
night to day would see a decrease in the stromal concentration of free Pi and ADP as ATP begins
to be manufactured. Previous examination of AtSLP1 enzyme properties found that it maintained
an approximate IC50 in line with physiologically relevant chloroplast Pi concentrations (Chapter
3). As well, despite diurnal fluctuations in AtSLP1 transcripts, AtSLP1 protein levels were
largely unchanging, except during the day when a small increase in AtSLP1 protein levels was
observed at around 8 h post continuous light exposure (Chapter 3). The interaction between CF1
ATP synthase β subunit and AtSLP1, as well as its phosphorylation-dependent inhibition by 143-3 proteins, coupled with AtSLP1 phosphatases in vitro biochemical characteristics, suggest
AtSLP1 could be involved in modulating CF1 ATP synthase activity. Additional
experimentation is required to verify this hypothesis (Figure 4.13).
4.4.2 AtSLP2 phosphatase is a novel client of redox relay protein AtMIA40
AtSLP2 represents a novel interaction partner of MIA40 in photosynthetic Eukaryotes, as
SLP2 phosphatases are absent in metazoans (Chapter 2). Previous examination of AtMIA40
insertional mutant plants found AtMIA40 responsible for importing the chaperone for superoxide
dismutase (Ccs1) and copper/zinc superoxide dismutase (CSD1) into the mitochondrial IMS as
well as CSD3 into the peroxisome (Carrie et al., 2010). Import of Ccs1 and CSD1 into the
mitochondrial IMS indicates a conserved MIA40 function in plants, as has been observed in
other eukaryotic systems such as humans and yeast (Kawamata and Manfredi, 2010). In addition
to these proteins, research in non-photosynthetic Eukaryotes has identified a number of
mitochondrial IMS proteins that are directly regulated by MIA40, in particular, cytochrome
oxidase c protein 17 (COX17) (Sideris et al., 2009) and translocase of inner membrane (TIM)
130
Figure 4.13: Model depicting how AtSLP1 may operate in vivo.
Given the activation of AtSLP1 by reductant in vitro, it may be possible that AtSLP1 is redox
regulated in vivo. Currently AtSLP1 has demonstrated inhibition by sodium pyrophosphate (PPi)
and phosphate (Pi) in vitro at physiologically relevant concentrations as well as an interaction
with the γ and β subunits of CF1 ATP synthase. Circled ‘P’ (yellow) represents phosphorylation
events which AtSLP1 may regulate. CF1 ATP synthase has been shown to dock 14-3-3 proteins
(Moorhead et al., 1999), with the A. thaliana γ and β subunits having been previously shown to
be phosphorylated on conserved serine and threonine residues (del Riego et al., 2006; Reiland et
al., 2009).
131
proteins, specifically Tim22, which is responsible for the integration of mitochondrial inner
membrane proteins (Fraga and Ventura, 2013), and others (Banci et al., 2009; Reddehase et al.,
2009; Kawamata and Manfredi, 2010; Darshi et al., 2012; Weckbecker et al., 2012). AtSLP2,
however, represents the first protein phosphatase identified to interact with MIA40.
MIA40 proteins have been found to catalyze the formation of disulphide bridges on target
protein substrates through their oxidoreductase activity, trapping the MIA40 substrate in the
mitochondrial IMS (Figure 4.14; (Banci et al., 2009; Sideris et al., 2009; Kawamata and
Manfredi, 2010)). COX and TIM proteins represent the first MIA40 substrates identified (Banci
et al., 2009; Sideris et al., 2009). Careful analysis found they maintain conserved dual Cx9C or
Cx3C motifs named the mitochondrial IMS sorting signal / IMS-targeting signal (MISS/ITS).
These dual cysteine-containing sequence stretches form anti-parallel alpha helices through
MIA40-catalyzed disulphide bridges (Banci et al., 2009; Sideris et al., 2009). This is achieved
via the cysteines of MIA40's active site (the CPC motif), which first forms disulphide bridge
intermediates with the target protein substrate (Banci et al., 2009), followed by the formation of
disulphide bridges on the target substrate and the release of reduced MIA40 all in the presence of
reduced glutathione (GSH) (Banci et al., 2009; Sideris et al., 2009). GSH has been shown to
enhance MIA40 activity in vitro (Bien et al., 2010), in addition to exerting an overall reducing
pressure on yeast MIA40 redox state in vivo (Kojer et al., 2012). GSH exerts a similar effect on
the oxidoreductases of the mammalian endoplasmic reticulum (Jessop and Bulleid, 2004), where
lack of GSH production results in compromised yeast protein folding under oxidizing conditions
(Cuozzo and Kaiser, 1999). In line with this, the activating effect of AtMIA40 on AtSLP2 was
132
Figure 4.14: Model depicting how AtSLP2 and AtMIA40 may operate in vivo.
Prior evidence from human and yeast MIA40 indicates that client proteins undergo oxidative
folding which traps them in the mitochondrial intermembrane space (IMS). This reaction was
also shown to be dependent on the presence of reductant in the form of reduced glutathione
(GSH).
133
most pronounced in the presence of 5 mM GSH; however, a measureable increase in AtSLP2
activity above baseline was also detected in assays conducted using reduced AtSLP2 in the
absence of GSH. These findings primarily support AtMIA40 activation of AtSLP2 through the
catalyzed formation of disulphide bridges to trap AtSLP2 in the IMS, but cannot discount the
alternative possibility that AtMIA40-GSH facilitates AtSLP2 activation solely through protein
isomerization of oxygen-induced disulphide bridges (Jessop and Bulleid, 2004; Kojer et al.,
2012).
MIA40s have also been found to govern the import of non-canonical CxnC motif
containing proteins such as Ccs1 (Reddehase et al., 2009; Kawamata and Manfredi, 2010). SLP2
phosphatases represent a non-canonical target of MIA40 oxidoreductase activity, as they do not
maintain either dual Cx9C or Cx3C motifs, but rather two regions of cysteines which form CxC
and Cx14C motifs based on linear sequence alignments. Since no protein structures are available
for a closely related SLP phosphatase ortholog of AtSLP2, structural alignments and/or
molecular modeling could not be performed to reveal the positioning of these motifs relative to
each other. Both cysteine containing motifs are located in the C-terminal half of the protein
beyond key AtSLP2 catalytic motifs. This likely means disulphide bridge formation induces
global conformational changes in protein structure resulting in enhanced AtSLP2 activity rather
than directly affecting enzymatic catalysis, as is found with PTP phosphatases like starch excess
4 (SEX4) (Silver et al., 2013). Interestingly, these CxnC motifs bracket two previously annotated
and highly conserved SLP protein phosphatase motifs whose functions remain unknown
(Chapter 2; (Andreeva and Kutuzov, 2004)).
The use of paralogous AtSLP1 as a control stems from its phylogenetic relatedness to
AtSLP2 and its analogous divergence from classic eukaryotic PPP protein phosphatases (Chapter
134
3; (Moorhead et al., 2009)). Phylogenetically, SLP1 and SLP2 protein phosphatases originated in
plants as a result of horizontal gene transfer from Myxobacteria early in photosynthetic
Eukaryote evolution (Chapter 2). Likely gene duplication of the incorporated progenitor SLP
phosphatase in early photosynthetic Eukaryote evolution then resulted in the proliferation of
distinct SLP1 and SLP2 protein phosphatase lineages (Chapter 2). Sequence alignment analysis
revealed SLP1 phosphatases maintain half of each of the conserved cysteine-containing CxnC
motifs identified in SLP2 phosphatases in similar sequence positions. These points rendered
AtSLP1 a logical control for both in vitro assays and TAP preparations (Chapter 3, (Andreeva
and Kutuzov, 2004)). In both in vitro assays and TAP pull-downs, AtSLP1 failed to associate
with AtMIA40, validating the observation of a specific AtSLP2-AtMIA40 complex. More
broadly, this highlights the likely diversity in SLP1 and SLP2 protein phosphatase function
within the plant cell.
The mitochondrial localization of AtSLP2, combined with its 4- (pNPP) to 20(phosphorylated peptides) fold increase in activity upon interaction with AtMIA40, and a
phosphorylated substrate preference indicative of Ser/Thr PPP protein phosphatases, suggests
reversible protein phosphorylation maintains a regulatory role within the mitochondrial IMS. No
studies to date have specifically examined the role of reversible protein phosphorylation in the
mitochondrial IMS of any organism despite targeted proteomics studies revealing the presence of
a number of protein kinases and protein phosphatases (Salvi et al., 2005; Pagliarini and Dixon,
2006; Vogtle et al., 2012; Duncan et al., 2013). In particular, Src, Lyn, Fyn, Csk and Fgr kinases
(Salvi et al., 2002) as well as SHP-2 phosphatase (Salvi et al., 2004) have been found in the
mitochondrial IMS of rat brain mitochondria (Salvi et al., 2005; Pagliarini and Dixon, 2006),
along with yeast PP2C phosphatase PTC5 (Vogtle et al., 2012) and Trypanosoma brunei dual
135
specificity and Asp-based phosphatase Tim50, which represents a key mitochondrial protein
import regulator (Duncan et al., 2013). Furthermore, recent elucidation of the IMS proteome of
yeast identified a number of IMS proteins that have been previously documented in whole
mitochondria phosphoproteomics studies to be phosphorylated (Vogtle et al., 2012). These
include: peptidyl-prolyl cis-trans isomerase (YDR155C; CPR1), thioredoxin reductase
(YDR353W; TRR1) and subunit VIB cytochrome c oxidase (YLR038C; COX12) (PhosphoPep
Ver 2.0). Given the prevalence of regulatory protein phosphorylation across Eukaryotes, it is
reasonable to predict that mitochondrial IMS reversible protein phosphorylation also represents a
conserved phenomenon.
4.4.3 Co-localization of both AtSLP2 and AtMIA40 to the mitochondria
Previous in silico analyses indicated that SLP2 phosphatases were cytosolic proteins
(Chapter 2). Despite the employment of several mitochondrial prediction algorithms, none
accounted for a potential mitochondrial inner membrane space (IMS) localization, likely because
proteins targeted to the IMS lack canonical mitochondrial targeting peptides (Chacinska et al.,
2008). AtMIA40 and AtSLP2 both lack a predicted mitochondrial targeting peptide (Chapter 3;
(Chacinska et al., 2008)). Although we previously noted some punctate staining for AtSLP2RFP, a cytosolic localization was concluded by its co-localization with the cytosolic GFP-only
marker construct in Vicia faba leaf cells (Chapter 3). As leaf cells are photosynthetic, it
represents a location where endogenous SLP2 phosphatases are not expressed, which may have
contributed to the deduced cytosolic localization. Here, through the combined isolation of
mitochondria and transient co-expression of fluorescent AtSLP2 and AtMIA40 in a nonphotosynthetic tissue known to express SLP2 phosphatases, a clear mitochondrial localization
136
was uncovered for both AtSLP2 and AtMIA40. This corroborates previous findings that
AtMIA40 is a mitochondrial-targeted protein (Carrie et al., 2010). With AtSLP2 and AtMIA40
both lacking a mitochondrial targeting sequence and AtSLP2 representing a client of AtMIA40,
which is exclusively found in the mitochondrial IMS of other Eukaryotes (Herrmann and
Riemer, 2012) and A. thaliana (Carrie et al., 2010), it is likely that AtSLP2 also resides within
the mitochondrial IMS. Characterization of AtMIA40 and other plant MIA40s found an
additional peroxisomal subcellular localization through a highly conserved C-terminal PTS1
motif (Carrie et al., 2010). AtSLP2 does not possess a C-terminal PTS1 motif and has not
exhibited peroxisomal localization under any circumstance (Chapter 3), indicating that AtMIA40
likely maintains a role in regulating diverse protein targets alternative to SLP2 phosphatases.
4.5 Conclusion
The employment of tandem affinity purification to investigate the AtSLP phosphatase
protein interactome has yielded a number of interesting findings. In addition to isolating the
protein interactome, the TAP protein expression and isolation methodology applied here
provided a means of comparing the phosphorylated peptide substrate preferences of bacteriallyexpressed and purified HIS6-AtSLP2 to in planta constructed AtSLP2. The AtSLP phosphatase
protein interactome differed between the AtSLP1 and AtSLP2 paralogs and was consistent with
their documented subcellular localizations. AtSLP1 was found to interact with CF1 ATP
synthase β and γ subunits of the chloroplast stroma, while AtSLP2 was found to interact with
AtMIA40 of the IMS. Reciprocal interaction analysis was only performed using AtMIA40 as it
was the most consistent and specific interactor identified through TAP pull-downs. Successful
reciprocal isolation of the AtMIA40-AtSLP2 protein complex substantiated this specific
137
interaction, and lends credence to the legitimacy of the remaining AtSLP interactors. Elucidation
of protein-protein interactors is central to characterizing the cellular role(s) of proteins, and
therefore represents a key intermediate step in fully understanding the function of a protein from
a broader biological perspective.
138
Chapter Five: Exploring the biological significance of AtSLP1 and AtSLP2: A
reverse genetic approach
5.1 Introduction
Careful examination of insertional gene-knockout mutant and/or over-expression plants is
essential to elucidating the biological functions of proteins (Furbank and Tester, 2011). This
provides both fundamental science and targeted agricultural biotechnology with a framework of
how a particular protein operates within the cellular environment by examining the effect it has
on a plant's phenotype (Furbank and Tester, 2011; Sozzani and Benfey, 2011). Identification of a
plant phenotype in a gene knockout or over-expression plant line can require conditional
circumstances, such as an abiotic stress (e.g. drought), while other phenotypes, such as
developmental / morphological perturbations (e.g. stunted growth), can be identified under
control conditions. Sometimes, even the application of experimental stresses may still miss
subtle phenotypes which are occurring at the cellular level. In particular, alterations in metabolic
flux and/or cell signaling may result in changes which lack visibly manifested organismal
phenotypes (Lu et al., 2008). However, continued development of genome sequencing and mass
spectrometry techniques / technologies has advanced the field of functional genomics (Hamilton
and Buell, 2012) and metabolomics (Schauer et al., 2008), creating new opportunities to identify
subtle plant phenotypes via targeted screening of gene knockout and/or over-expression plant
lines.
Despite the need to investigate biological processes in agriculturally relevant plants, A.
thaliana remains the foremost model organism for the elucidation of new biological information
on plant processes (Schauer et al., 2008). This is due to its short generation time (~6 weeks),
extensively annotated genome (first draft published in 2000) and ease of genetic manipulation
139
(Agrobacterium-mediated transfection), as well as the public availability of online resources
(Koornneef and Meinke, 2010). Here, A. thaliana was used as the organism of choice to
investigate the roles of SLP phosphatases in plants. Investigation of homozygous atslp1
knockout and 35S::AtSLP1 over-expression plant lines yielded a lack of notable results under
control conditions, and was not examined beyond standard growth parameters for conditional
phenotypes. Conversely, homozygous atslp2 knockout and 35S::AtSLP2 over-expression plant
lines presented dry seed and seed germination phenotypes under standard growth conditions,
which were subsequently explored in homozygous atmia40 plants, given the annotated
interaction between AtSLP2 and AtMIA40 (Chapter 4). Research surrounding AtSLP2 and
AtMIA40 function at the organismal level represents the focus of Chapter 5.
Seed germination is a process largely governed by the opposing activities of two key
phytohormones, abscisic acid (ABA) and gibberellic acid (GA) (Kucera et al., 2005; Muller et
al., 2006; Piskurewicz et al., 2008). ABA functions to establish dormancy in developing seeds
(Kucera et al., 2005; Holdsworth et al., 2008), while also maintaining a negative regulatory role
during seed germination by preserving seed dormancy (Kucera et al., 2005; Holdsworth et al.,
2008). Conversely, GA positively regulates seed germination by stimulating seed swelling,
endosperm rupture and testa cracking to facilitate radicle emergence and seedling establishment
(Holdsworth et al., 2008). Knockout plant lines deficient in either ABA or GA biosynthesis
and/or cell signaling components have been characterized, establishing the core proteins
involved in these processes (Kucera et al., 2005; Holdsworth et al., 2008). For instance, mutation
in ABA biosynthetic (aba1-1, aba2) and signaling (abi1 - abi5) proteins were found to exhibit a
lack of seed dormancy, while ABA over-producing mutants (cyp707a2) maintained enhanced
seed dormancy (Kucera et al., 2005). Similarly, GA biosynthetic mutant ga1 exhibited increased
140
dormancy and required exogenous GA to germinate, while DELLA transcription factor signaling
mutant rgl2 displayed reduced seed dormancy resulting from enhanced GA-induced gene
expression (Lee et al., 2002; Cao et al., 2005; Kucera et al., 2005). A loss of both GA
biosynthesis (ga1-3) and signaling through crosses with higher order rga1/rgl2/gai mutants
resulted in GA-independent germination (Cao et al., 2005).
Interestingly, at the center of ABA-related cell processes are Ser/Thr PPM/PP2C-family
phosphatases, which function as negative regulators of ABA signaling (Joshi-Saha et al., 2011).
Cellular ABA binds ABA receptor pyrabactin resistance (PYR/PYL/RCAR) proteins, relieving
PP2C phosphatase regulation of downstream Snf1-like related kinase 2 (SnRK2) and calciumdependent protein kinase (CDPK) targets. This frees these kinases to activate targets, including
ABF transcription factors and ion channels through protein phosphorylation events (Joshi-Saha
et al., 2011). To date no protein phosphatases have been implicated in regulating either GA
biosynthesis and/or signaling.
With seed development and germination essential to the daily lives of people world-wide
as a source of food, elucidating the cellular mechanisms which govern these processes maintains
both fundamental and biotechnological significance (Martinez-Andujar et al., 2012). As a key
driver of seed germination, understanding GA biosynthesis and signaling regulation is of
immense importance. Through a combination of biochemistry, cell biology and pharmacological
data presented here, the involvement of a Ser/Thr PPP protein phosphatase in regulating GArelated processes during seed germination has been shown for the first time. AtSLP2 was found
to be localized to the mitochondrial IMS through mTP-independent targeting, where it
specifically interacts with, and is activated by, mitochondrial IMS import and assembly protein
40 (AtMIA40) to negatively regulate seed germination through GA-related processes. Reciprocal
141
interaction analysis and in vitro protein phosphatase assays verified a specific interaction
between AtSLP2 and AtMIA40 (Chapter 4). Pharmacological screening of loss-of-function
atslp2-2 and atmia40 mutant plant lines provided evidence consistent with GA over-production
and/or signaling deficiencies, which was verified by quantitative PCR analysis of known GA
biosynthetic and signaling protein-encoding genes. The localization of both AtSLP2 and
AtMIA40 to the mitochondria, combined with their influence over seed GA-related processes,
represents a novel connection between plant cell metabolism and signaling.
5.2 Methods
5.2.1 Plant growth conditions and seed weight
Arabidopsis seeds used in non-pharmacological experimentation were surface-sterilized
as described in Chapter 4. Transgenic 35S::AtSLP over-expressors were selected on plates
containing 100 μg/mL kanamycin. Seeds were stratified for 2 d at 4°C in the dark for uniform
germination, and then transferred to continuous light for 5 - 7 d at 22°C until seedlings formed
true leaves. Developed seedlings were transplanted to soil and grown also as described in
Chapter 4. Conditions for seeds used in pharmacological studies involved dry seed sterilization
and stratification at 4oC for 2 d in the dark on MS agar plates containing 0.3, 1.0 µM ABA and
10 µM Uniconazole. Seeds were then germinated under 24 h light at 22oC at a light level of 125
µmol m-2s-1. Dry seed weight was measured for 50 seeds (n = 5) on a Sartorius MC-5
microbalance 5.1g (www.sartorius.dataweigh.com).
142
5.2.2 Molecular cloning and expression
At1g18480 (AtSLP2) and At1g07010 (AtSLP1) cGFP over-expression constructs were
created as previously mentioned (Chapter 4). Transformation of each construct into
Agrobacterium tumefaciens strain GV3101 (Agrobacterium) was performed to facilitate stable
integration of each construct into A. thaliana plants via the floral dip method previously
described by (Zhang et al., 2006).
5.2.3 Seed extraction
Seeds (50 mg) were extracted by micro-pestle on ice in a solution consisting of 50 mM
HEPES-NaOH pH 7.5, 150 mM NaCl, 5 % (v/v) glycerol in addition to 0.2 % (v/v) Triton X100, 1 mM PMSF, 1 mM benzamidine and sand added fresh the day of use. Seed lysates were
clarified by centrifugation at 4oC and 14000 x g. Protein estimations for each clarified seed lysate
were obtained using a Bradford protein assay read spectrophotometrically at 595 nm.
5.2.4 Plant DNA isolation and PCR genotyping
Four atslp1, two atslp2 and one atmia40 insertional mutant plant line(s) were obtained
from either the Arabidopsis Information Resource (TAIR; www.arabidopsis.org; SALK), the
Nottingham Arabidopsis Stock Center (NASC; www.arabidopsis.info; GABI-Kat) or the Riken
Bioresource Center Experimental Plant Division (RIKEN; www.brc.rike.jp/lab/epd/Eng/;
RATM). Respective plant lines included atslp1-1 (SALK_134367), atslp1-2 (SALK_129247),
atslp1-3 (RATM15-3180-1), atslp1-4 (GABI_313CO4), atslp2-1 (SALK_005557), atslp2-2
(RATM13-2055-1) and atmia40 (SALK_044358) (Figure 5.1). PCR screening of atslp1, atslp2
and atmia40 insertional mutants for homozygosity was conducted using primers outlined in
143
Appendix C.1. DNA was extracted from 14 d old rosette tissue and screened via PCR. PCR
conditions included 95oC for 5 min, [95oC 45 s, 55oC 45 s, 72oC 1.5 min] x 30 cycles, 72oC 10
min.
5.2.5 Immunoblot analysis
Immunoblotting for AtSLP1 and AtSLP2 expression was performed as previously
described (Chapters 3 and 4) to confirm PCR genotype data (Figure 5.2A and B). Affinity
purified polyclonal rabbit anti-AtSLP1 and -2 antibodies previously generated were used at 1.5
and 1.2 µg/mL, respectively (Chapter 3). Immunoblotting for AtMIA40 expression was
performed as previously described (Chapter 4) to confirm genotype data (Figure 5.2A and B;
Appendix C.2.) using a 1:1000 dilution of anti-AtMIA40 crude immune serum.
5.2.6 Quantitative PCR analysis
Total RNA from the various A. thaliana lines was isolated using a modified TRIzol
(Invitrogen) protocol. First-strand cDNA was synthesized from 5 μg total RNA using oligo
(dT)12-18 primer and SuperScript II Reverse Transcriptase (Invitrogen, USA) following
manufacturer’s instructions. The qPCR was performed using StepOnePlus Real-Time PCR
System (Applied Biosystems). Primer pairs are listed in Appendix C.3. Each PCR reaction
contained 1× Fast SYBR Green Master Mix (Applied Biosystems, USA), 200 nM of each
primer, and 0.5 μL cDNA in a final volume of 20 μL. PCR amplification was performed for 40
cycles at 95ºC, 3 s and 60ºC, 30 s with a preceding initial enzyme activation of 20 s at 95ºC.
144
Figure 5.1: AtSLP and AtMIA40 gene models depicting insertional mutant plant lines.
SALK lines were obtained from the Arabidopsis Information Resource (TAIR;
www.arabidopsis.org), GABI-kat line (GABI) was obtained from the Nottingham Arabidopsis
Stock Center (NASC; www.arabidopsis.info) and RATM lines were obtained from the Riken
Bioresource Center Experimental Plant Division (RIKEN; www.brc.rike.jp/lab/epd/Eng/).
Dashed line represents 5’-UTR region upstream of exon 1. Black triangles denote the point of TDNA insertion.
145
Figure 5.2: PCR and immunoblot analysis of AtSLP1 and AtSLP2 knockout and overexpression plant lines.
Genotyping of atslp1-3 (A) and atslp2-2 (B) mutant lines by PCR. Primers used are listed in
Appendix C.1. A number of positive insertional mutant plants were obtained as shown. Star
denotes homozygous insertional mutant line. Corroborative immunoblot analysis of various
AtSLP1 (C) and AtSLP2 (D) plant lines confirmed the elimination (knockout) or elevated
expression (over-expression) of each respective AtSLP. Italicized atslp denotes insertional
knockout plant line, WT NÖ denotes wild-type Nossen A. thaliana (NÖ) and italicized
35S::AtSLP denotes over-expression plant line. 35S::AtSLP constructs are of an approximately
26 kDa due to fusion with GFP. Star denotes homozygous insertional mutant line. All primers
used for genotyping atslp insertional mutant plant lines are listed in Appendix C.1. N.S. denotes
non-specific.
146
Relative expression levels were calculated and all quantifications were normalized using
ubiquitin 10 mRNA as an internal control. For each target gene, the reactions were carried out in
duplicate from two biological replicates.
5.2.7 Metabolite analysis
A standard set of metabolites was assessed for dry, mature atslp2-2, wild-type NÖ and
35S::AtSLP2 seeds by gas chromatography mass spectrometry through a collaborator (Dr. A.
Fernie) as previously described (Lisec et al., 2006). Presented here are results for the seed fatty
acid and amino acid content of each.
5.3 Results
5.3.1 Generation of homozygous insertional mutant and protein over-expression plant lines
Homozygous atslp1 and atslp2 plant lines were made in conjunction with 35S::AtSLP1
and 35S::AtSLP2 protein over-expression lines to elucidate a spectrum of A. thaliana phenotypic
effects. Homozygous atmia40 mutants were created in response to the identification of AtMIA40
as a bonafide interactor of AtSLP2 (Chapter 4). The atmia40 insertional plant line described here
functioned to independently corroborate phenotypic findings observed in atslp2 plants.
Originally atslp1 and 35S::AtSLP1 plant lines were created to identify plant phenotypes resulting
from the complete loss and/or over-expression of AtSLP1. No obvious phenotypes were
observed under standard conditions so further characterization was not pursued. All homozygous
insertional mutant and protein over-expressing plant lines were analyzed by immunoblot analysis
to confirm the loss or enhanced protein expression of AtSLP1, AtSLP2 or AtMIA40,
respectively (Figure 5.1 and 5.2; Chapter 4).
147
5.3.2 AtSLP2 and AtMIA40 function to negatively regulate seed germination
To elucidate the biological function of AtSLP2, insertional knockout (atslp2-2) and
constitutive AtSLP2 over-expression (35S::AtSLP2) plants were examined and found to exhibit a
number of unique seed-related phenotypes. Seed phenotype documentation was initiated through
the observation of a spectrum in silique size between atslp2-2 and 35S::AtSLP2 plants, which
qualitatively maintained larger and smaller siliques, respectively, relative to their wild-type
Nossen (NÖ) background (Figure 5.3A). Subsequent examination of mature seeds found that
atslp2-2 seeds possessed an elevated seed weight relative to NÖ and 35S::AtSLP2 seed
populations (Figure 5.3B). This increased seed weight phenotype of atslp2-2 was mirrored by
insertional atmia40 knockout plant seeds (Figure 5.3C). No effect on seed viability was noted in
either case, with each seed set exhibiting an equal ability to sow subsequent plant generations.
Upon germination, both atslp2-2 and atmia40 seeds exhibited an early seed swelling /
cracking (testa cracking) phenotype relative to their respective wild-type backgrounds (Figure
5.4 - 5.7), with atslp2-2 seeds maintaining the most pronounced phenotype (Figure 5.4 and 5.6).
Enhanced early testa cracking was accompanied by a spectrum of accelerated to delayed seed
germination rates observed in atslp2-2 and 35S::AtSLP2 seeds, respectively (Figure 5.4 and 5.6).
AtSLP2 and AtMIA40 protein levels were also examined during imbibition and germination
(Figure 5.8). Here 'seed imbibition' refers to seed water uptake and swelling at 4oC in the dark,
while 'seed germination' refers to seeds exposed to 24 h light at room temperature.
148
Figure 5.3: Silique size and seed weight.
(A) Representative depiction of maturing atslp2-2, wild-type NÖ and 35S:AtSLP2 siliques.
Qualitative visual comparison identified a consistent difference in silique size between atslp2,
WT NÖ and 35S::AtSLP2 plants. Siliques depicted were obtained from A. thaliana plants grown
under a 12 h light : 12 h dark photoperiod. The weight of 50 fully mature, dry atslp2-2 (B) and
atmia40 (C) seeds was compared to their respective wild-type backgrounds NÖ and Col-0.
35S::AtSLP2 over-expressing seeds were also measured against wild-type NÖ. The error bars
represent standard error (n = 5). Stars denote p < 0.05 when compared to seeds from each
respective wild-type background.
149
These two processes were explicitly separated to fully resolve when AtSLP2 and
AtMIA40 protein levels were maximal (Figure 5.8). AtSLP2 and AtMIA40 protein expression
was found to largely parallel each other, with AtSLP2 expression peaking early in imbibition (3 6 h) and steadily decreasing over the course of germination (Figure 5.8). AtMIA40 protein
expression was maintained in the dry seed through imbibition, followed by a steady decrease
over the germination time course (Figure 5.8).
5.3.3 An AtSLP2-AtMIA40 protein complex negatively regulates GA biosynthesis
The observed testa cracking and early germination phenotypes in atslp2-2 were
suggestive of a likely alteration in hormonal metabolism and/or signaling events. Seed
germination is governed by complex hormone metabolism and signaling mechanisms centering
around the antagonistic interaction displayed by ABA and GA, with either enhanced GA
biosynthesis/signaling or ABA under-production/insensitivity leading to rapid germination. To
elucidate which process AtSLP2 functions in, a pharmacological approach was undertaken to test
the rapid testa cracking and germination phenotypes of atslp2-2 in the presence of either
Uniconazole (GA biosynthetic inhibitor) or ABA (to ascertain ABA sensitivity). Uniconazole
(10 µM) completely inhibited the early testa cracking and germination phenotypes of both
atslp2-2 and atmia40 testa cracking, while 0.3 and 1.0 µM ABA delayed, but did not abolish,
atslp2-2 and atmia40 testa cracking (Figure 5.4 and 5.6). These observations indicate that
endogenous GA is required for the observed germination phenotypes, suggesting that GA levels
are increased in the absence of these proteins. In silico analysis of publically available
150
Figure 5.4: Depiction of atslp2-2, wild-type NÖ and 35S::AtSLP2 seeds germinated on 0.5 x
MS-Agar plates containing ABA or Uniconazole.
The atlsp2-2 seeds germinated earlier, exhibiting an accelerated rate of testa cracking, radicle
emergence and cotyledon emergence compared to wild-type NÖ and 35S::AtSLP2. Application
of 0.3 and 1.0 µM ABA slowed germination, while the application of 10 µM Uniconazole
completely inhibited germination. Testa cracking is depicted by red arrows, while enlarged
images of each panel show a close up of testa cracking. Control plates consisted of 0.5 x MS
only. All seeds were imbibed at 4oC for 48 h on each plate in the dark prior to germination under
24 h light at 23oC for the denoted number of days.
151
Figure 5.5: Depiction of atmia40 and wild-type Col-O seeds germinated on 0.5 x MS-Agar
plates containing ABA or Uniconazole.
The atmia40 seeds germinated slightly earlier, exhibiting an accelerated rate of testa cracking,
radicle emergence and cotyledon emergence compared to wild-type Col-O. The observable
phenotype was moderate relative to atslp2-2 seeds. Application of 0.3 and 1.0 µM ABA slowed
germination, while the application of 10 µM Uniconazole completely inhibited germination.
Testa cracking is depicted by red arrows, while enlarged images of each panel show a close-up
of testa cracking. Control plates consisted of 0.5 x MS only. All seeds were imbibed at 4oC for
48 h on each plate in the dark prior to germination under 24 h light at 23oC.
152
Figure 5.6: Quantitative analysis of seed testa cracking on 0.5 x MS-Agar plates containing
ABA or Uniconazole.
(A) Testa cracking exhibited by atslp2-2 (light grey; left), WT NÖ (black; middle) and
35S::AtSLP2 seeds (dark grey; right). Application of up to 1 µM ABA slowed, but did not
prevent day 1 testa cracking in the atslp2-2. (B) Testa cracking in atmia40 (light grey; left) and
WT Col-0 (black; right) seeds. Similar to atslp2-2 seeds, application of 1 µM ABA reduced but
did not prevent day 1 testa cracking in atmia40 seeds. Seed testa cracking was equally inhibited
by the application of 10 µM Uniconazole. Control plates consisted of 0.5 x MS only. Seeds were
imbibed at 4oC for 48 h on each plate type prior to germination under 24 h light at 23oC. Error
bars represent ± standard error (n = 3 plates of 100-150 seeds each). Stars denote 2-tailed
Student t-test (p < 0.05) when atslp2-2, atmia40 and 35S::AtSLP2 seeds were examined against
their respective WT NÖ (AtSLP2 related) and Col-0 (AtMIA40 related) backgrounds.
153
Figure 5.7: Quantitative analysis of seed radicle emergence on 0.5 x MS-Agar plates
containing ABA or Uniconazole.
(A) Radicle emergence by atslp2-2 (light grey), WT NÖ (dark grey) and 35S::AtSLP2 seeds
(medium grey) is shown. (B) Radicle emergence in atmia40 (light grey) and WT Col-0 (dark
grey) seeds. Similar to testa cracking, seed radicle emergence was equally inhibited across all
seed types. Control plates consisted of 0.5 x MS only. Seeds were imbibed at 4oC for 48 h on
each plate type prior to germination under 24 h light at 23oC. Error bars represent standard error
(n = 3 plates of 100-150 seeds each).
154
Figure 5.8: Immunoblot analysis of AtSLP2 and AtMIA40 protein expression in imbibed
and germinating seeds.
Wild-type NÖ seeds were imbibed at 4oC for the given time prior to extraction. Both AtSLP2
and AtMIA40 are most abundant early in seed germination. Antibody dilutions included 1.2
µg/mL AP anti-AtSLP2 and 1:1000 anti-AtMIA40 crude immune serum. Ponceau stained
transfer probed with the aforementioned antibodies depicts the loading in each lane. Each lane
contains 30 µg of total protein.
155
microarray data indicated that AtSLP2 transcripts were up-regulated in seeds following treatment
with 5 µM GA (Figure 5.9). Furthermore, GA-induced expression of AtSLP2 negatively
correlated with A. thaliana GA3 oxidase (GA3OX; At5g25900) and GID1A (At3g05120), while
positively correlating with DELLA transcription factors RGA1 (At1g14920) and RGL2
(At3g03450), indicating a role in the negative regulation of GA-related cellular processes (Figure
5.9).
Subsequent assessment of A. thaliana MIA40, GA3OX, GID1, RGA1 and RGL2
expression by quantitative RT-PCR over a 12 h imbibition time course corroborated previously
observed in silico data obtained through microarray analysis of A. thaliana seeds imbibed in the
presence of GA, while also revealing new information about AtMIA40 expression (Figure 5.10).
Over the imbibition time course, atslp2-2 seeds possessed significantly up-regulated expression
of RGL2, a key regulator of seed germination and a transcriptional repressor degraded in the
presence of GA (Figure 5.10; (Lee et al., 2002)). Accompanying up-regulation of RGL2
expression in atslp2-2 seeds was a significant decrease in RGL2 expression in the 35S::AtSLP2
seeds at 6 h and 12 h time points (Figure 5.10). Furthermore, over the imbibition time course,
GA3OX and GID1A expression was significantly down-regulated in 35S::AtSLP2 seeds at 6 h
and 12 h time points, which is consistent with repression of GA production (Figure 5.10).
Expression of MIA40 was also significantly induced following wild-type seed imbibition and
interestingly, in the absence of SLP2, MIA40 expression was constitutively up-regulated.
156
Figure 5.9: Relative transcript expression of GA-related biosynthetic and signaling
proteins from Biological Arabidopsis Resource (BAR).
(A) Changes in GA-related gene expression in response to seed imbibition in the presence of
ABA. (B) Changes in GA-related gene expression in response to seed imbibition in the presence
of GA. Gene identifiers (i.e. AtSLP2; At1g18480) are shown. Yellow represents no change,
while red and blue represent a relative increase and decrease in transcript expression
respectively. All data were obtained from microarray data compiled at BAR
(http://bar.utoronto.ca/).
157
Figure 5.10: Quantitative PCR analysis of GA biosynthetic and signaling genes from 0 h, 6
h and 12 h imbibed atslp2-2, wild-type and 35S::AtSLP2 seeds.
Seeds were imibibed for 0 h, 6 h and 12 h in water at 4oC in the dark followed by snap freezing.
(A-E) Transcriptional expression of A. thaliana GA3 oxidase (GA3OX), GA insensitive dwarf 1a
(GID1A), regulator of GA 1 (RGA1) and regulator of GA-like 2 (RGL2) as well as mitochondrial
intermembrane space import and assembly 40 (MIA40) was assessed. Error bars represent
standard error (n = 3). Individual stars denote a 2-tailed Student t-test (p < 0.05) when examined
against the respective WT time-point. Dual stars denote 2-tailed Student t-test (p < 0.05) when
examined against respective WT 0 h time point.
158
5.3.4 AtSLP2 influences seed fatty acid and amino acid content
With dry, mature atslp2-2 and 35S::AtSLP2 seeds found to weigh more and less than
wild-type NÖ seeds, respectively, metabolic profiling was undertaken to investigate how
AtSLP2 may be influencing seed processes which could result in the observed weight differences
(Figure 5.3). A range of metabolites were examined (sugars, polyols, amino acids, fatty acids)
with the most significant changes being noted for fatty acids (FA) and amino acids (AA).
Measurement of total seed FA (µg / mg seed tissue extracted) found it was reduced in atslp2-2
seeds and increased in 35S::AtSLP2 seeds relative to wild-type NÖ (Figure 5.11A). Further
examination of seed FA composition revealed a significant decrease in atslp2-2 seed 16:0, 18:0,
18:2, 18:3 and 20:1 FA content (µg / mg seed tissue extracted), while 35S::AtSLP2 seeds
increased their 16:0, 18:0, 18:2, 18:3, 20:0 and 20:1 FA content (Figure 5.11B). When examined
based on a molecule percent per mg seed tissue extracted, significant changes in only 16:0, 18:1,
18:2, 18:3 and 20:0 FAs were observed (Figure 5.11C).
In addition, examination of the AA composition of dry, mature atslp2-2 and 35S::AtSLP2
seeds relative to WT NÖ seeds uncovered a number of notable differences. In particular, a
significant increase in valine and isoleucine content was observed in atslp2-2 seeds, which
correspondingly decreased in 35S::AtSLP2 seeds (Figure 5.12). Additional AAs found to
increase in atslp2-2 seeds were the nitrogen-rich AAs arginine, lysine, asparagine and ornithine
as well as the aromatic AA tyrosine (Figure 5.12). Serine, aspartate and methionine also
exhibited an increase in atslp2-2 seeds, while phenylalanine and proline were found to decrease
(Figure 5.12). No notable changes were observed for pyroglutamic acid (glutamic acid &
glutamine) nor alanine and glycine (Figure 5.12).
159
Figure 5.11: Comparative analysis of atslp2-2 and 35S::AtSLP2 seed FA content and
composition.
(A) Total FA content (µg/mg seed) of atslp2-2, 35S::AtSLP2 and WT NÖ seeds. Single stars
denote p < 0.05 when examined relative to WT NÖ. (B) Breakdown of FA-type abundance (µg)
and (C) FA-type percent per mg atslp2-2, 35S::AtSLP2 and WT NÖ seed. The error bars
represent standard error (n = 3). Single stars denote p < 0.05 and double stars denote p < 0.01
when examined relative to WT NÖ. Significance was determined using a 2-tailed Student T-test.
160
Figure 5.12: Relative levels of AAs in atslp2-2, WT NÖ and 35S::AtSLP2 dry seeds.
Highlighted are branched AAs (blue), nitrogen rich AAs (red) and aromatic AAs (green). The
error bars represent standard error (n = 3). Stars denote p < 0.05 when compared to WT NÖ
using a 2-tailed Student T-test.
161
5.4 Discussion
5.4.1 Negative regulation of seed germination through GA metabolism
In exploring the biological function of AtSLP2, insertional atslp2-2 mutant seeds were
found to maintain a heavier dry seed weight relative to NÖ seeds. When germinated on control
0.5 x MS-Agar plates, atslp2-2 seeds exhibited accelerated testa cracking compared to NÖ seeds,
while 35S::AtSLP2 over-expressing seeds displayed delayed testa cracking. This indicated that
AtSLP2 was a regulator of seed germination. With seed germinative processes predominantly
governed by ABA and GA, this suggests that atslp2-2 seeds were either ABA insensitive / underproducing or GA-sensitive / over-producing. The lack of any dramatic ABA insensitivity and
maintenance of testa cracking in atslp2-2 and atmia40 seeds in the presence of ABA indicated
that the observed seed germination phenotype is likely due to enhanced GA production or altered
GA signaling. If atslp2-2 seeds were ABA insensitive, application of 0.3 µM and 1.0 µM ABA
should not have induced the concentration-dependent effect on seed germination that was
documented here (Koornneef et al., 1984; Koornneef et al., 1989), and if they were ABA underproducing they should have germinated on Uniconazole plates. Furthermore, Uniconazole
completely abrogated testa cracking and early germination of atslp2-2 and atmia40 seeds
indicating that GA is required for the observed germination phenotypes. Thus, the AtSLP2AtMIA40 complex likely functions to negatively regulate GA biosynthesis since GA signaling
mutants such as rgl2 and other DELLA protein (negative regulators) loss-of-function mutants
can germinate in the presence of GA inhibitors and confer GA phenotypes in the absence of
bioactive GA (Figure 5.13; (Sun and Kamiya, 1994; Lee et al., 2002; Cao et al., 2005)).
162
Figure 5.13: Model of AtSLP2-AtMIA40 protein complex function during seed
germination.
(A) Under wild-type conditions (Wild-type), AtMIA40 activates AtSLP2 to negatively regulate
GA biosynthesis and in doing so positively reinforces ABA inhibition of seed germination. (B)
Absence of AtSLP2 (atslp2-2) results in unimpeded GA biosynthetic processes leading to an
accelerated seed germination phenotype. (C) Over-expression of AtSLP2 (35S::AtSLP2) leads to
enhanced inhibition of GA-related cellular processes, which concomitantly enhances ABA
inhibition of seed germination. This leads to a delayed seed germination phenotype. (D) Absence
of AtMIA40 (atmia40) reduces the ability of AtSLP2 to negatively regulate GA biosynthesis.
This leads to the moderately accelerated seed germination phenotype observed with atmia40
seeds as AtSLP2 alone has been shown to maintain phosphatase activity in vitro (Chapter 3).
163
If GA biosynthesis was up regulated in atslp2-2 seeds, balancing of GA-induced
signaling would require a corresponding increase in the negative regulators to counter the
increased GA action. In accordance with this hypothesis, expression of GA-inducible RGL2, the
major determinant of seed germination (Lee et al., 2002; Stamm et al., 2012), was found to be
constitutively up-regulated in atslp2-2, indicating a system attempting to balance GA
biosynthetic and signaling outputs (Figure 5.10; (Lee et al., 2002)). In 35S::AtSLP2 seeds this
pattern was reversed with these seeds having significantly lower RGL2 expression than wild-type
following imbibition (Figure 5.10).
5.4.2 The AtSLP2-AtMIA40 protein complex functions to negatively regulate GA biosynthesis
When compared to atslp2-2 seeds, atmia40 seeds exhibited more moderate testa cracking
and early germination phenotype. This suggests that AtSLP2 likely represents the functional
driver of the AtSLP2-AtMIA40 complex in negatively regulating GA-related cellular processes.
Reinforcing this hypothesis, bacterially-expressed and purified AtSLP2 from E. coli maintained
detectable protein phosphatase activity, even in the absence AtMIA40 (Chapter 3), indicating
that atmia40 seeds likely possess some residual AtSLP2 protein phosphatase activity which
mitigates, to a certain extent, the absence of AtMIA40 and its requirement to fully activate
AtSLP2.
Furthermore, with both atslp2-2 and atmia40 seed phenotype data supporting a role for
mitochondrial-targeted AtSLP2 in regulating cytosolic GA-related processes, there remains a
need to resolve the intermediate mechanisms connecting the mitochondria to cytosolic GA.
Given the metabolic significance of mitochondria across all Eukaryotes, and that almost all
mitochondrial proteins are nuclear encoded and imported (Fraga and Ventura, 2013), these
164
intermediate mechanisms may include the regulation of mitochondrial protein import and/or
mitochondrial metabolism. Independently connecting the mitochondria to cytosolic GA-related
processes is the Nicotiana sylvestris cytoplasmic male sterile II (CMSII) mutant (Gutierres et al.,
1997). CmsII possesses a novel insertional mutation in the mitochondrial gene encoding NADH
dehydrogenase 7 (NAD7), accompanied by an absence of NAD9, which results in a faulty
mitochondrial electron transport chain (mETC) complex I (Gutierres et al., 1997). Similarly,
atmia40 plants were also shown to possess a defective mETC complex I resulting from reduced
mitochondrial NAD9, which exhibited a 40% decrease in complex I activity (Carrie et al., 2010).
Furthermore, metabolite profiling of leaves from cmsII plants uncovered a nitrogen-rich
phenotype, consisting of an elevated abundance of nitrogen-rich amino acids and a depletion of
starch stores and 2-oxoglutarate (Dutilleul et al., 2005). Subsequent characterization revealed
that topical application of bioactive GA could recover a wild-type growth phenotype in cmsII
shoots (Pellny et al., 2008). Furthermore, GA biosynthesis requires 2-oxoglutarate (Hedden and
Thomas, 2012), which is also a key mitochondrial metabolite required for nitrogen assimilation
(Bunik and Fernie, 2009). With both nitrogen assimilation and GA biosynthesis linked through
their requirement for 2-oxoglutarate and AtSLP2 potentially linked to electron transport chain
complex I through AtMIA40, it may be possible that mitochondrial-targeted AtSLP2 negatively
regulates cytosolic GA-related processes through the modulation of mitochondrial 2-oxoglutarate
production. Exploration of potential AtSLP2 integration into this network represents an
interesting future direction.
165
5.4.3 Influence of AtSLP on seed FA and AA composition
Both the deletion and over-expression of AtSLP2 resulted in abnormal seed FA content
and composition. Interestingly, when exogenous GA (500 µM) was continuously applied to
whole A. thaliana plants after bolting throughout plant development to silique maturity (Chen et
al., 2012), a decrease in the total seed FAs (µg / mg seed) was observed, which paralleled the
total FA decrease observed in atslp2-2 seeds (Figure 5.11A). GC-MS analysis of the
corresponding FA composition of seeds from GA-treated A. thaliana Col-0 plants revealed a
significant increase in 18:1 FAs in conjunction with a decrease in 18:2 and 18:3 FAs (Chen et al.,
2012). This exact FA fingerprint was observed here with atslp2-2 seeds (Figure 5.11C),
reinforcing the prospect that atslp2-2 seeds maintain excess GA resulting from a lack of AtSLP2
negative regulation. Observed changes in total seed FA content and seed FA composition were
complemented by a GA-stimulated increase in seed weight, where untreated A. thaliana Col-0
seeds were found to weigh approximately 1 mg / 50 seeds and seeds from GA-treated A. thaliana
Col-0 plants weighed approximately 1.55 mg / 50 seeds (Chen et al., 2012). This was paralleled
in atslp2-2 seeds from the NÖ ecotype which weighed approximately 1.4 mg / 50 seeds.
Examining the effect of GA on A. thaliana seed FA composition stemmed from the identification
of DELLA-regulated seed fatty acid reducer (SFAR) genes which function down-stream of GA
signaling to reduce seed FA content (Chen et al., 2012). It was found that over-expression of
SFAR genes lead to the same GA-stimulated seed FA fingerprint, while here, it was the absence
of AtSLP2 which presented the GA-stimulated phenotype. Overall, these observed effects on
seed FA content and composition indicate that AtSLP2 also plays a role during seed maturation.
Examination of atslp2-2 and 35S::AtSLP2 seed AA composition relative to wild-type NÖ
seeds was also performed (Figure 5.12). Of particular note was the dramatic increase in valine
166
and isoleucine in atslp2-2 seeds. Increase in valine and isoleucine concentrations has also been
observed in dark-treated A. thaliana seedlings lacking the mitochondrial-targeted enzymes 2hydroxyglutarate dehydrogenase (D2HGDH) and isovaleryl-CoA dehydrogenase (IVDH)
(Araujo et al., 2010). These proteins are generally characterized as part of a dark-induced
senescence system which responds to carbon starvation through the electron-transfer flavoprotein
/ electron-transfer flavoprotein:ubiquinone oxidoreductase (ETF/ETFQO) complex of the
mitochondrial inner membrane (Ishizaki et al., 2005). This complex represents an extension of
the electron transport chain, where in humans and plants alike it is responsible for the breakdown
of AAs as an alternative source of energy (Ishizaki et al., 2005; Araujo et al., 2010). Under
prolonged dark conditions, both d2hgdh and ivdh insertional mutant seedlings accumulated
significant amounts of branched (valine, isoleucine) and aromatic (tyrosine, tryptophan) AAs
relative to wild-type seedlings (Araujo et al., 2010). This was paralleled in dry, mature atslp2-2
seeds, indicating an inability under either circumstance to utilize these metabolites as an energy
source. It is possible that the significant accumulation of valine and isoleucine in atslp2-2 seeds
functions in conjunction with enhanced seed GA biosynthesis to accelerate atslp2-2 seed
germination.
Also of note, was the significant increase in nitrogen-rich AAs arginine, lysine and
asparagine (Figure 5.12). Nitrogen-rich AAs arginine and asparagine were also notably upregulated in the leaves of the Nicotiana sylvestris cytoplasmic male sterile II (CMSII) insertional
mutant mentioned above (Dutilleul et al., 2005), which maintains a dysfunctional mETC
complex I that was recoverable by the application of bioactive GA (Pellny et al., 2008). In
addition to being a resource that may enhance the germination of atslp2-2 seeds, the observed
elevation in nitrogen-rich AAs further relates AtSLP2 to both mETC function and cytosolic GA167
related processes. How exactly AtSLP2, a protein phosphatase, fits into these processes remains
to be resolved; however, possessing a mitochondrial IMS subcellular localization could suggest
AtSLP2 regulates either protein and/or metabolite import or mitochondrial matrix metabolic
processes through integral inner membrane proteins.
5.5 Conclusion
Unfortunately, the reverse genetics approach applied here did not uncover any
phenotypes relating to the biological function of AtSLP1. Conversely, a number of interesting
AtSLP2-related seed phenotypes were revealed. In particular, atslp2-2 seeds exhibited
accelerated germination indicative of GA over-production, which was supported by elevated
levels of RGL2 expression, a GA negative regulator. Further examination of atslp2-2 seed FA
and AA composition noted significant content and compositional changes indicative of perturbed
mitochondrial function. In addition to seeds, a potential role exists for AtSLP2 in roots, which
was not explored here. Future research should look to examine roots from the atslp2-2 and
35S::AtSLP2 plant lines created. This may reveal additional biological roles for AtSLP2.
.
168
Chapter Six: Rhizobiales-like phosphatase 2 from Arabidopsis thaliana is a
novel phosphotyrosine-specific PPP protein phosphatase
6.1 Introduction
Reversible protein phosphorylation, mediated by protein kinases and phosphatases, is a
prolific regulatory mechanism key to the proper functioning of eukaryotic cells. Given the
extensive catalog of proteins controlled by this mechanism (Olsen et al., 2010), connecting the
biochemical characteristics of protein kinases and phosphatases to their cellular function is of
central importance. The PPP, PPM/PP2C, PTP and Asp-based protein phosphatase families of A.
thaliana collectively encode ~150 protein phosphatases (Kerk et al., 2008), with PPP and
PPM/PP2C protein phosphatases maintaining a catalytic mechanism comprised of a tertiary
structural fold that coordinates metal cations to assist in protein dephosphorylation (Egloff et al.,
1995; Shi, 2009). Both PPP and PPM/PP2C protein phosphatases from Eukaryotes have been
shown to specifically target pSer and pThr residues on protein substrates (Kerk et al., 2008).
The remaining protein phosphatase families are the PTP and Asp-based protein
phosphatases. Both the PTP and Asp-based protein phosphatases employ catalytic motifs
generated from a secondary fold in protein structure. These include HCx5R and DxDxT motifs,
respectively (Denu et al., 1996; Pannifer et al., 1998; Shi, 2009; Zhang et al., 2010). The PTP
phosphatases operate independent of metal cation co-factors, while Asp-based protein
phosphatases require Mg2+ (Shi, 2009; Zhang et al., 2010). PTP phosphatases employ a reduced
cysteine to catalyze the removal of phosphate from pTyr residues of target protein substrates
(Denu et al., 1996; Pannifer et al., 1998; Kerk et al., 2008). Some PTP phosphatases (DSP
phosphatases) maintain the ability to dephosphorylate pSer/pThr residues as well as pTyr or
other non-protein substrates via the same metal-free PTP phosphatase catalytic mechanism
169
(Vilela et al., 2010; Silver et al., 2013; Tonks, 2013). To date, A. thaliana genome analysis has
only identified a single classic PTP protein phosphatase (SSU72), compared to humans, who
possess 38 (Kerk et al., 2008). Despite the low abundance of classic PTP protein phosphatases,
phosphoproteomic studies have consistently found levels of protein tyrosine phosphorylation
(Sugiyama et al., 2008; Mithoe et al., 2012) in A. thaliana and Oryza sativa (O. sativa) which
parallel in abundance to those found in humans (Sugiyama et al., 2008). Interestingly, many of
the prokaryotic Ser/Thr protein phosphatases characterized to date have been found to maintain
dual substrate specificity (Lai and Le Moual, 2005; Pereira et al., 2011; Arora et al., 2012).
Given the prokaryotic heritage of SLP and RLPH phosphatases, research presented within this
chapter looks to define the substrate specificities of A. thaliana SLP and RLPH PPP protein
phosphatases.
The Shewanella-like (SLP) phosphatases, were found to be evolutionarily related to PPP
protein phosphatases (Chapter 2; (Andreeva and Kutuzov, 2004)). Bioinformatic analysis
revealed that they form two highly conserved and phylogenetically distinct sub-groups (Group I,
SLP1 and Group II, SLP2 phosphatases) across all plants (Chapter 2). Specific biochemical
characterization of the A. thaliana (At) SLP1 and SLP2 phosphatases further uncovered unique
biochemical properties and subcellular localizations distinct from other eukaryotic PPP protein
phosphatases (Chapters 2 and 3). This included a complete insensitivity to classic PPP protein
phosphatase inhibitors, in addition to chloroplastic (AtSLP1) and mitochondrial (AtSLP2)
subcellular locations, respectively (Chapters 3 and 4).
The second group of bacterial-like protein phosphatases is the Rhizobiales-like (RLPH)
phosphatases, which were named due to their phylogenetic relatedness to phosphatases encoded
by these bacterial groups (Andreeva and Kutuzov, 2004). Here, I present a biochemical
170
characterization of A. thaliana RLPH phosphatase 2 (AtRLPH2). Analyses uncover a complete
insensitivity to classic PPP protein phosphatase inhibitors, in addition to a catalytic reaction
mechanism highly resistant to inhibition by EDTA or EGTA. As well, through systematic
screening of a set of phosphorylated peptides, AtRLPH2 was found to uniquely maintain a
phosphatase activity solely directed towards pTyr peptides. This is the first example of a
Eukaryotic PPP protein phosphatase displaying specific pTyr activity, and may offer to help
bridge the gap between the prevalence of pTyr in plants and lack of classic PTP protein
phosphatases.
6.2 Materials and Methods
6.2.1 Bioinformatics
Genevestigator (www.genevestigator.com) was used to obtain Affimatrix 22k microarray
data for AtRLPH1 and AtRLPH2 transcriptional expression across different plant tissues.
AtRLPH2 orthologs from other plants were identified, cataloged in Chapter 2 and aligned here
against representative PPP protein phosphatases using ClustalX2 (http://www.clustal.org/). The
presented alignment was assembled using Genedoc as previous described (Chapter 2).
6.2.2 Molecular cloning of AtRLPH2
Fluorescent, tandem affinity purification (TAP) and recombinant protein expression
construct creation utilized full-length At3g09970 (AtRLPH2) obtained from The Arabidopsis
Information Resource (http://www.arabidopsis.org/). AtRLPH2 was cloned with Gatewaycompatible primers for insertion into pDONR221 (Invitrogen). Subsequently, AtRLPH2 was
subcloned into the plant expression vectors pB7RWG2 (http://gateway.psb.ugent.be/) to create a
171
C-terminal RFP (cRFP) fusion construct, and pYL436 to create a C-terminal TAP construct
(Rubio et al 2005). AtRLPH2 was also homologously recombined into pET-DEST42 to create a
C-terminal V5-HIS6 fusion construct (AtRLPH2-HIS6). All cloning steps were performed using
DH5α E. coli. Isolated plasmid DNA was then transformed into the bacterial strain BL21 (DE3)
CodonPlus-RIL E. coli (Agilent Technologies) for expression and subsequent purification of
AtRLPH2-HIS6 protein. AtSLP1-TAP (Chapter 4) and HIS6-AtSLP1 (Chapter 3) utilized here,
were created previously.
6.2.3 Transient expression of fluorescent AtRLPH2 constructs and microscopy
AtRLPH2-RFP was transiently co-expressed with either GFP-only (p2FGW7) to
illuminate the nucleus and cytosol, or YFP-tagged Histone 2B (H2B), to illuminate the nucleus
only (Boisnard-Lorig et al., 2001). All co-expression experimentation was performed using Vicia
faba epidermal leaf cells as previously described (Chapter 3). Microscopic imaging was
conducted using a Leica DMIRE2 spectral confocal and multiphoton microscope with a Leica
TCS SP2 acoustic optical beam splitter (Leica Microsystems), with all cells and leaves visualized
using a 63 x water immersion lens. The excitation/emission wavelengths (nm) employed were as
follows:
GFP,
488/505-515;
YFP,
488/515-525;
RFP,
594/610-650;
chlorophyll
autofluorescence, 488 and 594/685 to 715. Subsequent image processing was performed using
the freely accessible MacBiophotonics ImageJ software (http://www.macbiophotonics.
ca/downloads.htm).
172
6.2.4 Heterologous protein expression and purification
AtRLPH2-HIS6 was expressed in BL21 (DE3) CodonPlus-RIL E. coli (Agilent
Technologies) grown in LB supplemented with 1 % glucosamine at 22oC, 200 rpm and induced
with 0.1 mM IPTG for 18 h. Bacteria were pelleted at 4,000 x g for 15 min and re-suspended in 1
x extraction buffer (50 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 5 % [v/v] glycerol, and 10
mM imidazole) followed by snap freezing and storage at -80oC prior to use. The day of
purification saw the addition of 1 % (v/v) Tween-20, 20 mM imidazole, 1 mM PMSF, 1 mM
benzamidine, 2 µg/mL leupeptin, and 5 µg/mL pepstatin upon thawing. Extraction involved
mechanical lysis via French press at 3 x 16,000 p.s.i. (Sim-Aminco Spectronic Instruments).
Crude lysates were clarified at 40,000 x g for 30 min at 4oC. Supernatants were removed, and an
additional 1 mM PMSF and 1 mM benzamidine were added prior to incubation with NiNTAagarose matrix (Qiagen) end-over-end at 4oC for 1.5 h. Matrix was captured in a column via
gravity sedimentation and washed with 500 cv of Buffer A (1 x extraction buffer containing 1 M
NaCl, 1 % [v/v] Tween-20, and 20 mM imidazole) followed by 100 cv of Buffer B (1 x
extraction buffer). NiNTA-bound proteins were eluted in 1x extraction buffer containing 500
mM imidazole, pH 7.5. Protein eluates were concentrated using a 30,000 Da molecular weight
cut-off Amicon concentrator (Millipore). NiNTA-pure AtRLPH2 eluate was concentrated to 300
µL and subjected to two sequential concentration / re-suspension steps involving Mono-Q (MQ)
Buffer A (50 mM HEPES-NaOH pH 7.5, 50 mM NaCl and 5 % [v/v] glycerol). Concentrated
and MQ Buffer A exchanged AtRLPH2-HIS6 eluate was loaded onto a Mono-Q 5/50 anion
exchange column using a fast liquid protein chromatography (FPLC) system at 0.5 mL/min. The
unbound MQ fraction contained pure AtRLPH2-HIS6. This fraction was concentrated, proteins
resolved by SDS-PAGE, aliquoted, snap frozen in liquid nitrogen, and stored at -80oC for
173
subsequent use. HIS6-AtSLP1 was purified using NiNTA as described previously described
(Chapter 3).
6.2.5 Tandem affinity purification (TAP) isolation of AtSLP1 and AtRLPH2
AtSLP1 and AtRLPH2 TAP-tagged constructs were transformed into Agrobacterium
GV3101 and subsequently used to transfect A. thaliana via the floral dip method as previously
described (Zhang et al., 2006) and performed in Chapter 4. AtSLP1- and AtRLPH2-TAP
expressing A. thaliana plants were grown as described in Chapter 4. AtSLP1- and AtRLPH2TAP protein was isolated in parallel, each from 20 g of rosette tissue, as previously described
using 400 µL of settled NiNTA (Templeton et al., 2011). Each TAP-purified protein was
employed in enzyme assays immediately after the final NiNTA purification step, where it was
washed, but not eluted as previously described (Chapter 4).
6.2.6 Enzymatic analysis
Enzymatic assessment of AtRLPH2 was conducted using two phosphatase assays. The
first involved use of the small molecule phosphatase substrate pNPP (Sigma) while the second
measured phosphatase-catalyzed phosphate release from phosphorylated peptide substrates
derived from known phospho-proteins using malachite green reagent (Baykov et al., 1988).
Assays employing pNPP as a phosphatase substrate were used to assess: AtRLPH2 pH
activity optima, metal cation dependency (EDTA, EGTA, Mn2+, Fe3+, Zn2+, Ca2+ and Mg2+) and
inhibitor sensitivity (MCLR, OA, NaF, sodium orthovanadate (NaOV), ATP, ADP, AMP, Pi,
PPi, glucose 6-phosphate (Glu-6P) and glycerol 3-phosphate (Gly-3P)). Standard pNPP assay
conditions included pre-incubation of 150 ng AtRLPH2 with an experimental variable in 1x
174
dilution buffer consisting of 100 mM HEPES-NaOH pH7.5, 150 mM NaCl (totaling a volume of
20 µL) for 10 min at 30oC. Each assay was then supplemented with 130 µL of 1x dilution buffer
containing 5 mM pNPP followed by an additional 10 min incubation, shaking at 30oC. All pNPP
assays were then quenched with 150 µL of 2 M NaOH. Cleavage of pNPP was measured
spectrophotometrically at 405 nm.
Malachite Green assays were performed as previously described (Baykov et al., 1988)
alternatively using a 10 % ammonium molybdate solution. Each assay employed 1 µg of
recombinant phosphatase protein incubated for 1 h at 30oC in 160 µL of 1 x dilution buffer
containing each respective experimental phosphorylated peptide substrate (as described in
Chapter 4). Addition of 40 µL malachite green solution quenched the reaction to reveal
phosphatase catalyzed increase in free phosphate relative to control assays lacking protein
phosphatase. Quenched assays were left at room temperature (23oC) for 10 min prior to
spectrophotometric assessment at 630 nm. Amount of free phosphate was calculated using a
standard curve derived from known amounts of potassium phosphate.
Assays performed using TAP purified AtSLP1 and AtRLPH2 employed 65 µL of protein
phosphatase coupled NiNTA isolated from 20 g of rosette tissue as described above. Each
aliquot of coupled NiNTA matrix was re-suspended in 235 µL of 1 x dilution buffer containing
the respective phosphorylated peptide and totaling 300 µL. Each assay mixture was incubated for
1 h in a 30oC water bath. NiNTA matrix was pelleted and 160 µL of reaction mix was removed
and quenched with 40 µL of malachite green solution. Quenched assays were evaluated
spectrophotometrically as outlined above.
175
6.3 Results
Pursuit of AtRLPH2, and not AtRLPH1, for biochemical characterization was driven by
the observed transcriptional expression profile of AtRLPH2 versus AtRLPH1 across A. thaliana
tissue types (Figure 6.1). Interestingly, AtRLPH1 exhibited almost no transcript expression,
while AtRLPH2 exhibited an almost ubiquitous tissue expression profile (Figure 6.1), suggesting
RLPH1 may in fact be an inactive gene.
6.3.1 AtRLPH2 is a dual-localized nuclear and cytosolic protein
Transient co-expression of AtRLPH2-cRFP with GFP- and YFP-tagged marker
constructs in Vicia faba leaf epidermal pavement cells demonstrated that AtRLPH2 is a duallocalized nuclear / cytosolic protein (Figure 6.2). The observed dual nuclear / cytosolic
subcellular localization confirmed in silico subcellular localization findings presented in Chapter
2.
6.3.2 Purification of recombinant HIS6-tagged AtRLPH2
Expression and purification of AtRLPH2-HIS6 from E. coli using sequential NiNTA
affinity purification and Mono-Q (MQ) anion exchange provided pure protein for biochemical
characterization (Figure 6.3). Anion exchange chromatography eliminated a number of nonspecific co-purifying bacterial proteins, yielding a significantly more pure AtRLPH2 protein
fraction (Figure 6.3A). Immunoblotting of purified AtRLPH2 with V5-epitope antibodies
identified the purified protein as AtRLPH2 (Figure 6.3B), which was supported by MALDITOF peptide mass fingerprinting. Subsequent calibrated size exclusion chromatography using a
176
Figure 6.1: AtRLPH1 and 2 transcriptional tissue expression profile.
Compiled microarray transcript expression data for AtRLPH1 (At3g09960) and AtRLPH2
(At3g09970) was obtained from Genevestigator (www.genevestigator.com). Expression is
indicated by a gradient of white (low expression) to red (high expression) as a percentage of the
top 1% genes expressed in each tissue. The numbers denote the number of total arrays used in
determining the displayed level of relative gene expression for AtRLPH1 and AtRLPH2.
177
Figure 6.2: Subcellular localization of AtRLPH2.
Transient co-expression of AtRLPH2-RFP with (A) GFP-only and (B) histone 2B-YFP (H2BYFP) fluorescent marker constructs in Vicia faba leaf epidermal pavement cells. AtRLPH2-RFP
consistently exhibited dual subcellular targeting to the cytosol and nucleus. Scale bar = 20 µm.
178
Figure 6.3: Purification of AtRLPH2-HIS6 from E. coli.
(A) Colloidal blue stained 12 % SDS-PAGE depicting 5 µg of NiNTA and Mono-Q HIS6-V5tagged AtRLPH2 containing eluates. (B) Anti-V5 tag western immunoblot (1:500)
demonstrating mono-specific detection of HIS6-V5-tagged AtRLPH2 (100 ng). The anti-V5
immunoreactive band was also confirmed as AtRLPH2 by mass spectrometry.
179
Superdex 200 gel filtration column, resolved an in-solution molecular mass of 49.1 kDa, despite
a denatured SDS-PAGE mass of approximately 40 kDa (Figure 6.3 and Figure 6.4).
6.3.3 Enzymatic characterization of AtRLPH2
Bacterially-expressed and purified AtRLPH2 was found to maintain a pH optimum of 7.5
- 7.8 (Figure 6.5A) with a specific activity of approximately 20 nmol Pi min-1mg-1 towards the
artificial phosphatase substrate pNPP (Figure 6.5B). Screening of known metal cation co-factors
of various protein and non-proteinaceous phosphatases revealed a lack of metal cation-dependent
phosphatase activity, with highest activity observed in the control condition containing 5 mM
EDTA (Figure 6.5B). AtRLPH2 activity assays in the presence of 0.5 mM Mn2+, Fe3+, Ca2+ and
Mg2+ all exhibited slightly lower activity, while Zn2+ demonstrated an inhibitory effect (Figure
6.5C). Lack of AtRLPH2 metal-dependent activity was further supported by activity assays
conducted with up to 50 mM EDTA or EGTA (Figure 6.5D). Alignment of AtRLPH2 orthologs
from across the kingdom Plantae against classic PPP protein phosphatases PP1 (TOPP1) and
PP2A (PP2A-1) from A. thaliana and the related Shewanella-like protein phosphatases, also
from A. thaliana (AtSLP1 and AtSLP2), identified two highly conserved cysteine residues in the
active site of RLPH phosphatases (Figure 6.6), indicating a potential metal-independent PTP
phosphatase catalytic mechanism (Figure 6.7), even though the PPP phosphatase metal-binding
residues are present. Inhibition assays conducted using hydrogen peroxide (H2O2) and Nethylmaleimide (NEM) failed to inhibit AtRLPH2 at concentrations specific to a PTP
phosphatase catalytic mechanism (Figure 6.8).
180
Figure 6.4: Calibrated Superdex 200 size exclusion chromatography of AtRLPH2-HIS6.
The observed molecular mass of 49.1 kDa is consistent with a monomeric protein. Vo = void
volume. Inset represents a Colloidal blue stained gel of the peak fractions. Each lane contains 30
µL of a 1 mL fraction.
181
Figure 6.5: AtRLPH2 pH activity profile, metal cation dependency, and sensitivity to metal
chelators EDTA and EGTA.
(A) AtRLPH2 pH activity profile was assessed from pH 5.5 to 9. Activity assays were conducted
in the absence of metal cations. (B) Substrate saturation of AtRLPH2 using the artificial
phosphatase substrate pNPP. (C) AtRLPH2 was assayed in the presence of (Mn2+, Fe3+, Ca2+,
Zn2+ or Mg2+) and absence of metal cations (control). Highest phosphatase activity was detected
in the control condition containing 5 mM EDTA. (D) Assays containing increasing amounts of
EDTA and EGTA. All assays were performed using 150 ng of purified AtRLPH2 and the
phosphatase substrate pNPP. Phosphatase activity was assessed through pNPP cleavage. Error
bars depict ± standard error (n = 3).
182
Figure 6.6: ClustalX alignment of representative RLPH phosphatases from across
photosynthetic Eukaryotes.
Highlighted are cysteines conserved across RLPH phosphatases near or in active site motifs
(labeled motif 1 and 2). These cysteine residues may influence AtRLPH2 protein phosphatase
activity. Gene identifiers corresponding to the list of RLPH phosphatase depicted here can be
found in Appendix A.2.
183
Figure 6.7: Cartoon depiction of PPP, PPM and PTP protein phosphatase motifs relative to
AtRLPH2.
Based on the maintenance of highly conserved PPP Ser/Thr protein phosphatase catalytic motifs
GDxHG, GDxVDRG, GNHE and HGG, AtRLPH2 is a PPP protein phosphatase. Amino acids
labeled in green and blue are important in metal ion coordination and enzyme catalysis. Cysteine
(C) labeled in red depicts the catalytic cysteine of SEX4. Additional points of interest for
AtRLPH2 include the lack of a C-terminal SAPNYC motif responsible for coordinating OA and
MCLR inhibition. PP2Cs represents PPM Ser/Thr protein phosphatases (Shi, 2009) and starch
excess 4 (SEX4) represents a PTP/DSP phosphatase maintaining the canonical PTP protein
phosphatase catalytic motif (Silver et al., 2013; Tonks, 2013).
184
Figure 6.8: Effect of hydrogen peroxide (H2O2) and N-ethylmaleimide (NEM) on AtRLPH2
phosphatase activity.
Both H2O2 and NEM failed to inhibit AtRLPH2 at the micromolar concentrations indicative of a
PTP and/or DSP phosphatase (Silver et al., 2013; Tonks, 2013).
185
Sodium pyrophosphate (PPi) is a traditional phosphatase inhibitor and displays potent
inhibition of AtRLPH2 with an IC50 of approximately 100 nM (Figure 6.9A). Additional
phosphate-containing compounds ATP, ADP, AMP, Pi, Glu-6P and Gly-3P were also evaluated
for their inhibitory effect on AtRLPH2 (Figure 6.9B). Using pNPP as a substrate, AtRLPH2
exhibited micromolar (µM) to millimolar (mM) sensitivity to ATP, ADP, Pi and AMP (Figure
6.9B). Glu-6P and Gly-3P had no notable inhibitory effect on AtRLPH2 activity up to a 2 mM
concentration (Figure 6.9B). As well, phosphate analogs NaF and NaOV were examined for their
inhibitory effect on AtRLPH2 (Figure 6.10). NaF and NaOV inhibit PPP and PTP protein
phosphatases, respectively. Both NaF and NaOV substantially reduced AtRLPH2 activity to 5
and 20 percent of maximum when using up to 100 mM (Figure 6.10A) and 10 mM (Figure
6.10B) concentrations, respectively (Figure 6.5A and B).
The naturally occurring PPP protein phosphatase-specific small molecule inhibitors OA
and MCLR were also examined for their inhibitory effect on AtRLPH2. Up to 150 nM OA failed
to inhibit AtRLPH2 (Figure 6.10C), while increasing amounts of MCLR exerted an activating
effect on AtRLPH2 (Figure 6.10D). Coinciding with this lack of sensitivity to OA and MCLR
was a noted lack of the key C-terminal SAPNYC motif responsible for coordinating OA and
MCLR in other PPP protein phosphatases (Figure 6.6).
6.3.4 AtRLPH2 is a phosphotyrosine-specific PPP protein phosphatase
Utilization of pSer, pThr and pTyr phosphorylated peptides to decipher the substrate
preferences of PP1, PP2A, PP2B and PP2C has previously been employed (Donella Deana et al.,
1990; Donella-Deana et al., 1991; Donella-Deana et al., 1994) . Therefore, using our own
186
Figure 6.9: AtRLPH2 sensitivity to inhibition by phosphate-containing small molecules.
AtRLPH2-HIS6 was incubated with increasing amounts of (A) sodium pyrophosphate and (B)
ATP (black circles), ADP (grey squares), AMP (grey triangles), Pi (black diamonds), Glu-6P
(black squares; dashed black line), Gly-3P (grey circles; dashed grey line). All assays were
performed using 150 ng of purified AtRLPH2 and the phosphatase substrate pNPP. Phosphatase
activity was assessed through pNPP cleavage. Error bars represent standard error (n = 3).
187
Figure 6.10: AtRLPH2 sensitivity to classic PPP and PTP protein phosphatase small
molecule inhibitors.
AtRLPH2-HIS6 incubated with increasing amounts of (A) NaF, (B) NaOV, (C) OA and (D)
MCLR in conjunction with the phosphatase substrate pNPP. Inhibition by the phosphate analogs
NaF and NaOV is indicative of a PPP and PTP protein phosphatase, respectively. Phosphatase
activity was assessed through pNPP cleavage. All assays were performed using 150 ng of
purified AtRLPH2. Error bars represent standard error (n = 3).
188
phosphorylated peptides consisting of pSer, pThr, pTyr and pThr/pTyr peptides, the substrate
specificity of AtRLPH2 was evaluated (Table 6.1 and Figure 6.11). The activity of bacteriallyexpressed and purified AtRLPH2 towards both phosphorylated peptide and non-peptide
substrates was measured by the detection of enzyme-catalyzed release of phosphate from each
phospho-substrate (Table 6.1 and Figure 6.11). AtRLPH2 exhibited exceptional preference for
pTyr- and pThr/pTyr-containing peptides, with almost no activity toward pSer and pThr peptides
(Table 6.1 and Figure 6.11). Highest activity was towards the dually phosphorylated pThr/pTyr
mitogen-activated protein (MAP) kinase peptides p38beta and p38 with specific activities of
144278.0 ± 1459.9 and 168610.4 ± 641.3 pmol Pi min-1mg-1 respectively (Table 6.1 and Figure
6.11). Lowest AtRLPH2 activity was towards pSer- and pThr-containing peptides RRP1B and
Ki67 with 996.8 ± 8.8 and 1478.6 ± 3.6 pmol Pi min-1mg-1 specific activities, respectively (Table
6.1 and Figure 6.11). No measurable activity was detected when employing non-phospho-peptide
substrates (Table 6.1).
Phylogenetically related Shewanella-like PPP protein phosphatase AtSLP1 was expressed
and purified from bacteria and examined in conjunction with AtRLPH2 to assess its preference
for pSer, pThr, pTyr and pThr/pTyr phosphorylated peptides (Figure 6.11). Unlike AtRLPH2,
AtSLP1 exhibited a broader specificity profile for phosphorylated peptide substrates, effectively
dephosphorylating pSer, pThr, pTyr and pThr/pTyr phosphorylated peptides (Figure 6.11).
AtSLP1 also demonstrated an enhanced preference for the pThr/pTyr phosphorylated peptides
p38 and p38beta over any singly phosphorylated peptide, maintaining a specific activity of
208287.8 ± 3276.2 and 205614.1 ± 4814.7 pmol Pi min-1mg-1, respectively (Figure 6.11).
189
Table 6.1: Malachite green dephosphorylation assay examining AtRLPH2 phosphatase
activity towards phosphorylated peptide substrates.
Assays were performed using either 500 µM phosphorylated peptide or 5 mM metabolite and 1
µg purified AtRLPH2. Grey highlighted area represents dual pTyr (pY) and pThr (pT)
phosphorylated peptides. The phosphorylated protein from which each corresponding
phosphorylated peptide was derived is listed. Values are followed by ± standard error (n = 3).
190
Figure 6.11: Phosphorylated substrate preferences of purified A. thaliana bacterial-like
PPP protein phosphatases.
A. thaliana SLP1 (AtSLP1 – black bars) and RLPH2 (AtRLPH2 – grey bars) were expressed
and purified as HIS6-tagged fusion proteins in E. coli. Purified phosphatases were incubated
with 500 µM of the designated phosphorylated peptide at 30oC for 1 h to determine their
phospho-substrate preference. Phosphatase activity was determined using a stop time malachite
green assay to detect phosphatase catalyzed phosphate release. Error bars represent standard
error (n = 3).
191
Isolation of over-expressed, in planta constructed AtSLP1 and AtRLPH2 via tandem
affinity purification (TAP) was performed to verify the phosphorylated peptide substrate
preferences observed when employing recombinant, bacterially-produced protein (Figure 6.12).
The deduced activities for each TAP isolated protein further substantiated the robust preference
of AtRLPH2 for pTyr-containing peptides, while also demonstrating that AtSLP1 has a less
pronounced specificity for pTyr-phosphorylated peptide substrates relative to its recombinant,
bacterially-produced counterpart. Despite this, AtSLP1 still maintained measurable phosphatase
activity towards pTyr phosphorylated peptides, but with a marked preference for pThr-containing
phosphorylated peptides (Figure 6.12).
6.4 Discussion
6.4.1 Bridging the gap: AtRLPH2 is a phosphotyrosine-specific PPP protein phosphatase
Phosphoproteomic research efforts in plants have revealed a similar ratio of tyrosine
phosphorylation events as found in mammals with 82.7% (84.8%) pSer, 13.1% (12.3%) pThr
and 4.2% (2.9%) pTyr in A. thaliana (O. sativa), respectively, compared to 86.4% pSer, 11.8%
pThr and 1.8% pTyr in humans (Sugiyama et al., 2008; Nakagami et al., 2010; Olsen et al.,
2010). Genomic studies indicate A. thaliana maintains only a single classic PTP protein
phosphatase relative to the 38 encoded by humans and therefore should not be able to maintain
an equivalent level of protein tyrosine phosphorylation (Kerk et al., 2008), suggesting that
reversible protein tyrosine phosphorylation may alternatively be directed by either PPP protein
phosphatases, such as the bacterial-like PPP protein phosphatases AtSLP1 and AtRLPH2, or
DSP phosphatases that fall under the PTP phosphatase class.
192
Figure 6.12: Phosphorylated substrate preferences of TAP-purified A. thaliana bacteriallike PPP-family protein phosphatases.
Constitutively-expressed AtSLP1-TAP and AtRLPH2-TAP were isolated from 20 g of A.
thaliana rosette leaf tissue as per Templeton et al., 2011. Incubation of each TAP purified
protein with either 500 µM BRCA1 (pThr – black bars) or rat SAPK3 (pTyr – grey bars)
phospho-peptides revealed each protein phosphatase's substrate preference. Phosphatase activity
was determined using a stop time malachite green assay to detect phosphatase-catalyzed
phosphate release. Although assayed, AtRLPH2-TAP displayed no measurable activity against
the pThr BRCA1 peptide. Error bars represent standard error (n = 3).
193
With RLPH phosphatases suggested to have entered plants through a horizontal gene
transfer event involving Planctomycetes bacteria it is possible they may have maintained many
of their ancestral biochemical properties (Chapter 2; (Andreeva and Kutuzov, 2004)). Studies
examining bacterial protein phosphorylation in Salmonella enterica and Bacillus anthracis using
phosphorylated peptide substrates have revealed two PP2C phosphatases which seem to possess
a dual specificity for pSer/pThr and pTyr (Lai and Le Moual, 2005; Arora et al., 2012).
Furthermore, characterization of bacterial PPP protein phosphatases has also found dual
specificity towards pSer/pThr and pTyr despite maintaining a PPP protein phosphatase catalytic
fold (Tsuruta and Aizono, 1999, 2000; Pereira et al., 2011). These combined findings may
represent the tip of a widespread phenomenon across prokaryotes and render it possible that
either dual specificity or sole pTyr-specific protein phosphatase capabilities may also extend to
uncharacterized phosphatases (e.g. PP2C phosphatases) in plants in addition to the bacterial PPP
protein phosphatases discussed here. Interestingly, plants encode considerably more PP2C
protein phosphatases when compared to humans (76 PP2Cs in A. thaliana versus 18 PP2Cs in
humans; (Kerk et al., 2008; Xue et al., 2008; Fuchs et al., 2013)).
Further validation of substrate specificity results obtained using bacterially-expressed and
purified AtSLP1 and AtRLPH2 was pursued using in planta constructed versions of each
phosphatase TAP-isolated from A. thaliana rosette leaf tissue. Only a slightly reduced preference
for pTyr-containing peptides was observed for TAP isolated AtSLP1, with the initial dualspecificity towards pSer/pThr and pTyr peptides remaining intact. TAP-isolated AtRLPH2
preference for pTyr remained unchanged. These corroborative phosphatase assays involving in
planta constructed versions of both AtSLP1 and AtRLPH2 proteins were performed, as it was
previously observed that protein phosphatase substrate specificity can differ between endogenous
194
and recombinant versions of the same phosphatase (MacKintosh et al., 1996). In this study,
bacterially-produced and purified PP1 phosphatase exhibited activity towards pTyr, in addition
to pSer/pThr, while the endogenous PP1 purified from rabbit skeletal muscle was pSer/pThr
specific. This was not observed with either AtSLP1 nor AtRLPH2, which exhibited no major
difference in phosphorylated substrate preference between their recombinant and in planta
protein forms. However, previous examination of the AtSLP1 paralog AtSLP2 via identical
enzymatic analysis exhibited a marked substrate specificity difference between its recombinant
and in planta forms (Chapter 4). Recombinant AtSLP2 showed a broad dual specificity activity
profile, while endogenous AtSLP2 was pSer/pThr specific (Chapter 4).
6.4.2 Function of AtRLPH2 protein phosphatase
With the largest proportion of pTyr-containing proteins found in the A. thaliana nucleus
and/or cytosol (Sugiyama et al., 2008; Mithoe et al., 2012) and AtRLPH2 localized to these
compartments, it may be possible that AtRLPH2 regulates signal transduction events that result
in its movement between the nucleus and cytosol. In particular, MAP kinases are key proteins
responsible for translating developmental and extracellular environmental cues (e.g.
physiological stress) into intracellular signaling cascades which modify gene expression (Bartels
et al., 2010). They are also proteins which maintain pTyr as part of their activation loop (TxY)
(Bartels et al., 2010). Involvement in either of these roles would fit with AtRLPH2's dual
localization to the nucleus and cytosol. Current evidence indicates that MAPK phosphatases in
plants are comprised of both PP2C and DSP phosphatases (Vilela et al., 2010). Coupled with a
pH optimum consistent with the cytosolic environment and the diversity of phosphatases
regulating MAP kinase signaling, AtRLPH2 may play a role in regulating the phosphorylation of
195
these protein targets. However, given the prevalence of pTyr-containing proteins outside of MAP
kinases, this hypothesis remains one of many (Sugiyama et al., 2008; Nakagami et al., 2010;
Olsen et al., 2010).
AtRLPH2 also exhibited an enhanced sensitivity to pyrophosphate (PPi) compared to
AtSLP1, AtSLP2 and A. thaliana TOPP (PP1) phosphatases (Chapter 3; (Stubbs et al., 2001)).
Findings originally indicated that AtRLPH2 may not be a protein phosphatase based on its high
affinity for PPi, but rather, a novel pyrophosphatase (May et al., 2011). This was reminiscent of
DSP phosphatases which maintain a PTP phosphatase catalytic HCx5R motif, but exclusively
dephosphorylate non-proteinaceous substrates such as starch and phosphoinositides (Kerk et al.,
2008; Silver et al., 2013; Tonks, 2013). Further examination using phosphorylated metabolites
with a demonstrated inhibitory effect, including PPi, failed to elicit any phosphatase activity as a
substrate (Table 6.1). This indicated that the observed affinity of AtRLPH2 for these nonproteinaceous substrates was likely due to competition for active site occupancy in enzyme
assays conducted with pNPP.
Given its high affinity for PPi, AtRLPH2 may possess a cellular role in regulating PPirelated metabolic and/or signaling processes, in particular, enhanced sensitivity to PPi may tie
AtRLPH2 to stress adaptation or metabolism (Plaxton, 1996; Mustroph et al., 2005). In addition
to signaling, a large proportion of pTyr-containing proteins in A. thaliana were found to regulate
protein metabolism and other metabolic processes, providing precedence for a connection
between energy sensing and protein tyrosine phosphorylation (Sugiyama et al., 2008). AtRLPH2
also exhibited an increasing sensitivity to phosphate-containing compounds AMP, Pi, ADP and
ATP, with no effect observed with Glu-6P or Gly-3P, further emphasizing a potential link
between AtRLPH2 and the cellular energy status of the cell. Additional experimentation is still
196
required to identify the in vivo substrates of AtRLPH2 and to verify the significance of its
sensitivity to PPi. The prokaryotic heritage of plant RLPH phosphatases suggests a selection
pressure to remain unaltered in photosynthetic Eukaryotes, which could be indicative of a
conserved role for AtRLPH2 in regulating core and/or ancient cellular processes.
Like other photosynthetic Eukaryote PPP protein phosphatases, AtRLPH2 was sensitive
to NaF inhibition (Pallen et al., 1985; Stubbs et al., 2001), in addition to inhibition by the PTP
protein phosphatase inhibitor NaOV (Swarup et al., 1982; Pallen et al., 1985). With each of these
compounds possessing specific inhibitory capabilities against their respective protein
phosphatase families, it was unique to find that each compound was able to inhibit AtRLPH2 in
a concentration-dependent manner. This supports the notion that AtRLPH2 is a pTyr specific
protein phosphatase which maintains a PPP protein phosphatase active site fold capable of
accommodating bulkier pTyr-containing proteins.
6.4.3 AtRLPH2 maintains an ancient insensitivity to naturally occurring protein phosphatase
inhibitors
Given the ancient prokaryotic lineage of RLPH phosphatases, along with previous
biochemical findings involving related bacterial-like PPP phosphatases AtSLP1, AtSLP2
(Chapter 3) and PbSHLP1 (Patzewitz et al., 2013), it has become apparent that the bacterial
phosphatases of Eukaryotes maintain a conserved insensitivity to OA and MCLR. A possible
explanation for this observation may be that these phosphatases were under heavy selection
pressure in Prokaryotes to maintain OA and MCLR insensitivity. This may have conveyed a
survival advantage in environments containing competing organisms such as OA-producing
dinoflagellates (Bialojan and Takai, 1988) and MCLR-producing cyanobacteria (MacKintosh et
197
al., 1990). The lack of OA and MCLR inhibition can be directly related to the lack of a key Cterminal SAPNYC motif which has been shown to be central in coordinating both inhibitors in
PP1 and PP2A (Goldberg et al., 1995; Maynes et al., 2001). Whether the lack of OA and MCLR
sensitivity in these bacterial-like protein phosphatases is of specific biological significance to
photosynthetic Eukaryotes or an evolutionary by-product of their prokaryotic origin has yet to be
resolved.
6.4.4 AtRLPH2 catalysis: Cysteine-mediated or metal-dependent catalytic mechanism
Lack of detectable metal cation dependency and enzymatic assay quenching by EDTA or
EGTA in vitro suggested that AtRLPH2 may be metal cation dependent despite possessing the
entire complement of PPP protein phosphatase catalytic motifs required to coordinate metal
cations. Sensitivity to Zn2+ inhibition was observed however, indicating a capacity for metal
coordination similar to other classic PPP protein phosphatases (Shi, 2009) and suggesting that in
solution metal cations can access the active site in vitro. Interestingly, an alignment of plant
RLPH2 phosphatases uncovered a conserved cysteine present in active site motif 2 where other
PPP phosphatases would possess hydrophobic residues. Subsequent biochemical exploration of
this cysteines involvement in a PTP-like phosphatase catalytic mechanism produced results
inconsistent with a metal-independent PTP phosphatase. Both classic PTP protein phosphatases
and DSP phosphatases utilize a conserved HCx5R redox responsive catalytic motif which
maintains a low micromolar (µM) IC50 response to oxidizing agents H2O2 and NEM (Tanner et
al., 2011; Silver et al., 2013). In addition to being purified in the absence of reductant,
incorporation of these compounds into AtRLPH2 assays failed to achieve complete inhibition,
even at millimolar (mM) concentrations. This result, combined with the observed inhibition of
198
AtRLPH2 by Zn2+ cations and conservation of metal cation-binding residues, collectively
indicates that AtRLPH2 likely utilizes a metal cation-mediated catalytic mechanism consistent
with other PPP protein phosphatases.
6.5 Conclusion
Presented here for the first time is a pTyr-specific PPP protein phosphatase. This finding
helps to bridge the current disconnect between the high abundance of protein tyrosine
phosphorylation in plants and the lack of pTyr-specific protein phosphatases. Furthermore,
findings presented here open the door to the larger possibility that reversible pTyr specific
protein phosphorylation in plants is regulated by a number of unconventional protein
phosphatases such as AtSLP1 and AtRLPH2, as well as PPM/PP2C protein phosphatases and
DSP phosphatases. Given the massive family expansion of PPM/PP2C protein phosphatases in
photosynthetic Eukaryotes relative to other eukaryotic organisms, future investigations may
reveal an unique bacterial origin of some PPM/PP2C protein phosphatases which result in dual
pSer/pThr- and pTyr-specific protein phosphatase activity. As well, the dual cytosolic/nuclear
localization of AtRLPH2 may indicate a role in signal transduction between these two
compartments. Further research is required to identify its interaction partners and endogenous
phosphorylated protein substrates. This exciting new finding of a pTyr-specific PPP protein
phosphatase brings a fresh perspective to regulatory protein phosphorylation in plants, and
should serve as a model for the further identification and characterization of unconventional
pTyr protein phosphatases.
199
Chapter Seven: Perspectives and Future Directions
7.1 Summary
With reversible protein phosphorylation representing one of the most well characterized
post-translational modifications known, it is amazing that new protein kinases and phosphatases
are being indentified in extensively examined organisms like A. thaliana. The goal of this Ph.D.
thesis was to characterize two recently resolved members of the PPP protein phosphatase family,
the SLP and RLPH phosphatases (Andreeva and Kutuzov, 2004; Kerk et al., 2008), to offer
insight into their evolutionary heritage, subcellular localizations and biochemical properties, as
well as protein interactors and biological function. Careful dissection of their evolution trajectory
uncovered a mixed mechanism of acquisition by Eukaryotes involving a combination of classic
mitochondrial endosymbiosis and horizontal gene transfer mechanisms (Chapter 2). Initial in
silico subcellular prediction algorithms were largely corroborated by transient expression of
fluorescent fusion proteins in various tissues (Chapters 2 and 3). This found SLP1 and SLP2
phosphatases to be chloroplast and cytosol targeted, respectively (Chapters 2 and 3), while
RLPH2 phosphatases were found to be nuclear / cytosolic targeted (Chapters 2 and 6). By means
of mitochondrial isolation from A. thaliana cell culture, AtSLP2 presented a notable exception in
that it was found to be exclusively mitochondrial (Chapter 4). This was later corroborated by
transient expression in tissues known to contain endogenous AtSLP2, demonstrating the SLP2
phosphatases are mitochondrial rather than cytosolic. As well, all A. thaliana bacterial-like PPP
protein phosphatases exhibited a common insensitivity to inhibitors OA and MCLR, which may
represent a remnant of their prokaryotic origin (Chapter 3). Employment of phosphorylated
peptide substrates uncovered additional layers of complexity by identifying the phosphorylated
substrate preferences of AtSLP1, AtSLP2 and AtRLPH2. Here, I found that AtSLP1, AtSLP2
200
and AtRLPH2 represent dual-specificity, Ser/Thr-specific and Tyr-specific protein phosphatases
respectively (Chapters 4 and 6).
In the case of SLP phosphatases, I was also able to elucidate a protein interactome using
TAP pull-downs, revealing AtSLP1 and AtSLP2 to maintain completely unique sets of protein
interaction partners, indicative of independent cellular and biological functions (Chapter 4).
AtSLP1 was found to interact with CF1 ATP synthase β and γ subunits of the chloroplast stroma,
while AtSLP2 interacted specifically with AtMIA40 protein of the mitochondrial intermembane
space. Unfortunately, reciprocal validation of these interactions was only achieved between
AtMIA40 and AtSLP2, while the verification of an AtSLP1 - CF1 ATP synthase interaction
remains incomplete. Verification of a specific AtSLP2 - AtMIA40 interaction was followed by
careful plant phenotype analysis of atslp2-2 and 35S::AtSLP2 plants which resulted in the
identification of a pronounced early germination phenotype in atslp2-2 seeds. Corroborating
evidence for this phenotype was found using atmia40 seeds, which exhibited an identical, but
more moderate, early germination phenotype (Chapter 5). Additional pharmacological
characterization involving the topical application of ABA and Uniconazole indicated that this
phenotype was a result of enhanced GA production in the atslp2-2 plant, suggesting AtSLP2 to
be a negative regulator of GA biosynthesis. This discovery was reminiscent of the key role of
PP2C phosphatases in ABA signaling, rendering AtSLP2 an exciting new candidate for the
regulation of plant phytohormone-related processes.
Overall, the research I present throughout this thesis functions as a solid foundation from
which future studies of the bacterial-like protein phosphatases from A. thaliana and beyond can
be based. Given this, I outline below what I believe are some of the logical next steps to take in
further exploring the bacterial-like PPP protein phosphatases.
201
7.2 Novel PPP phosphatase of the chloroplast: SLP1 phosphatases
The documented interaction between AtSLP1 and CF1 ATP synthase β and γ subunits
requires validation through either independent or reciprocal protein-protein interaction analysis.
This would likely prove easiest through the application of immunoprecipitation. Here, antibodies
to the soluble CF1 ATP synthase of A. thaliana could be employed to obtain an AtSLP1 chloroplast ATP synthase complex from either rosette tissue or isolated chloroplast extracts
harvested from plants during the day and extracted in the presence of phosphatase inhibitors.
Subsequent immunoblotting for AtSLP1 with specific antibodies developed in this thesis would
circumvent the need for mass spectrometry. Based on available literature, immunoprecipitation
should be able to be applied under more stringent conditions and in the presence of phosphatase
inhibitors to isolate intact and phosphorylated A. thaliana CF1 ATP synthase (del Riego et al.,
2006; Reiland et al., 2009). This could then also be used in conjunction with bacteriallyexpressed and purified AtSLP1 to assess CF1 ATP synthase β and γ subunits as a potential
phosphorylated substrate of AtSLP1. A number of in vitro assay readouts could be employed to
validate this possibility. These include malachite green enzyme assays, Pro-Q diamond
phosphoprotein stain (Invitrogen) or quantitative mass spectrometry.
Furthermore, with standard growth conditions failing to identify an obvious atslp1 /
35S::AtSLP1 plant phenotype, application of experimental growth conditions should be explored.
Given the putative interaction identified between AtSLP1 and chloroplast ATP synthase,
photoperiod could be envisioned as a logical starting point. With A. thaliana growth examined
here under a 12 h light / 12 h dark photoperiod, 'long' day and 'short' day experimentation could
be conducted using 16 h light / 8 h dark and 8 h light / 16 h dark photoperiods. With AtSLP1
202
representing the first chloroplast targeted PPP protein phosphatase identified to date, and the
chloroplast possessing a number of phosphorylated proteins, elucidating the cellular function of
AtSLP1 could be of fundamental importance.
7.3 Connecting AtSLP2 to GA biosynthesis
The fortuitous connection uncovered here between AtSLP2 and GA biosynthesis requires
further characterization. First and foremost, understanding this connection requires identifying
the phosphorylated substrate of AtSLP2. Given its unique location in the mitochondrial IMS
along with AtMIA40, a direct regulatory connection between AtSLP2 and cytosolic GA
metabolism seems unlikely, but given that mitochondria are of central importance to plant cell
function, other indirect connections remain viable possibilities worthy of exploration (Araujo et
al., 2012). One of the primary functions of mitochondria in plants is to provide carbon skeletons,
through the TCA cycle, to the cytosol for anabolic construction of a wide array of metabolites.
This is powered largely through pyruvate which feeds directly into the TCA cycle. Analogous to
auxotrophic systems (e.g. humans), plant mitochondria can also utilize amino acids as an
alternative carbon source when carbon is in short supply (Ishizaki et al., 2005; Araujo et al.,
2012), in particular, carbon rich amino acids such as the branched amino acids valine, leucine
and isoleucine (Ishizaki et al., 2005; Araujo et al., 2010; Araujo et al., 2012). With atslp2-2 seeds
exhibiting massively elevated valine and isoleucine levels, it has become evident that loss of
AtSLP2 perturbs homeostatic levels of branched amino acids possibly by preventing their import
into the mitochondria for degradation. Although FA are not broken down in the mitochondria of
plants, they too exhibited a significant change in atslp2-2 seeds, which may also have resulted
from disfunctional mitochondrial processes. With AtSLP2's subcellular compartmentation
203
relegated to the mitochondrial IMS, there are only a few mechanistic possibilities for how it
exerts control over GA-related processes, amino acid degradation and FA biosynthesis. These
options include: direct regulation of an inner membrane transporter(s), involvement in regulating
mitochondrial matrix protein import or direct regulation of an integral mitochondrial inner
membrane protein, which exerts influence over matrix metabolic processes. Identifying
AtSLP2's substrate will be key to resolving which of these hypotheses are true.
7.4 The SLP phosphatase of Schizosaccharomyces pombe
Also called fission yeast, Schizosaccharomyces pombe (S. pombe) represents a model,
unicellular eukaryotic organism, that is ideal for examining DNA damage, DNA replication and
cell division (Simanis, 1995; Gould and Simanis, 1997). It has a sequenced genome, a
comprehensive and freely accessible online resources such as PomBase (www.pombase.org) and
a library of available knockout yeast strains for reverse genetics. In particular, the SpSLP
phosphatase SPCC1840.0c, noted in Chapter 2, is more similar to the SLP3 phosphatases of
Green Algae than it is to the SLP1 and SLP2 phosphatases of plants, representing a more ancient
derivation of the overall SLP protein phosphatase ancestry. With all the tools already in place for
the S. pombe inclined researcher, and SLP1 and 2 phosphatase characterization already underway
from A. thaliana, the characterization of SpSLP may prove interesting and complementary.
Contributing to the potentially unique nature of SpSLP is its ER subcellular localization
(Matsuyama et al., 2006). To date, independent functions seem to exist for both AtSLP1 and
AtSLP2, rendering the characterization of ER-targeted SpSLP likely to yield yet another
independent SLP phosphatase function.
204
7.5 Bridging the gap: the AtRLPH2 is a phosphotyrosine-specific PPP protein
Phosphoproteomics has revealed that both A. thaliana and O. sativa maintain amounts of
protein tyrosine phosphorylation comparable to that found in humans (Sugiyama et al., 2008;
Nakagami et al., 2010), but lack the corresponding abundance of protein tyrosine kinases and
phosphatases (Kerk et al., 2008). This thesis presented results involving A. thaliana RLPH2
which demonstrated it to be a novel, pTyr-specific, bacterial-like PPP protein phosphatase.
Given this, I believe it imperative to characterize the protein interactome of AtRLPH2. This will
provide an understanding of AtRLPH2's cellular function through the proteins it interacts with.
As well, I would accompany these experiments with the reverse genetics approach previously
applied to AtSLP1 and AtSLP2 involving both insertional mutants and over-expressing plant
lines. This will assist in uncovering the broader biological role of AtRLPH2. Finding that
AtRLPH2 was a pTyr-specific PPP protein phosphatase suggests that tyrosine phosphorylation in
plants may be facilitated by unconventional protein tyrosine kinases and phosphatases. From a
phosphatase perspective this may involve DSP and/or PP2C phosphatases. DSP phosphatases
possess an identical catalytic motif to the classic PTP phosphatases, rendering them capable of
dephosphorylating pTyr, while PP2C phosphatases, which are a group classically comprised of
Ser/Thr phosphatases, have extensively proliferated across the genomes of plants.
205
Literature Cited:
Abascal F, Zardoya R, Posada D (2005) ProtTest: selection of best-fit models of protein
evolution. Bioinformatics 21: 2104-2105
Acuna R, Padilla BE, Florez-Ramos CP, Rubio JD, Herrera JC, Benavides P, Lee SJ, Yeats
TH, Egan AN, Doyle JJ, Rose JK (2012) Adaptive horizontal transfer of a bacterial
gene to an invasive insect pest of coffee. Proc Natl Acad Sci U S A 109: 4197-4202
Ahn CS, Han JA, Lee HS, Lee S, Pai HS (2011) The PP2A regulatory subunit Tap46, a
component of the TOR signaling pathway, modulates growth and metabolism in plants.
Plant Cell 23: 185-209
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997)
Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.
Nucleic Acids Res 25: 3389-3402
Andreeva AV, Kutuzov MA (1999) RdgC/PP5-related phosphatases: novel components in
signal transduction. Cellular Signalling 11: 555-562
Andreeva AV, Kutuzov MA (2001) Nuclear localization of the plant protein Ser/Thr
phosphatase PP7. Molecular cell biology research communications : MCBRC 4: 345-352
Andreeva AV, Kutuzov MA (2001) PPP family of protein Ser/Thr phosphatases: two distinct
branches? Molecular Biology and Evolution 18: 448-452
Andreeva AV, Kutuzov MA (2004) Widespread presence of "bacterial-like" PPP phosphatases
in eukaryotes. BMC Evolutionary Biology 4: 47
Andreeva AV, Kutuzov MA (2008) Protozoan protein tyrosine phosphatases. International
Journal of Parasitology 38: 1279-1295
Andreeva AV, Kutuzov MA (2009) PPEF/PP7 protein Ser/Thr phosphatases. Cellular and
Molecular Life Sciences : CMLS 66: 3103-3110
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support
methods demonstrates accuracy, power, and robustness of fast likelihood-based
approximation schemes. Syst Biol 60: 685-699
Araujo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, Witt S, Obata T,
Schauer N, Graham IA, Leaver CJ, Fernie AR (2010) Identification of the 2hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors
linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria.
Plant Cell 22: 1549-1563
Araujo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR (2012) Metabolic control
and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant
tissues. Plant Cell Environment 35: 1-21
Arora G, Sajid A, Arulanandh MD, Singhal A, Mattoo AR, Pomerantsev AP, Leppla SH,
Maiti S, Singh Y (2012) Unveiling the novel dual specificity protein kinases in Bacillus
anthracis: identification of the first prokaryotic dual specificity tyrosine phosphorylationregulated kinase (DYRK)-like kinase. Journal of Biological Chemistry 287: 26749-26763
Ballesteros I, Dominguez T, Sauer M, Paredes P, Duprat A, Rojo E, Sanmartin M,
Sanchez-Serrano JJ (2012) Specialized functions of the PP2A subfamily II catalytic
subunits PP2A-C3 and PP2A-C4 in the distribution of auxin fluxes and development in
Arabidopsis. The Plant Journal
206
Banci L, Bertini I, Cefaro C, Ciofi-Baffoni S, Gallo A, Martinelli M, Sideris DP, Katrakili
N, Tokatlidis K (2009) MIA40 is an oxidoreductase that catalyzes oxidative protein
folding in mitochondria. Nature Structural & Molecular Biology 16: 198-206
Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S (2002) Extensive feature detection
of N-terminal protein sorting signals. Bioinformatics 18: 298-305
Barajas-Lopez Jde D, Kremnev D, Shaikhali J, Pinas-Fernandez A, Strand A (2013) PAPP5
Is Involved in the Tetrapyrrole Mediated Plastid Signalling during Chloroplast
Development. PloS One 8: e60305
Bartels S, Gonzalez Besteiro MA, Lang D, Ulm R (2010) Emerging functions for plant MAP
kinase phosphatases. Trends in Plant Science 15: 322-329
Bayer RG, Stael S, Rocha AG, Mair A, Vothknecht UC, Teige M (2012) Chloroplastlocalized protein kinases: a step forward towards a complete inventory. Journal of
Experimental Botany 63: 1713-1723
Baykov AA, Evtushenko OA, Avaeva SM (1988) A malachite green procedure for
orthophosphate determination and its use in alkaline phosphatase-based enzyme
immunoassay. Analytical Biochemistry 171: 266-270
Belbahri L, Calmin G, Mauch F, Andersson JO (2008) Evolution of the cutinase gene family:
evidence for lateral gene transfer of a candidate Phytophthora virulence factor. Gene 408:
1-8
Bialojan C, Takai A (1988) Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein
phosphatases. Specificity and kinetics. Biochemical Journal 256: 283-290
Bien M, Longen S, Wagener N, Chwalla I, Herrmann JM, Riemer J (2010) Mitochondrial
disulfide bond formation is driven by intersubunit electron transfer in Erv1 and proofread
by glutathione. Molecular Cell 37: 516-528
Blakeslee JJ, Zhou HW, Heath JT, Skottke KR, Barrios JA, Liu SY, DeLong A (2008)
Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem
organization and stress response in roots. Plant Physiology 146: 539-553
Bligny R, Gardestrom P, Roby C, Douce R (1990) 31P NMR studies of spinach leaves and
their chloroplasts. J Biol Chem 265: 1319-1326
Boden M, Hawkins J (2005) Prediction of subcellular localization using sequence-biased
recurrent networks. Bioinformatics 21: 2279-2286
Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas
C, Haseloff J, Berger F (2001) Dynamic analyses of the expression of the
HISTONE::YFP fusion protein in arabidopsis show that syncytial endosperm is divided
in mitotic domains. Plant Cell 13: 495-509
Bollen M, Peti W, Ragusa MJ, Beullens M (2010) The extended PP1 toolkit: designed to
create specificity. Trends in Biochemical Sciences 35: 450-458
Boto L (2010) Horizontal gene transfer in evolution: facts and challenges. Proc Biol Sci 277:
819-827
Bozzo GG, Raghothama KG, Plaxton WC (2002) Purification and characterization of two
secreted purple acid phosphatase isozymes from phosphate-starved tomato (Lycopersicon
esculentum) cell cultures. Eur J Biochem 269: 6278-6286
Brautigan DL (2013) Protein Ser/ Thr phosphatases - the ugly ducklings of cell signalling. The
FEBS Journal 280: 324-325
207
Brautigan DL, Gruppuso PA, Mumby M (1986) Protein phosphatase type-1 and type-2
catalytic subunits both bind inhibitor-2 and monoclonal immunoglobulins. J Biol Chem
261: 14924-14928
Breitkreutz A, Choi H, Sharom JR, Boucher L, Neduva V, Larsen B, Lin ZY, Breitkreutz
BJ, Stark C, Liu G, Ahn J, Dewar-Darch D, Reguly T, Tang X, Almeida R, Qin ZS,
Pawson T, Gingras AC, Nesvizhskii AI, Tyers M (2010) A global protein kinase and
phosphatase interaction network in yeast. Science 328: 1043-1046
Bunik VI, Fernie AR (2009) Metabolic control exerted by the 2-oxoglutarate dehydrogenase
reaction: a cross-kingdom comparison of the crossroad between energy production and
nitrogen assimilation. The Biochemical Journal 422: 405-421
Bunney TD, van Walraven HS, de Boer AH (2001) 14-3-3 protein is a regulator of the
mitochondrial and chloroplast ATP synthase. Proc Natl Acad Sci U S A 98: 4249-4254
Cao D, Hussain A, Cheng H, Peng J (2005) Loss of function of four DELLA genes leads to
light- and gibberellin-independent seed germination in Arabidopsis. Planta 223: 105-113
Carrie C, Giraud E, Duncan O, Xu L, Wang Y, Huang S, Clifton R, Murcha M, Filipovska
A, Rackham O, Vrielink A, Whelan J (2010) Conserved and novel functions for
Arabidopsis thaliana MIA40 in assembly of proteins in mitochondria and peroxisomes.
The Journal of Biological Chemistry 285: 36138-36148
Caunt CJ, Keyse SM (2013) Dual-specificity MAP kinase phosphatases (MKPs): Shaping the
outcome of MAP kinase signalling. The FEBS Journal 280: 489-504
Ceulemans H, Stalmans W, Bollen M (2002) Regulator-driven functional diversification of
protein phosphatase-1 in eukaryotic evolution. BioEssays 24: 371-381
Chacinska A, Guiard B, Muller JM, Schulze-Specking A, Gabriel K, Kutik S, Pfanner N
(2008) Mitochondrial biogenesis, switching the sorting pathway of the intermembrane
space receptor Mia40. The Journal of Biological Chemistry 283: 29723-29729
Chatzi A, Tokatlidis K (2012) The Mitochondrial Intermembrane Space: A Hub for Oxidative
Folding Linked to Protein Biogenesis. Antioxidants & Redox Signaling
Chen GI, Gingras AC (2007) Affinity-purification mass spectrometry (AP-MS) of
serine/threonine phosphatases. Methods 42: 298-305
Chen M, Du X, Zhu Y, Wang Z, Hua S, Li Z, Guo W, Zhang G, Peng J, Jiang L (2012)
Seed Fatty Acid Reducer acts downstream of gibberellin signalling pathway to lower
seed fatty acid storage in Arabidopsis. Plant Cell Environment 35: 2155-2169
Chen MS, Silverstein AM, Pratt WB, Chinkers M (1996) The tetratricopeptide repeat domain
of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes
and acts as a dominant negative mutant. Journal of Biological Chemistry 271: 3231532320
Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PT (1994) A novel
human protein serine/threonine phosphatase, which possesses four tetratricopeptide
repeat motifs and localizes to the nucleus. EMBO J 13: 4278-4290
Cho US, Xu W (2007) Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme.
Nature 445: 53-57
Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C,
Kianianmomeni A, Burglin TR, Frech C, Turcotte B, Kopec KO, Synnott JM, Choo
C, Paponov I, Finkler A, Soon Heng Tan C, Hutchins AP, Weinmeier T, Rattei T,
Chu JS, Gimenez G, Irimia M, Rigden DJ, Fitzpatrick DA, Lorenzo-Morales J,
208
Bateman A, Chiu CH, Tang P, Hegemann P, Fromm H, Raoult D, Greub G,
Miranda-Saavedra D, Chen N, Nash P, Ginger ML, Horn M, Schaap P, Caler L,
Loftus B (2013) Genome of Acanthamoeba castellanii highlights extensive lateral gene
transfer and early evolution of tyrosine kinase signaling. Genome Biol 14: R11
Claros MG, Vincens P (1996) Computational method to predict mitochondrially imported
proteins and their targeting sequences. Eur J Biochem 241: 779-786
Cohen P (2000) The regulation of protein function by multisite phosphorylation--a 25 year
update. Trends Biochem Sci 25: 596-601
Cohen P (2002) The origins of protein phosphorylation. Nature Cell Biology 4: E127-130
Cohen P, Holmes CF, Tsukitani Y (1990) Okadaic acid: a new probe for the study of cellular
regulation. Trends in Biochemical Science 15: 98-102
Cohen PT (1997) Novel protein serine/threonine phosphatases: variety is the spice of life.
Trends Biochem Sci 22: 245-251
Cohen PT, Philp A, Vazquez-Martin C (2005) Protein phosphatase 4--from obscurity to vital
functions. FEBS Letter 579: 3278-3286
Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator.
Genome Res 14: 1188-1190
Cuozzo JW, Kaiser CA (1999) Competition between glutathione and protein thiols for
disulphide-bond formation. Nature Cell Biology 1: 130-135
Dai M, Xue Q, McCray T, Margavage K, Chen F, Lee JH, Nezames CD, Guo L, Terzaghi
W, Wan J, Deng XW, Wang H (2013) The PP6 Phosphatase Regulates ABI5
Phosphorylation and Abscisic Acid Signaling in Arabidopsis. The Plant Cell
Dai M, Zhang C, Kania U, Chen F, Xue Q, McCray T, Li G, Qin G, Wakeley M, Terzaghi
W, Wan J, Zhao Y, Xu J, Friml J, Deng XW, Wang H (2012) A PP6-type phosphatase
holoenzyme directly regulates PIN phosphorylation and auxin efflux in Arabidopsis. The
Plant Cell 24: 2497-2514
Dancheck B, Ragusa MJ, Allaire M, Nairn AC, Page R, Peti W (2011) Molecular
investigations of the structure and function of the protein phosphatase 1-spinophilininhibitor 2 heterotrimeric complex. Biochemistry 50: 1238-1246
Darshi M, Trinh KN, Murphy AN, Taylor SS (2012) Targeting and import mechanism of
coiled-coil helix coiled-coil helix domain-containing protein 3 (ChChd3) into the
mitochondrial intermembrane space. The Journal of Biological Chemistry 287: 3948039491
Dawson RM (1998) The toxicology of microcystins. Toxicology 36: 953-962
de la Fuente van Bentem S, Vossen JH, de Vries KJ, van Wees S, Tameling WI, Dekker
HL, de Koster CG, Haring MA, Takken FL, Cornelissen BJ (2005) Heat shock
protein 90 and its co-chaperone protein phosphatase 5 interact with distinct regions of the
tomato I-2 disease resistance protein. The Plant Journal 43: 284-298
de la Fuente van Bentem S, Vossen JH, Vermeer JE, de Vroomen MJ, Gadella TW, Jr.,
Haring MA, Cornelissen BJ (2003) The subcellular localization of plant protein
phosphatase 5 isoforms is determined by alternative splicing. Plant Physiology 133: 702712
De Munter S, Kohn M, Bollen M (2013) Challenges and opportunities in the development of
protein phosphatase-directed therapeutics. ACS Chemical Biology 8: 36-45
209
del Riego G, Casano LM, Martin M, Sabater B (2006) Multiple phosphorylation sites in the
beta subunit of thylakoid ATP synthase. Photosynthetic Research 89: 11-18
DeLong A (2006) Switching the flip: protein phosphatase roles in signaling pathways. Current
Opinion in Plant Biology 9: 470-477
Denu JM, Lohse DL, Vijayalakshmi J, Saper MA, Dixon JE (1996) Visualization of
intermediate and transition-state structures in protein-tyrosine phosphatase catalysis. Proc
Natl Acad Sci U S A 93: 2493-2498
Denu JM, Stuckey JA, Saper MA, Dixon JE (1996) Form and function in protein
dephosphorylation. Cell 87: 361-364
Derelle E, Ferraz C, Rombauts S, Rouze P, Worden AZ, Robbens S, Partensky F, Degroeve
S, Echeynie S, Cooke R, Saeys Y, Wuyts J, Jabbari K, Bowler C, Panaud O, Piegu
B, Ball SG, Ral JP, Bouget FY, Piganeau G, De Baets B, Picard A, Delseny M,
Demaille J, Van de Peer Y, Moreau H (2006) Genome analysis of the smallest freeliving eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci U
S A 103: 11647-11652
Di Rubbo S, Irani NG, Russinova E (2011) PP2A phosphatases: the "on-off" regulatory
switches of brassinosteroid signaling. Science Signaling 4: pe25
Donella-Deana A, Krinks MH, Ruzzene M, Klee C, Pinna LA (1994) Dephosphorylation of
phosphopeptides by calcineurin (protein phosphatase 2B). European Journal of
Biochemistry 219: 109-117
Donella-Deana A, Meyer HE, Pinna LA (1991) The use of phosphopeptides to distinguish
between protein phosphatase and acid/alkaline phosphatase activities: opposite specificity
toward phosphoseryl/phosphothreonyl substrates. Biochim Biophys Acta 1094: 130-133
Donella Deana A, Mac Gowan CH, Cohen P, Marchiori F, Meyer HE, Pinna LA (1990) An
investigation of the substrate specificity of protein phosphatase 2C using synthetic
peptide substrates; comparison with protein phosphatase 2A. Biochim Biophys Acta
1051: 199-202
Dong L, Ermolova NV, Chollet R (2001) Partial purification and biochemical characterization
of a heteromeric protein phosphatase 2A holoenzyme from maize (Zea mays L.) leaves
that dephosphorylates C4 phosophoenolpyruvate carboxylase. Planta 213: 379-389
Dorrell RG, Smith AG (2011) Do red and green make brown?: perspectives on plastid
acquisitions within chromalveolates. Eukaryot Cell 10: 856-868
Douglas P, Moorhead GB, Ye R, Lees-Miller SP (2001) Protein phosphatases regulate DNAdependent protein kinase activity. Journal of Biological Chemistry 276: 18992-18998
Douglas P, Zhong J, Ye R, Moorhead GB, Xu X, Lees-Miller SP (2010) Protein phosphatase
6 interacts with the DNA-dependent protein kinase catalytic subunit and
dephosphorylates gamma-H2AX. Molecular and Cellular Biology 30: 1368-1381
Duncan MR, Fullerton M, Chaudhuri M (2013) Tim50 in Trypanosoma brucei possesses a
dual specificity phosphatase activity and is critical for mitochondrial protein import. The
Journal of Biological Chemistry 288: 3184-3197
Dutilleul C, Lelarge C, Prioul JL, De Paepe R, Foyer CH, Noctor G (2005) Mitochondriadriven changes in leaf NAD status exert a crucial influence on the control of nitrate
assimilation and the integration of carbon and nitrogen metabolism. Plant Physiology
139: 64-78
Eddy SR (1998) Profile hidden Markov models. Bioinformatics 14: 755-763
210
Egloff MP, Cohen PT, Reinemer P, Barford D (1995) Crystal structure of the catalytic subunit
of human protein phosphatase 1 and its complex with tungstate. Journal of Molecular
Biology 254: 942-959
Egloff MP, Johnson DF, Moorhead G, Cohen PT, Cohen P, Barford D (1997) Structural
basis for the recognition of regulatory subunits by the catalytic subunit of protein
phosphatase 1. EMBO J 16: 1876-1887
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization
of proteins based on their N-terminal amino acid sequence. J Mol Biol 300: 1005-1016
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for
predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8: 978-984
Embley TM, Martin W (2006) Eukaryotic evolution, changes and challenges. Nature 440: 623630
Eubel H, Heazlewood JL, Millar AH (2007) Isolation and subfractionation of plant
mitochondria for proteomic analysis. Methods in Molecular Biology 355: 49-62
Farkas I, Dombradi V, Miskei M, Szabados L, Koncz C (2007) Arabidopsis PPP family of
serine/threonine phosphatases. Trends in Plant Science 12: 169-176
Forreiter C, Kirschner M, Nover L (1997) Stable transformation of an Arabidopsis cell
suspension culture with firefly luciferase providing a cellular system for analysis of
chaperone activity in vivo. Plant Cell 9: 2171-2181
Fraga H, Ventura S (2013) Oxidative folding in the mitochondrial intermembrane space in
human health and disease. International Journal of Molecular Sciences 14: 2916-2927
Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants.
The FEBS Journal 280: 681-693
Fuerst JA, Sagulenko E (2011) Beyond the bacterium: planctomycetes challenge our concepts
of microbial structure and function. Nat Rev Microbiol 9: 403-413
Fukao Y, Hayashi M, Nishimura M (2002) Proteomic analysis of leaf peroxisomal proteins in
greening cotyledons of Arabidopsis thaliana. Plant Cell Physiol 43: 689-696
Furbank RT, Tester M (2011) Phenomics--technologies to relieve the phenotyping bottleneck.
Trends in Plant Science 16: 635-644
Futami R, Llorens C, Vicente-Ripolles M, Moya A (2008) The Alignment Format Converter
Server 1.0. Biotechvana Bioinformatics
Gennidakis S, Rao S, Greenham K, Uhrig RG, O'Leary B, Snedden WA, Lu C, Plaxton
WC (2007) Bacterial- and plant-type phosphoenolpyruvate carboxylase polypeptides
interact in the hetero-oligomeric Class-2 PEPC complex of developing castor oil seeds.
The Plant Journal 52: 839-849
Genoud T, Santa Cruz MT, Kulisic T, Sparla F, Fankhauser C, Metraux JP (2008) The
protein phosphatase 7 regulates phytochrome signaling in Arabidopsis. PloS One 3:
e2699
Gentry MS, Roma-Mateo C, Sanz P (2013) Laforin, a protein with many faces: glucan
phosphatase, adapter protein, et alii. FEBS J 280: 525-537
Girzalsky W, Platta HW, Erdmann R (2009) Protein transport across the peroxisomal
membrane. Biol Chem 390: 745-751
Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, Kuriyan J (1995) Threedimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1.
Nature 376: 745-753
211
Goldman BS, Nierman WC, Kaiser D, Slater SC, Durkin AS, Eisen JA, Ronning CM,
Barbazuk WB, Blanchard M, Field C, Halling C, Hinkle G, Iartchuk O, Kim HS,
Mackenzie C, Madupu R, Miller N, Shvartsbeyn A, Sullivan SA, Vaudin M,
Wiegand R, Kaplan HB (2006) Evolution of sensory complexity recorded in a
myxobacterial genome. Proc Natl Acad Sci U S A 103: 15200-15205
Gould KL, Simanis V (1997) The control of septum formation in fission yeast. Genes
Development 11: 2939-2951
Gray MW (1998) Rickettsia, typhus and the mitochondrial connection. Nature 396: 109-110
Gray MW (2012) Mitochondrial evolution. Cold Spring Harb Perspect Biol 4: a011403
Groth G, Pohl E (2001) The structure of the chloroplast F1-ATPase at 3.2 A resolution. Journal
of Biological Chemistry 276: 1345-1352
Gutierres S, Sabar M, Lelandais C, Chetrit P, Diolez P, Degand H, Boutry M, Vedel F, de
Kouchkovsky Y, De Paepe R (1997) Lack of mitochondrial and nuclear-encoded
subunits of complex I and alteration of the respiratory chain in Nicotiana sylvestris
mitochondrial deletion mutants. Proc Natl Acad Sci U S A 94: 3436-3441
Hamilton JP, Buell CR (2012) Advances in plant genome sequencing. The Plant Journal 70:
177-190
Hammet A, Pike BL, McNees CJ, Conlan LA, Tenis N, Heierhorst J (2003) FHA domains as
phospho-threonine binding modules in cell signaling. IUBMB Life 55: 23-27
Hastie CJ, Borthwick EB, Morrison LF, Codd GA, Cohen PT (2005) Inhibition of several
protein phosphatases by a non-covalently interacting microcystin and a novel
cyanobacterial peptide, nostocyclin. Biochim Biophys Acta 1726: 187-193
Hedden P, Thomas SG (2012) Gibberellin biosynthesis and its regulation. The Biochemical
Journal 444: 11-25
Heidari B, Matre P, Nemie-Feyissa D, Meyer C, Rognli OA, Moller SG, Lillo C (2011)
Protein phosphatase 2A B55 and A regulatory subunits interact with nitrate reductase and
are essential for nitrate reductase activation. Plant Physiology 156: 165-172
Hell K (2008) The Erv1-Mia40 disulfide relay system in the intermembrane space of
mitochondria. Biochimica et Biophysica Acta 1783: 601-609
Heroes E, Lesage B, Gornemann J, Beullens M, Van Meervelt L, Bollen M (2013) The PP1
binding code: a molecular-lego strategy that governs specificity. The FEBS journal 280:
584-595
Herrmann JM, Riemer J (2012) Mitochondrial disulfide relay: redox-regulated protein import
into the intermembrane space. Journal of Biological Chemistry 287: 4426-4433
Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, Leitner A, Beck
M, Hartl FU, Ban N, Malmstrom L, Aebersold R (2012) Structural probing of a
protein phosphatase 2A network by chemical cross-linking and mass spectrometry.
Science 337: 1348-1352
Hoch JA (2000) Two-component and phosphorelay signal transduction. Current Opinion in
Microbiology 3: 165-170
Holdsworth MJ, Bentsink L, Soppe WJ (2008) Molecular networks regulating Arabidopsis
seed maturation, after-ripening, dormancy and germination. The New Phytologist 179:
33-54
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K (2007)
WoLF PSORT: protein localization predictor. Nucleic Acids Res 35: W585-587
212
Hunter T, Pawson T (2012) The evolution of protein phosphorylation. Preface. Philosophical
transactions of the Royal Society of London 367: 2512
Hurley TD, Yang J, Zhang L, Goodwin KD, Zou Q, Cortese M, Dunker AK, DePaoliRoach AA (2007) Structural basis for regulation of protein phosphatase 1 by inhibitor-2.
Journal of Biological Chemistry 282: 28874-28883
Iki T, Yoshikawa M, Meshi T, Ishikawa M (2012) Cyclophilin 40 facilitates HSP90-mediated
RISC assembly in plants. EMBO J 31: 267-278
Ingebritsen TS, Cohen P (1983) Protein phosphatases: properties and role in cellular regulation.
Science 221: 331-338
Ishizaki K, Larson TR, Schauer N, Fernie AR, Graham IA, Leaver CJ (2005) The critical
role of Arabidopsis electron-transfer flavoprotein:ubiquinone oxidoreductase during
dark-induced starvation. Plant Cell 17: 2587-2600
Ito J, Taylor NL, Castleden I, Weckwerth W, Millar AH, Heazlewood JL (2009) A survey of
the Arabidopsis thaliana mitochondrial phosphoproteome. Proteomics 9: 4229-4240
Janouskovec J, Horak A, Obornik M, Lukes J, Keeling PJ (2010) A common red algal origin
of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci U S A
107: 10949-10954
Jeffares DC, Penkett CJ, Bahler J (2008) Rapidly regulated genes are intron poor. Trends in
Genetics 24: 375-378
Jessop CE, Bulleid NJ (2004) Glutathione directly reduces an oxidoreductase in the
endoplasmic reticulum of mammalian cells. Journal of Biological Chemistry 279: 5534155347
Jia Z, Barford D, Flint AJ, Tonks NK (1995) Structural basis for phosphotyrosine peptide
recognition by protein tyrosine phosphatase 1B. Science 268: 1754-1758
Jonassen EM, Heidari B, Nemie-Feyissa D, Matre P, Lillo C (2011) Protein phosphatase 2A
regulatory subunits are starting to reveal their functions in plant metabolism and
development. Plant Signaling & Behavior 6: 1216-1218
Joshi-Saha A, Valon C, Leung J (2011) A brand new START: abscisic acid perception and
transduction in the guard cell. Science Signaling 4: re4
Kalanon M, McFadden GI (2010) Malaria, Plasmodium falciparum and its apicoplast.
Biochem Soc Trans 38: 775-782
Kanekatsu M, Saito H, Motohashi K, Hisabori T (1998) The beta subunit of chloroplast ATP
synthase (CF0CF1-ATPase) is phosphorylated by casein kinase II. Biochemistry and
Molecular Biology International 46: 99-105
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple
sequence alignment based on fast Fourier transform. Nucleic Acids Res 30: 3059-3066
Kawaguchi M, Hiroi J, Miya M, Nishida M, Iuchi I, Yasumasu S (2010) Intron-loss
evolution of hatching enzyme genes in Teleostei. BMC Evol Biol 10: 260
Kawamata H, Manfredi G (2010) Import, maturation, and function of SOD1 and its copper
chaperone CCS in the mitochondrial intermembrane space. Antioxidants & Redox
Signaling 13: 1375-1384
Keeling PJ (2009) Role of horizontal gene transfer in the evolution of photosynthetic eukaryotes
and their plastids. Methods Mol Biol 532: 501-515
Keeling PJ, Palmer JD (2008) Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet
9: 605-618
213
Kennelly PJ (2002) Protein kinases and protein phosphatases in prokaryotes: a genomic
perspective. FEMS Microbiol Lett 206: 1-8
Kerk D, Templeton G, Moorhead GB (2008) Evolutionary radiation pattern of novel protein
phosphatases revealed by analysis of protein data from the completely sequenced
genomes of humans, green algae, and higher plants. Plant Physiology 146: 351-367
Kim EE, Wyckoff HW (1991) Reaction mechanism of alkaline phosphatase based on crystal
structures. Two-metal ion catalysis. J Mol Biol 218: 449-464
Kim TW, Guan S, Burlingame AL, Wang ZY (2011) The CDG1 kinase mediates
brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and
GSK3-like kinase BIN2. Molecular Cell 43: 561-571
Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Burlingame AL, Wang ZY (2009)
Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear
transcription factors. Nature Cell Biology 11: 1254-1260
Kloeker S, Reed R, McConnell JL, Chang D, Tran K, Westphal RS, Law BK, Colbran RJ,
Kamoun M, Campbell KS, Wadzinski BE (2003) Parallel purification of three catalytic
subunits of the protein serine/threonine phosphatase 2A family (PP2A(C), PP4(C), and
PP6(C)) and analysis of the interaction of PP2A(C) with alpha4 protein. Protein
Expression and Purification 31: 19-33
Klumpp S, Thissen MC, Krieglstein J (2006) Protein phosphatases types 2Calpha and 2Cbeta
in apoptosis. Biochem Soc Trans 34: 1370-1375
Kohzuma K, Dal Bosco C, Meurer J, Kramer DM (2013) Light- and Metabolism-related
Regulation of the Chloroplast ATP Synthase Has Distinct Mechanisms and Functions.
Journal of Biological Chemistry 288: 13156-13163
Kojer K, Bien M, Gangel H, Morgan B, Dick TP, Riemer J (2012) Glutathione redox
potential in the mitochondrial intermembrane space is linked to the cytosol and impacts
the Mia40 redox state. EMBO J 31: 3169-3182
Koonin EV (2010) The origin and early evolution of eukaryotes in the light of phylogenomics.
Genome Biol 11: 209
Koornneef M, Hanhart CJ, Hilhorst HW, Karssen CM (1989) In Vivo Inhibition of Seed
Development and Reserve Protein Accumulation in Recombinants of Abscisic Acid
Biosynthesis and Responsiveness Mutants in Arabidopsis thaliana. Plant Physiology 90:
463-469
Koornneef M, Meinke D (2010) The development of Arabidopsis as a model plant. The Plant
Journal 61: 909-921
Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic
acid-insensitive mutants of Arabidopsis thaliana. Physiological Plant 61: 377-383
Kotting O, Kossmann J, Zeeman SC, Lloyd JR (2010) Regulation of starch metabolism: the
age of enlightenment? Current Opinion in Plant Biology 13: 321-329
Krah A, Pogoryelov D, Meier T, Faraldo-Gomez JD (2010) On the structure of the protonbinding site in the F(o) rotor of chloroplast ATP synthases. J Mol Biol 395: 20-27
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed
dormancy release and germination. Seed Science Research 15: 281–307
Kutuzov MA, Andreeva AV (2002) Protein Ser/Thr phosphatases with kelch-like repeat
domains. Cellular Signalling 14: 745-750
214
Kutuzov MA, Andreeva AV (2008) Protein Ser/Thr phosphatases of parasitic protozoa. Mol
Biochem Parasitol 161: 81-90
Kutuzov MA, Andreeva AV (2012) Prediction of biological functions of Shewanella-like
protein phosphatases (Shelphs) across different domains of life. Funct Integr Genomics
12: 11-23
Kutuzov MA, Bennett N, Andreeva AV (2001) Interaction of plant protein Ser/Thr
phosphatase PP7 with calmodulin. Biochemical and Biophysical Research
Communications 289: 634-640
Kutuzov MA, Evans DE, Andreeva AV (1998) Expression and characterization of PP7, a novel
plant protein Ser/Thr phosphatase distantly related to RdgC/PPEF and PP5. FEBS Letter
440: 147-152
Lai SM, Le Moual H (2005) PrpZ, a Salmonella enterica serovar Typhi serine/threonine protein
phosphatase 2C with dual substrate specificity. Microbiology 151: 1159-1167
Lang BF, Gray MW, Burger G (1999) Mitochondrial genome evolution and the origin of
eukaryotes. Annu Rev Genet 33: 351-397
Lartillot N, Lepage T, Blanquart S (2009) PhyloBayes 3: a Bayesian software package for
phylogenetic reconstruction and molecular dating. Bioinformatics 25: 2286-2288
Lazarow PB (2006) The import receptor Pex7p and the PTS2 targeting sequence. Biochim
Biophys Acta 1763: 1599-1604
Le Corguille G, Pearson G, Valente M, Viegas C, Gschloessl B, Corre E, Bailly X, Peters
AF, Jubin C, Vacherie B, Cock JM, Leblanc C (2009) Plastid genomes of two brown
algae, Ectocarpus siliculosus and Fucus vesiculosus: further insights on the evolution of
red-algal derived plastids. BMC Evol Biol 9: 253
Lee S, Cheng H, King KE, Wang W, He Y, Hussain A, Lo J, Harberd NP, Peng J (2002)
Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene
whose expression is up-regulated following imbibition. Genes & Development 16: 646658
Lehti-Shiu MD, Shiu SH (2012) Diversity, classification and function of the plant protein
kinase superfamily. Philosophical transactions of the Royal Society of London. 367:
2619-2639
Leivar P, Antolin-Llovera M, Ferrero S, Closa M, Arro M, Ferrer A, Boronat A, Campos
N (2011) Multilevel control of Arabidopsis 3-hydroxy-3-methylglutaryl coenzyme A
reductase by protein phosphatase 2A. Plant Cell 23: 1494-1511
Li H, Lin D, Dhonukshe P, Nagawa S, Chen D, Friml J, Scheres B, Guo H, Yang Z (2011)
Phosphorylation switch modulates the interdigitated pattern of PIN1 localization and cell
expansion in Arabidopsis leaf epidermis. Cell Research 21: 970-978
Lima T, Auchincloss AH, Coudert E, Keller G, Michoud K, Rivoire C, Bulliard V, de
Castro E, Lachaize C, Baratin D, Phan I, Bougueleret L, Bairoch A (2009) HAMAP:
a database of completely sequenced microbial proteome sets and manually curated
microbial protein families in UniProtKB/Swiss-Prot. Nucleic Acids Res 37: D471-478
Lingard MJ, Gidda SK, Bingham S, Rothstein SJ, Mullen RT, Trelease RN (2008)
Arabidopsis PEROXIN11c-e, FISSION1b, and DYNAMIN-RELATED PROTEIN3A
cooperate in cell cycle-associated replication of peroxisomes. Plant Cell 20: 1567-1585
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass
spectrometry-based metabolite profiling in plants. Nature Protocols 1: 387-396
215
Liu G, Zhang J, Larsen B, Stark C, Breitkreutz A, Lin ZY, Breitkreutz BJ, Ding Y, Colwill
K, Pasculescu A, Pawson T, Wrana JL, Nesvizhskii AI, Raught B, Tyers M, Gingras
AC (2010) ProHits: integrated software for mass spectrometry-based interaction
proteomics. Nature Biotechnology 28: 1015-1017
Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and
maltose metabolism. Plant Physiol 138: 2280-2291
Lu Y, Savage LJ, Ajjawi I, Imre KM, Yoder DW, Benning C, Dellapenna D, Ohlrogge JB,
Osteryoung KW, Weber AP, Wilkerson CG, Last RL (2008) New connections across
pathways and cellular processes: industrialized mutant screening reveals novel
associations between diverse phenotypes in Arabidopsis. Plant Physiology 146: 14821500
MacKintosh C (1992) Regulation of spinach-leaf nitrate reductase by reversible
phosphorylation. Biochim Biophys Acta 1137: 121-126
MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA (1990) Cyanobacterial
microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from
both mammals and higher plants. FEBS Letter 264: 187-192
MacKintosh C, Coggins J, Cohen P (1991) Plant protein phosphatases. Subcellular
distribution, detection of protein phosphatase 2C and identification of protein
phosphatase 2A as the major quinate dehydrogenase phosphatase. Biochemical Journal
273 ( Pt 3): 733-738
MacKintosh C, Garton AJ, McDonnell A, Barford D, Cohen PT, Tonks NK, Cohen P
(1996) Further evidence that inhibitor-2 acts like a chaperone to fold PP1 into its native
conformation. FEBS Letter 397: 235-238
MacKintosh C, MacKintosh RW (1994) Inhibitors of protein kinases and phosphatases. Trends
in Biochemical Sciences 19: 444-448
MacKintosh RW, Dalby KN, Campbell DG, Cohen PT, Cohen P, MacKintosh C (1995) The
cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase
1. FEBS Lett 371: 236-240
Malyan A (2010) Nucleotide binding to noncatalytic sites is essential for ATP-dependent
stimulation and ADP-dependent inactivation of the chloroplast ATP synthase.
Photosynthic Research: 243-248
Manning G, Plowman GD, Hunter T, Sudarsanam S (2002) Evolution of protein kinase
signaling from yeast to man. Trends in Biochemical Science 27: 514-520
Martinez-Andujar C, Martin RC, Nonogaki H (2012) Seed traits and genes important for
translational biology--highlights from recent discoveries. Plant Cell Physiology 53: 5-15
Matsuda S, Vert JP, Saigo H, Ueda N, Toh H, Akutsu T (2005) A novel representation of
protein sequences for prediction of subcellular location using support vector machines.
Protein Sci 14: 2804-2813
Matsuyama A, Arai R, Yashiroda Y, Shirai A, Kamata A, Sekido S, Kobayashi Y,
Hashimoto A, Hamamoto M, Hiraoka Y, Horinouchi S, Yoshida M (2006) ORFeome
cloning and global analysis of protein localization in the fission yeast
Schizosaccharomyces pombe. Nature Biotechnology 24: 841-847
May A, Berger S, Hertel T, Kock M (2011) The Arabidopsis thaliana phosphate starvation
responsive gene AtPPsPase1 encodes a novel type of inorganic pyrophosphatase.
Biochim Biophys Acta 1810: 178-185
216
Mayer WE, Schuster LN, Bartelmes G, Dieterich C, Sommer RJ (2011) Horizontal gene
transfer of microbial cellulases into nematode genomes is associated with functional
assimilation and gene turnover. BMC Evol Biol 11: 13
Maynes JT, Bateman KS, Cherney MM, Das AK, Luu HA, Holmes CF, James MN (2001)
Crystal structure of the tumor-promoter okadaic acid bound to protein phosphatase-1.
Journal of Biological Chemistry 276: 44078-44082
McCarty RE (1992) A PLANT BIOCHEMIST'S VIEW OF H+-ATPases AND ATP
SYNTHASES. Journal of Experimental Biology 172: 431-441
Meek S, Morrice N, MacKintosh C (1999) Microcystin affinity purification of plant protein
phosphatases: PP1C, PP5 and a regulatory A-subunit of PP2A. FEBS Letter 457: 494498
Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG,
Ohno C, Zhang J, Huang F, Schwab R, Weigel D, Meyerowitz EM, Luschnig C,
Offringa R, Friml J (2007) Antagonistic regulation of PIN phosphorylation by PP2A
and PINOID directs auxin flux. Cell 130: 1044-1056
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for
inference of large phylogenetic trees. In Proceedings of the Gateway Computing
Environments Workshop (GCE). IEEE, New Orleans, LA, pp 1-8
Mithoe SC, Boersema PJ, Berke L, Snel B, Heck AJ, Menke FL (2012) Targeted quantitative
phosphoproteomics approach for the detection of phospho-tyrosine signaling in plants.
Journal of Proteome Research 11: 438-448
Moller SG, Kim YS, Kunkel T, Chua NH (2003) PP7 is a positive regulator of blue light
signaling in Arabidopsis. Plant cell 15: 1111-1119
Moorhead G, Douglas P, Cotelle V, Harthill J, Morrice N, Meek S, Deiting U, Stitt M,
Scarabel M, Aitken A, MacKintosh C (1999) Phosphorylation-dependent interactions
between enzymes of plant metabolism and 14-3-3 proteins. The Plant Journal 18: 1-12
Moorhead GB, De Wever V, Templeton G, Kerk D (2009) Evolution of protein phosphatases
in plants and animals. . Biochemical Journal 417
Moorhead GB, Trinkle-Mulcahy L, Ulke-Lemee A (2007) Emerging roles of nuclear protein
phosphatases. Nature reviews. Molecular cell biology 8: 234-244
Mora-Garcia S, Vert G, Yin Y, Cano-Delgado A, Cheong H, Chory J (2004) Nuclear protein
phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in
Arabidopsis. Genes Development 18: 448-460
Muller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed
germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium
sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell
Physiology 47: 864-877
Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S (2005) Low levels of
pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to
decreased vitality under oxygen deficiency. Ann Bot 96: 717-726
Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M,
Ishihama Y, Shirasu K (2010) Large-scale comparative phosphoproteomics identifies
conserved phosphorylation sites in plants. Plant Physiology 153: 1161-1174
Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for colocalization studies in Arabidopsis and other plants. Plant Journal 51: 1126-1136
217
Nguyen TH, Brechenmacher L, Aldrich JT, Clauss TR, Gritsenko MA, Hixson KK,
Libault M, Tanaka K, Yang F, Yao Q, Pasa-Tolic L, Xu D, Nguyen HT, Stacey G
(2012) Quantitative Phosphoproteomic Analysis of Soybean Root Hairs Inoculated with
Bradyrhizobium japonicum. Molecular & Cellular Proteomics : MCP 11: 1140-1155
Nicholas KB, Nicholas HBJ, Deerfield DWI (1997) GeneDoc: analysis and visualization of
genetic variation. EMBNEW.news 4: 1-4
Ogawa D, Abe K, Miyao A, Kojima M, Sakakibara H, Mizutani M, Morita H, Toda Y,
Hobo T, Sato Y, Hattori T, Hirochika H, Takeda S (2011) RSS1 regulates the cell
cycle and maintains meristematic activity under stress conditions in rice. Nature
Communications 2: 278
Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (2006) Global, in
vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127: 635648
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J,
Jensen TS, Nigg EA, Brunak S, Mann M (2010) Quantitative phosphoproteomics
reveals widespread full phosphorylation site occupancy during mitosis. Science signaling
3: ra3
Pagliarini DJ, Dixon JE (2006) Mitochondrial modulation: reversible phosphorylation takes
center stage? Trends in Biochemical Sciences 31: 26-34
Pallen CJ, Valentine KA, Wang JH, Hollenberg MD (1985) Calcineurin-mediated
dephosphorylation of the human placental membrane receptor for epidermal growth
factor urogastrone. Biochemistry 24: 4727-4730
Pannifer AD, Flint AJ, Tonks NK, Barford D (1998) Visualization of the cysteinyl-phosphate
intermediate of a protein-tyrosine phosphatase by x-ray crystallography. Journal of
Biological Chemistry 273: 10454-10462
Park JH, Lee SY, Kim WY, Jung YJ, Chae HB, Jung HS, Kang CH, Shin MR, Kim SY,
Su'udi M, Yun DJ, Lee KO, Kim MG (2011) Heat-induced chaperone activity of
serine/threonine protein phosphatase 5 enhances thermotolerance in Arabidopsis thaliana.
The New Phytologist 191: 692-705
Patzewitz EM, Guttery DS, Poulin B, Ramakrishnan C, Ferguson DJ, Wall RJ, Brady D,
Holder AA, Szoor B, Tewari R (2013) An ancient protein phosphatase, SHLP1, is
critical to microneme development in Plasmodium ookinetes and parasite transmission.
Cell Reports 3: 622-629
Patzewitz EM, Guttery DS, Poulin B, Ramakrishnan C, Ferguson DJ, Wall RJ, Brady D,
Holder AA, Szoor B, Tewari R (2013) An Ancient Protein Phosphatase, SHLP1, Is
Critical to Microneme Development in Plasmodium Ookinetes and Parasite
Transmission. Cell Rep 3: 622-629
Pellny TK, Van Aken O, Dutilleul C, Wolff T, Groten K, Bor M, De Paepe R, Reyss A, Van
Breusegem F, Noctor G, Foyer CH (2008) Mitochondrial respiratory pathways
modulate nitrate sensing and nitrogen-dependent regulation of plant architecture in
Nicotiana sylvestris. The Plant Journal 54: 976-992
Pereira SF, Goss L, Dworkin J (2011) Eukaryote-like serine/threonine kinases and
phosphatases in bacteria. Microbiol Mol Biol Rev 75: 192-212
218
Perez J, Castaneda-Garcia A, Jenke-Kodama H, Muller R, Munoz-Dorado J (2008)
Eukaryotic-like protein kinases in the prokaryotes and the myxobacterial kinome. Proc
Natl Acad Sci U S A 105: 15950-15955
Peti W, Nairn AC, Page R (2013) Structural basis for protein phosphatase 1 regulation and
specificity. The FEBS Journal 280: 596-611
Petsalaki EI, Bagos PG, Litou ZI, Hamodrakas SJ (2006) PredSL: a tool for the N-terminal
sequence-based prediction of protein subcellular localization. Genomics Proteomics
Bioinformatics 4: 48-55
Piskurewicz U, Jikumaru Y, Kinoshita N, Nambara E, Kamiya Y, Lopez-Molina L (2008)
The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by
stimulating abscisic acid synthesis and ABI5 activity. Plant cell 20: 2729-2745
Plaxton WC (1989) Molecular and immunological characterization of plastid and cytosolic
pyruvate kinase isozymes from castor-oil-plant endosperm and leaf. Eur J Biochem 181:
443-451
Plaxton WC (1996) The Organization and Regulation of Plant Glycolysis. Annu Rev Plant
Physiol Plant Mol Biol 47: 185-214
Poole AM, Penny D (2007) Evaluating hypotheses for the origin of eukaryotes. Bioessays 29:
74-84
Raymond JA, Kim HJ (2012) Possible role of horizontal gene transfer in the colonization of sea
ice by algae. PLoS One 7: e35968
Reddehase S, Grumbt B, Neupert W, Hell K (2009) The disulfide relay system of
mitochondria is required for the biogenesis of mitochondrial Ccs1 and Sod1. Journal of
Molecular Biology 385: 331-338
Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W,
Baginsky S (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel
chloroplast kinase substrates and phosphorylation networks. Plant Physiology 150: 889903
Remmert M, Biegert A, Hauser A, Soding J (2012) HHblits: lightning-fast iterative protein
sequence searching by HMM-HMM alignment. Nat Methods 9: 173-175
Rolland N, Curien G, Finazzi G, Kuntz M, Marechal E, Matringe M, Ravanel S,
Seigneurin-Berny D (2012) The biosynthetic capacities of the plastids and integration
between cytoplasmic and chloroplast processes. Annu Rev Genet 46: 233-264
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L,
Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic
inference and model choice across a large model space. Syst Biol 61: 539-542
Roy J, Cyert MS (2009) Cracking the phosphatase code: docking interactions determine
substrate specificity. Science signaling 2: re9
Roy LM, Barkan A (1998) A SecY homologue is required for the elaboration of the chloroplast
thylakoid membrane and for normal chloroplast gene expression. Journal of Cell Biology
141: 385-395
Rubio V, Shen Y, Saijo Y, Liu Y, Gusmaroli G, Dinesh-Kumar SP, Deng XW (2005) An
alternative tandem affinity purification strategy applied to Arabidopsis protein complex
isolation. The Plant Journal 41: 767-778
Russell JA, Roy MK, Sanford JC (1992) Physical trauma and tungsten toxicity reduce the
efficiency of biolistic transformation. Plant Physiol 98: 1050-1056
219
Ryu JS, Kim JI, Kunkel T, Kim BC, Cho DS, Hong SH, Kim SH, Fernandez AP, Kim Y,
Alonso JM, Ecker JR, Nagy F, Lim PO, Song PS, Schafer E, Nam HG (2005)
Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing
phytochrome stability and affinity for a signal transducer. Cell 120: 395-406
Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A (2002) Characterization
and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria.
Biochim Biophys Acta 1589: 181-195
Salvi M, Brunati AM, Toninello A (2005) Tyrosine phosphorylation in mitochondria: a new
frontier in mitochondrial signaling. Free Radical Biology & Medicine 38: 1267-1277
Salvi M, Stringaro A, Brunati AM, Agostinelli E, Arancia G, Clari G, Toninello A (2004)
Tyrosine phosphatase activity in mitochondria: presence of Shp-2 phosphatase in
mitochondria. Cellular and Molecular Life Sciences : CMLS 61: 2393-2404
Schauer N, Semel Y, Balbo I, Steinfath M, Repsilber D, Selbig J, Pleban T, Zamir D, Fernie
AR (2008) Mode of inheritance of primary metabolic traits in tomato. Plant Cell 20: 509523
Schein AI, Kissinger JC, Ungar LH (2001) Chloroplast transit peptide prediction: a peek inside
the black box. Nucleic Acids Res 29: E82
Schliebner I, Pribil M, Zuhlke J, Dietzmann A, Leister D (2008) A Survey of Chloroplast
Protein Kinases and Phosphatases in Arabidopsis thaliana. Current Genomics 9: 184-190
Schonknecht G, Chen WH, Ternes CM, Barbier GG, Shrestha RP, Stanke M, Brautigam
A, Baker BJ, Banfield JF, Garavito RM, Carr K, Wilkerson C, Rensing SA,
Gagneul D, Dickenson NE, Oesterhelt C, Lercher MJ, Weber AP (2013) Gene
transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote.
Science 339: 1207-1210
Seibel NM, Eljouni J, Nalaskowski MM, Hampe W (2007) Nuclear localization of enhanced
green fluorescent protein homomultimers. Anal Biochem 368: 95-99
Seifried A, Schultz J, Gohla A (2013) Human HAD phosphatases: structure, mechanism, and
roles in health and disease. The FEBS Journal 280: 549-571
Sents W, Ivanova E, Lambrecht C, Haesen D, Janssens V (2013) The biogenesis of active
protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase
specificity. The FEBS Journal 280: 644-661
Sheppeck JE, 2nd, Gauss CM, Chamberlin AR (1997) Inhibition of the Ser-Thr phosphatases
PP1 and PP2A by naturally occurring toxins. Bioorg Med Chem 5: 1739-1750
Shi Y (2009) Serine/threonine phosphatases: mechanism through structure. Cell 139: 468-484
Sideris DP, Petrakis N, Katrakili N, Mikropoulou D, Gallo A, Ciofi-Baffoni S, Banci L,
Bertini I, Tokatlidis K (2009) A novel intermembrane space-targeting signal docks
cysteines onto Mia40 during mitochondrial oxidative folding. The Journal of Cell
Biology 187: 1007-1022
Siegl G, MacKintosh C, Stitt M (1990) Sucrose-phosphate synthase is dephosphorylated by
protein phosphatase 2A in spinach leaves. Evidence from the effects of okadaic acid and
microcystin. FEBS Letter 270: 198-202
Silver DM, Silva LP, Issakidis-Bourguet E, Glaring MA, Schriemer DC, Moorhead GB
(2013) Insight into the redox regulation of the phosphoglucan phosphatase SEX4
involved in starch degradation. The FEBS Journal 280: 538-548
220
Simanis V (1995) The control of septum formation and cytokinesis in fission yeast. Semin Cell
Biol 6: 79-87
Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: A tool for rapidly screening proteomes
for N-terminal targeting sequences. Proteomics 4: 1581-1590
Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman
SC, Smith AM (2004) Diurnal changes in the transcriptome encoding enzymes of starch
metabolism provide evidence for both transcriptional and posttranscriptional regulation
of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687-2699
Snel B, Bork P, Huynen MA (2002) Genomes in flux: the evolution of archaeal and
proteobacterial gene content. Genome Res 12: 17-25
Sozzani R, Benfey PN (2011) High-throughput phenotyping of multicellular organisms: finding
the link between genotype and phenotype. Genome Biology 12: 219
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML
Web servers. Syst Biol 57: 758-771
Stamm P, Ravindran P, Mohanty B, Tan EL, Yu H, Kumar PP (2012) Insights into the
molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis
thaliana. BMC Plant Biology 12: 179
Stefansson B, Ohama T, Daugherty AE, Brautigan DL (2008) Protein phosphatase 6
regulatory subunits composed of ankyrin repeat domains. Biochemistry 47: 1442-1451
Stock JB, Stock AM, Mottonen JM (1990) Signal transduction in bacteria. Nature 344: 395400
Stubbs MD, Tran HT, Atwell AJ, Smith CS, Olson D, Moorhead GB (2001) Purification and
properties of Arabidopsis thaliana type 1 protein phosphatase (PP1). Biochim Biophys
Acta 1550: 52-63
Sugiyama N, Nakagami H, Mochida K, Daudi A, Tomita M, Shirasu K, Ishihama Y (2008)
Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in
Arabidopsis. Mol Syst Biol 4: 193
Sun TP, Kamiya Y (1994) The Arabidopsis GA1 locus encodes the cyclase ent-kaurene
synthetase A of gibberellin biosynthesis. Plant cell 6: 1509-1518
Sun X, Kang X, Ni M (2012) Hypersensitive to red and blue 1 and its modification by protein
phosphatase 7 are implicated in the control of Arabidopsis stomatal aperture. PLoS
Genetics 8: e1002674
Swarup G, Cohen S, Garbers DL (1982) Inhibition of membrane phosphotyrosyl-protein
phosphatase activity by vanadate. Biochemical and Biophysical Research
Communications 107: 1104-1109
Szurmak B, Strokovskaya L, Mooney BP, Randall DD, Miernyk JA (2003) Expression and
assembly of Arabidopsis thaliana pyruvate dehydrogenase in insect cell cytoplasm.
Protein Expression and Purification 28: 357-361
Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV,
Dixon JE (2012) Secreted kinase phosphorylates extracellular proteins that regulate
biomineralization. Science 336: 1150-1153
Takemiya A, Ariyoshi C, Shimazaki K (2009) Identification and functional characterization of
inhibitor-3, a regulatory subunit of protein phosphatase 1 in plants. Plant Physiology 150:
144-156
221
Takemiya A, Kinoshita T, Asanuma M, Shimazaki K (2006) Protein phosphatase 1 positively
regulates stomatal opening in response to blue light in Vicia faba. Proc Natl Acad Sci U S
A 103: 13549-13554
Takemiya A, Yamauchi S, Yano T, Ariyoshi C, Shimazaki K (2013) Identification of a
regulatory subunit of protein phosphatase 1 which mediates blue light signaling for
stomatal opening. Plant & Cell Physiology 54: 24-35
Tamura K, Fukao Y, Iwamoto M, Haraguchi T, Hara-Nishimura I (2010) Identification and
characterization of nuclear pore complex components in Arabidopsis thaliana. Plant Cell
22: 4084-4097
Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng
Z, Gampala SS, Gendron JM, Jonassen EM, Lillo C, DeLong A, Burlingame AL,
Sun Y, Wang ZY (2011) PP2A activates brassinosteroid-responsive gene expression and
plant growth by dephosphorylating BZR1. Nature Cell Biology 13: 124-131
Tanner JJ, Parsons ZD, Cummings AH, Zhou H, Gates KS (2011) Redox regulation of
protein tyrosine phosphatases: structural and chemical aspects. Antioxidants & Redox
Signaling 15: 77-97
Templeton GW, Nimick M, Morrice N, Campbell D, Goudreault M, Gingras AC,
Takemiya A, Shimazaki K, Moorhead GB (2011) Identification and characterization of
AtI-2, an Arabidopsis homologue of an ancient protein phosphatase 1 (PP1) regulatory
subunit. Biochemical Journal 435: 73-83
Tirichine L, Bowler C (2011) Decoding algal genomes: tracing back the history of
photosynthetic life on Earth. The Plant Journal 66: 45-57
Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nature
Reviews. Molecular Cell Biology 7: 833-846
Tonks NK (2013) Protein tyrosine phosphatases - from housekeeping enzymes to master
regulators of signal transduction. The FEBS Journal 280: 346-378
Tran HT, Uhrig RG, Nimick M, Moorhead GB (2012) Interfacing protein lysine acetylation
and protein phosphorylation: Ancient modifications meet on ancient proteins. Plant
Signaling & Behavior 7: 901-903
Tran HT, Ulke A, Morrice N, Johannes CJ, Moorhead GB (2004) Proteomic characterization
of protein phosphatase complexes of the mammalian nucleus. Molecular & Cellular
Proteomics : MCP 3: 257-265
Trotta A, Wrzaczek M, Scharte J, Tikkanen M, Konert G, Rahikainen M, Holmstrom M,
Hiltunen HM, Rips S, Sipari N, Mulo P, Weis E, von Schaewen A, Aro EM,
Kangasjarvi S (2011) Regulatory subunit B'gamma of protein phosphatase 2A prevents
unnecessary defense reactions under low light in Arabidopsis. Plant Physiology 156:
1464-1480
Tseng TS, Briggs WR (2010) The Arabidopsis rcn1-1 mutation impairs dephosphorylation of
Phot2, resulting in enhanced blue light responses. Plant Cell 22: 392-402
Tsuruta H, Aizono Y (1999) Enzymatical properties of psychrophilic phosphatase I. J Biochem
125: 690-695
Tsuruta H, Aizono Y (2000) Cloning of phosphatase I gene from a psychrophile, Shewanella
sp., and some properties of the recombinant enzyme. J Biochem 127: 143-149
Ubersax JA, Ferrell JE, Jr. (2007) Mechanisms of specificity in protein phosphorylation. Nat
Rev Mol Cell Biol 8: 530-541
222
Uhrig RG, O'Leary B, Spang HE, MacDonald JA, She YM, Plaxton WC (2008)
Coimmunopurification of phosphorylated bacterial- and plant-type phosphoenolpyruvate
carboxylases with the plastidial pyruvate dehydrogenase complex from developing castor
oil seeds. Plant Physiology 146: 1346-1357
Vilela B, Pages M, Lumbreras V (2010) Regulation of MAPK signaling and cell death by
MAPK phosphatase MKP2. Plant Signaling & Behavior 5: 1497-1500
Villarino A, Duran R, Wehenkel A, Fernandez P, England P, Brodin P, Cole ST, ZimnyArndt U, Jungblut PR, Cervenansky C, Alzari PM (2005) Proteomic identification of
M. tuberculosis protein kinase substrates: PknB recruits GarA, a FHA domain-containing
protein, through activation loop-mediated interactions. J Mol Biol 350: 953-963
Virshup DM, Shenolikar S (2009) From promiscuity to precision: protein phosphatases get a
makeover. Mol Cell 33: 537-545
Vogtle FN, Burkhart JM, Rao S, Gerbeth C, Hinrichs J, Martinou JC, Chacinska A,
Sickmann A, Zahedi RP, Meisinger C (2012) Intermembrane space proteome of yeast
mitochondria. Molecular & Cellular Proteomics : MCP 11: 1840-1852
Walker G, Dorrell RG, Schlacht A, Dacks JB (2011) Eukaryotic systematics: a user's guide
for cell biologists and parasitologists. Parasitology 138: 1638-1663
Weckbecker D, Longen S, Riemer J, Herrmann JM (2012) Atp23 biogenesis reveals a
chaperone-like folding activity of Mia40 in the IMS of mitochondria. EMBO J 31: 43484358
Wen F, Wang J, Xing D (2012) A protein phosphatase 2A catalytic subunit modulates blue
light-induced chloroplast avoidance movements through regulating actin cytoskeleton in
Arabidopsis. Plant & Cell Physiology 53: 1366-1379
Wenzl P, Wong L, Kwang-won K, Jefferson RA (2005) A functional screen identifies lateral
transfer of beta-glucuronidase (gus) from bacteria to fungi. Mol Biol Evol 22: 308-316
Wu G, Wang X, Li X, Kamiya Y, Otegui MS, Chory J (2011) Methylation of a phosphatase
specifies dephosphorylation and degradation of activated brassinosteroid receptors.
Science Signaling 4: ra29
Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y (2006)
Structure of the protein phosphatase 2A holoenzyme. Cell 127: 1239-1251
Xue T, Wang D, Zhang S, Ehlting J, Ni F, Jakab S, Zheng C, Zhong Y (2008) Genome-wide
and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC
Genomics 9: 550
Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, Hirozane-Kishikawa
T, Gebreab F, Li N, Simonis N, Hao T, Rual JF, Dricot A, Vazquez A, Murray RR,
Simon C, Tardivo L, Tam S, Svrzikapa N, Fan C, de Smet AS, Motyl A, Hudson
ME, Park J, Xin X, Cusick ME, Moore T, Boone C, Snyder M, Roth FP, Barabasi
AL, Tavernier J, Hill DE, Vidal M (2008) High-quality binary protein interaction map
of the yeast interactome network. Science 322: 104-110
Zhang J, Zhang Z, Brew K, Lee EY (1996) Mutational analysis of the catalytic subunit of
muscle protein phosphatase-1. Biochemistry 35: 6276-6282
Zhang M, Liu J, Kim Y, Dixon JE, Pfaff SL, Gill GN, Noel JP, Zhang Y (2010) Structural
and functional analysis of the phosphoryl transfer reaction mediated by the human small
C-terminal domain phosphatase, Scp1. Protein Science 19: 974-986
223
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated
transformation of Arabidopsis thaliana using the floral dip method. Nature Protocols 1:
641-646
Zhang X, Ozawa Y, Lee H, Wen YD, Tan TH, Wadzinski BE, Seto E (2005) Histone
deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine
phosphatase 4. Genes Development 19: 827-839
224
Appendix A.1. SLP Phylogenetic Tree and Alignment Sequence Information
225
226
227
228
229
Appendix A.2. RLPH Phylogenetic Tree and Alignment Sequence Information
230
231
232
Appendix B. Phylogenetic Tree Node Scores
233
Appendix C.1. Cloning and Genotyping Primers
234
Appendix C.2. Plant Genotyping
Genotyping was performed as outlined in the Materials and Methods in Chapter 5. Primers
used are outlined in Appendix C.1.
235
Appendix C.3. Primers for qPCR of GA Related Genes
236