* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download In the prevailing view Conducitn is usually considered to act as a
Hedgehog signaling pathway wikipedia , lookup
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell growth wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
List of types of proteins wikipedia , lookup
Wnt signaling pathway wikipedia , lookup
Beta-catenin wikipedia , lookup
© 2014. Published by The Company of Biologists Ltd. Negative feedback regulation of the Wnt pathway by conductin/axin2 involves insensitivity to upstream signalling Dominic B. Bernkopf, Michel V. Hadjihannas, and Jürgen Behrens Nikolaus-Fiebiger-Center, Chair of Experimental Medicine II, Friedrich-Alexander Universität Erlangen-Nürnberg, [email protected] Keywords: axin / conductin / Dvl / negative feedback / Wnt Journal of Cell Science Accepted manuscript Glückstr. 6, D-91054 Erlangen, Germany 1 JCS Advance Online Article. Posted on 6 November 2014 Abstract Axin and conductin/axin2 are structurally related inhibitors of Wnt/-catenin signalling that promote degradation of -catenin. Whereas axin is constitutively expressed, conductin is a Wnt target gene implicated in negative feedback regulation. Here we show that axin and conductin differ in their functional interaction with the upstream Wnt pathway component Dvl. Conductin shows reduced binding to Dvl2 compared to axin, and degradation of - Journal of Cell Science Accepted manuscript catenin by conductin is only poorly blocked by Dvl2. We propose that insensitivity to Dvl is an important feature of conductin´s role as a negative feedback regulator of Wnt signalling. Introduction Stabilisation of -catenin is a key step in the Wnt/-catenin signalling pathway allowing catenin to stimulate transcription of Wnt target genes in conjunction with TCF transcription factors (Clevers and Nusse, 2012). In the absence of Wnts, -catenin is earmarked for ubiquitination and proteasomal degradation by phosphorylation in a destruction complex consisting of the tumor suppressor APC, the kinases GSK3 and CK1 and the scaffolding proteins axin or conductin/axin2 (Stamos and Weis, 2013). Binding of Wnt ligands to their receptors frizzled and LRP5/6 leads to membrane recruitment of axin proteins by the frizzledassociated phosphoprotein Dvl (dishevelled) ultimately resulting in the inhibition of -catenin phosphorylation and degradation (Bilic et al., 2007; Cliffe et al., 2003; Cselenyi et al., 2008; Smalley et al., 1999; Wu et al., 2009; Zeng et al., 2008). Axin molecules form dynamic oligomers by head-to-tail interaction of their DIX domains (Fiedler et al., 2011; Kishida et al., 1999). These complexes, often seen as cytoplasmic puncta upon overexpression, are thought to provide high avidity interaction sites for -catenin and other destruction complex components. Specific mutations of the DIX domain that abolish the head-to-tail interaction dissolve axin oligomers and render axin ineffective in -catenin degradation (Fiedler et al., 2011). Dvl also has a DIX domain through which it binds to axin. Dvl can interfere with axin function in different ways. Recruitment of axin by Dvl leads to inhibition of GSK3 activity by phosphorylated LRP5/6 receptors (Cselenyi et al., 2008; Wu et al., 2009; Zeng et al., 2008). In addition, the DIX-DIX domain interactions of Dvl and axin disturb self-assembly of axin homopolymers and thereby interfere with -catenin degradation (Fiedler et al., 2011; Kishida et al., 1999; Schwarz-Romond et al., 2007). 2 Axin and conductin are related proteins that share key sequence elements (Behrens et al., 1998; Fagotto et al., 1999). In contrast to axin, which is constitutively expressed, conductin is a direct Wnt target gene upregulated after activation of the pathway (Jho et al., 2002; Lustig et al., 2002). Therefore, it is assumed that conductin acts as a negative feedback regulator of Wnt signalling. However, it is unclear how conductin escapes upstream inhibition by activated Wnt signalling to remain active in -catenin degradation. In principle, conductin might accumulate to levels sufficiently high to saturate available binding sites at Wnt Journal of Cell Science Accepted manuscript receptor/Dvl complexes and might thus be able to continue degrading -catenin. Alternatively, or in addition, conductin might be less susceptible to upstream inhibition. We provide evidence that favours the latter mechanism by showing (i) that Wnt stimulation leads to only a modest increase in conductin protein compared to axin levels, and (ii) that conductin interacts less efficiently with Dvl2 than axin making it largely resistant to inhibition by Dvl2. Thus, functional rather than expression differences determine the differential roles of axin and conductin as constitutive versus inducible inhibitors of Wnt signalling. Results and discussion The relative amounts of axin and conductin under Wnt signalling were determined by Western blotting of extracts from Wnt3a treated MDA-MB-231 cells. In line with previous reports, the amount of conductin increased after Wnt3a treatment for 6 hrs whereas that of axin decreased (Fig. 1A, lanes 1,2; (Lustig et al., 2002; Yamamoto et al., 1999)). Normalisation of the Western blot signals on serial dilutions of GFP-Conductin and GFPAxin present on the same blots allowed comparison of axin and conductin levels (Fig. 1A, lanes 3-6 and 7-10). This showed that conductin levels were lower than axin levels under resting conditions and, surprisingly, remained significantly lower after Wnt3a-induced upregulation of conductin and downregulation of axin (Fig. 1A, bar chart). These results largely exclude strong accumulation of conductin as a decisive factor for negative feedback regulation. We also compared axin and conductin levels in three colorectal carcinoma cell lines which exhibit constitutively high conductin expression due to activation of Wnt signalling by mutations of APC (DLD1, SW480) or -catenin (HCT116) (Lustig et al., 2002). In all cell lines axin levels exceeded those of conductin (Fig. 1B) confirming that conductin is only moderately upregulated by Wnt signalling. 3 We next compared the potency of axin and conductin in degrading -catenin. Transfection of axin in SW480 cells led to a stronger reduction of endogenous -catenin than transfection of conductin (Fig. 1C, D) over a range of different expression levels (Fig. 1E). Notably, axin was present in puncta, whereas conductin was more diffusely distributed, possibly explaining its lower activity in degrading -catenin (Fig. 1C). Similarly, transiently expressed -catenin was strongly reduced by coexpression of axin but less by coexpression of conductin (Fig. 1F). Journal of Cell Science Accepted manuscript To compare the individual contribution of endogenous axin and conductin to the inhibition of Wnt signalling, we knocked down their expression in MDA-MB-231 cells using siRNAs and monitored nuclear -catenin levels by immunofluorescence staining. Depletion of axin or conductin led to an increase in -catenin staining intensity in the absence of exogenous Wnt ligands (Fig. 2A) suggesting that both factors are active in degrading -catenin. Of note, MDA-MB-231 cells express endogenous Wnts (Bafico et al., 2004) which might stimulate the pathway to a certain extent but are apparently not sufficient to fully block axin proteins. Wnt3a treatment increased -catenin intensity, similar to treatment with the GSK3 inhibitor BIO, whereas knockdown of -catenin reduced staining. Importantly, knockdown of conductin further increased the Wnt3a-induced -catenin staining intensity, whereas knockdown of axin did not (Fig. 2A). The Wnt3a-induced increase of dephosphorylated catenin (ABC) was augmented by knockdown of conductin but not axin in three different cell lines, at similar knockdown rates (Fig. 2B-D). Moreover, knockdown of conductin led to a stronger increase of Wnt3a-induced TCF/-catenin reporter activity than knockdown of axin (Fig. 2D). Thus, despite its lower expression level and activity conductin appears to retain negative regulatory activity under sustained Wnt activation whereas axin activity is more strongly inhibited. Recruitment of axin/conductin proteins to frizzled receptors by Dvl is widely considered the initial step towards inhibition of the -catenin destruction complex (MacDonald et al., 2009; Metcalfe and Bienz, 2011). We therefore tested whether axin and conductin might differ in their interaction with Dvl. For this, recruitment of axin and conductin by CAAX-tagged Dvl2, a membrane-associated modification of Dvl2, was determined by cell fractionation (Fig. 3A; (Smalley et al., 1999)). Axin was equally distributed between membrane and cytoplasmic fractions, and coexpression of Dvl2-CAAX increased the presence of axin in the membrane 4 fraction (Fig. 3A, lanes 1-4). Membrane recruitment of axin was strongly reduced when its DIX domain was mutated (construct axinM3 (Fiedler et al., 2011)) demonstrating dependence on the specific DIX-DIX mediated interaction with Dvl2 (Fig. 3A, lanes 5-8). In contrast to axin, conductin was mainly present in the cytoplasmic fraction and only poorly recruited to the membrane by Dvl2-CAAX (Fig. 3A, lanes 9-12). Importantly, replacement of the DIX domain of conductin with that of axin generating CdtAxinDIX resulted in increased membrane recruitment of this protein by Dvl2-CAAX (Fig. 3A, lanes 13-16). These results show that conductin exhibits weaker interaction with Dvl2 than axin which is determined by the DIX Accepted manuscript domains of axin and conductin. The punctate pattern of GFP-Axin in the cytoplasm was partially resolved by Dvl2-CAAX resulting in colocalisation of both proteins at the plasma membrane (Fig. 3B, upper panels). In contrast, GFP-AxinCdtDIX containing the DIX domain of conductin did not become membrane associated by Dvl2-CAAX (Fig. 3B, lower panels). Like axin, Dvl2 localises in cytoplasmic puncta, and axin colocalises with Dvl2 in such puncta (Fig. 3C). The DIX domain mutant axinM3 did not form puncta (Fiedler et al., 2011) Journal of Cell Science but, surprisingly, abolished puncta formation by Dvl2 (Fig. 3C). The M3 mutation impairs the head interaction surface of the axin DIX domain preventing its homopolymerisation, but leaves the tail interaction surface intact for interaction with Dvl2 (Fiedler et al., 2011). We propose that this results in blunting of Dvl2 polymers leading to smaller complexes no longer visible as puncta (see scheme). Conductin alone shows a diffuse staining pattern (cf. Fig. 1C), and colocalised with Dvl2 in puncta, albeit with a lower efficiency than axin as indicated by its remaining partially diffuse staining pattern (Fig. 3C). ConductinM3 remained diffuse in the presence of Dvl2 but in contrast to axinM3 did not dissolve Dvl2 puncta. We propose that conductinM3 integrates to a lower extent in Dvl2 polymers than axinM3 and therefore fails to disrupt these polymers. These data support differences in the strength of interaction of axin and conductin with Dvl2. We next analysed whether -catenin degradation by axin and conductin is differently inhibited by Dvl2. As readout we used immunofluorescence staining of endogenous -catenin in SW480 cells (Fig. 4A). Axin efficiently degraded -catenin whereas conductin was less efficient resulting in residual -catenin staining (Fig. 4A, a,f). Coexpression of Dvl2-CAAX but not of the DIX domain mutant Dvl2M2-CAAX reduced -catenin degradation by axin (Fig. 4A, b,c). Importantly, Dvl2-CAAX only weakly affected degradation by conductin (Fig. 5 4A, g). Axin containing the DIX domain of conductin (GFP-AxinCdtDIX) became largely resistant to inhibition by Dvl2-CAAX (Fig. 4A, d,e), whereas conductin containing the DIX domain of axin (GFP-CdtAxinDIX) became more sensitive to inhibition by Dvl2-CAAX (Fig. 4A, h,i). These results are quantified in Fig. 4B,C. Similarly, degradation of transiently expressed -catenin by coexpressed axin was more efficiently inhibited by Dvl2-CAAX than degradation by conductin. Conversely, -catenin degradation by AxinCdtDIX was unaffected by Dvl2-CAAX whereas degradation by CdtAxinDIX was blocked by Dvl2-CAAX (Fig. 4D,E). Journal of Cell Science Accepted manuscript Together these results show that the different capability of Dvl2 to interfere with axin and conductin depends on the respective DIX-DIX domain interactions. A bioinformatical analysis predicted ten amino acids likely to be required for specificity of DIX domain interactions (Ehebauer and Arias, 2009). Four of these amino acids differ between axin and conductin and may mediate differential interaction with Dvl (Fig. S1). Like Dvl2, Dvl1 and Dvl3 inhibited -catenin degradation by axin but not degradation by AxinCdtDIX (Fig. 4F). In line with differential inhibition of axin versus conductin by Dvl2, knockdown of conductin but not of axin further increased Dvl2 stimulated activity of the TCF/-catenin dependent reporter (Fig. 4G). Our results shed light on the molecular basis of negative feedback regulation in Wnt signalling by showing that the amounts of conductin in Wnt stimulated cells remained lower than those of axin and that conductin is relatively insensitive to Dvl2. This rules out mere overproduction of conductin as the basis of negative pathway regulation and favours the idea that conductin´s capacity as a negative feedback regulator is based mainly on its reduced responsiveness towards upstream signalling. It remains to be determined whether the qualitative rather than quantitative feedback mode revealed here has specific consequences for the regulation of Wnt signalling. Replacement of axin by knock-in of the conductin/axin2 cDNA generated viable mice suggesting that compensatory mechanism can neutralise functional differences between axin and conductin in vivo (Chia and Costantini, 2005). The separation of tasks between axin and conductin as constitutive and inducible negative regulators of Wnt signalling, respectively, is remarkable and contrasts with feedback modes in other pathways in which negative regulators act in both, constitutive and inducible modes, e.g. patched in hedgehog signalling. While the reasons for this are not clear, we found that conductin was less active in -catenin degradation than axin. It is conceivable that a gradual 6 suppression of signalling by conductin is favoured over an abrupt block by axin to allow for temporal and spatial fine tuning of Wnt pathway activity. Materials and Methods Cell culture, transfections, and treatments Cells were grown in DMEM /10% fetal bovine serum /1% penicillin/streptomycin at Journal of Cell Science Accepted manuscript 37°C/10% CO2. siRNAs against axin (Tanneberger et al., 2011), -catenin and conductin (Hadjihannas et al., 2006) were transfected with Oligofectamine (Invitrogen), and plasmids with polyethylenimine (Sigma) (HEK293T, U2OS cells) or Lipofectamine 2000 (Invitrogen) (SW480 cells). Cells were treated with Wnt3a-conditioned medium (Willert et al., 2003) 48h after siRNA transfection. (2’Z,3’E)-6-Bromoindirubin-3′-oxime (BIO) was obtained from Sigma. TCF/-catenin dependent pBAR reporter activity (Major et al., 2007) was determined in HEK293T cells. Molecular biology To generate GFP-AxinCdtDIX and GFP-CdtAxinDIX amino acids 719-832 of rat axin and amino acids 700-840 of mouse conductin were exchanged. The M2 mutant of Dvl2 and M3 mutants of axin and conductin were generated using the Quickchange Site-Directed mutagenesis kit (Stratagene) according to (Schwarz-Romond et al., 2007) and (Fiedler et al., 2011), respectively. Cell lysis, fractionation and Western blotting Whole cell extracts were prepared in Triton X-100-based lysis buffer or hypotonic buffer (Fig. 2C upper panels, (Lustig et al., 2002)) or Passive Lysis Buffer (Promega) (Fig. 2D). Cytoplasmic and membrane fractions were obtained using the ProteoJET™ Membrane Protein Extraction Kit (Fermentas). Western blotting was performed as described (Tanneberger et al., 2011). Primary antibodies: mouse anti--actin, rabbit anti-Flag, rabbit anti-HA, rabbit anti-PanCadherin (all from Sigma), rabbit anti-axin (Cell Signaling), mouse anti-conductin (C/G7; (Lustig et al., 2002)), mouse anti-active--catenin (ABC; Millipore), mouse anti-GFP (Roche) and rat anti--tubulin (Serotec). Densitometric analysis was performed with AIDA 2D Densitometry (raytest). For comparison of axin and conductin 7 amounts in Fig. 1A and B signal ratios obtained with anti-axin and anti-conductin antibodies were normalised to those obtained with anti-GFP antibodies. Immunofluorescence microscopy Methanol-fixed cells were stained as described (Hadjihannas et al., 2006). Images were acquired on an Axioplan2 microscope using Metamorph (Zeiss). For intensitiy measurements, images were acquired at constant exposure times, and background-free intensities of the Journal of Cell Science Accepted manuscript nuclear regions (Fig. 2) or of whole cells (Fig. 1,4) were determined. Statistics p-Values were determined using unpaired, two-tailed t-tests (Fig. 1D, 2D and 4B,F,G) or significance was tested after ANOVA by post hoc testing based on the Bonferroni method (Fig. 2A). Acknowledgements We thank A. Kikuchi, M. Bienz, T. Dale, and D. Sussman for reagents and M. Brückner for technical assistance. This study was supported by EFI funding of the Friedrich-Alexander Universität Erlangen-Nürnberg and the KFO257 of the DFG to J.B. Author Contribution D.B.B. and M.V.H. designed and performed experiments, D.B.B., M.V.H., and J.B. analysed data, and D.B.B. and J.B. wrote the manuscript. Competing interests The authors declare no competing interests. 8 References Bafico, A., Liu, G., Goldin, L., Harris, V. and Aaronson, S. A. (2004). An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell 6, 497-506. Journal of Cell Science Accepted manuscript Behrens, J., Jerchow, B. A., Wurtele, M., Grimm, J., Asbrand, C., Wirtz, R., Kuhl, M., Wedlich, D. and Birchmeier, W. (1998). Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280, 5969. Bilic, J., Huang, Y. L., Davidson, G., Zimmermann, T., Cruciat, C. M., Bienz, M. and Niehrs, C. (2007). Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619-22. Chia, I. V. and Costantini, F. (2005). Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol 25, 4371-6. Clevers, H. and Nusse, R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192-205. Cliffe, A., Hamada, F. and Bienz, M. (2003). A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13, 9606. Cselenyi, C. S., Jernigan, K. K., Tahinci, E., Thorne, C. A., Lee, L. A. and Lee, E. (2008). LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc Natl Acad Sci U S A 105, 8032-7. Ehebauer, M. T. and Arias, A. M. (2009). The structural and functional determinants of the Axin and Dishevelled DIX domains. BMC Struct Biol 9, 70. Fagotto, F., Jho, E., Zeng, L., Kurth, T., Joos, T., Kaufmann, C. and Costantini, F. (1999). Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol 145, 741-56. Fiedler, M., Mendoza-Topaz, C., Rutherford, T. J., Mieszczanek, J. and Bienz, M. (2011). Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating beta-catenin. Proc Natl Acad Sci U S A 108, 1937-42. Hadjihannas, M. V., Bruckner, M., Jerchow, B., Birchmeier, W., Dietmaier, W. and Behrens, J. (2006). Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci U S A 103, 10747-52. Jho, E. H., Zhang, T., Domon, C., Joo, C. K., Freund, J. N. and Costantini, F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22, 1172-83. 9 Kishida, S., Yamamoto, H., Hino, S., Ikeda, S., Kishida, M. and Kikuchi, A. (1999). DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol 19, 4414-22. Lustig, B., Jerchow, B., Sachs, M., Weiler, S., Pietsch, T., Karsten, U., van de Wetering, M., Clevers, H., Schlag, P. M., Birchmeier, W. et al. (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22, 1184-93. Journal of Cell Science Accepted manuscript MacDonald, B. T., Tamai, K. and He, X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17, 9-26. Metcalfe, C. and Bienz, M. (2011). Inhibition of GSK3 by Wnt signalling--two contrasting models. J Cell Sci 124, 3537-44. Schwarz-Romond, T., Fiedler, M., Shibata, N., Butler, P. J., Kikuchi, A., Higuchi, Y. and Bienz, M. (2007). The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol 14, 484-92. Smalley, M. J., Sara, E., Paterson, H., Naylor, S., Cook, D., Jayatilake, H., Fryer, L. G., Hutchinson, L., Fry, M. J. and Dale, T. C. (1999). Interaction of axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. Embo J 18, 2823-35. Stamos, J. L. and Weis, W. I. (2013). The beta-catenin destruction complex. Cold Spring Harb Perspect Biol 5, a007898. Tanneberger, K., Pfister, A. S., Kriz, V., Bryja, V., Schambony, A. and Behrens, J. (2011). Structural and functional characterization of the Wnt inhibitor APC membrane recruitment 1 (Amer1). J Biol Chem 286, 19204-14. Willert, K., Brown, J. D., Danenberg, E., Duncan, A. W., Weissman, I. L., Reya, T., Yates, J. R., 3rd and Nusse, R. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448-52. Wu, G., Huang, H., Garcia Abreu, J. and He, X. (2009). Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 4, e4926. Yamamoto, H., Kishida, S., Kishida, M., Ikeda, S., Takada, S. and Kikuchi, A. (1999). Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem 274, 10681-4. Zeng, X., Huang, H., Tamai, K., Zhang, X., Harada, Y., Yokota, C., Almeida, K., Wang, J., Doble, B., Woodgett, J. et al. (2008). Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135, 367-75. 10 Figure Legends Fig. 1. Expression levels and -catenin degradation capacity of axin and conductin (A, B) Western blotting for conductin (Cdt), axin and GFP in lysates of MDA-MB-231 cells treated with Wnt3a for 6h or left untreated (A, lanes 1,2), in lysates of colorectal cancer cells HCT116, SW480 and DLD1 (B, lanes 1-3), and in serially diluted lysates of HEK293T cells transfected with human GFP-Conductin or GFP-Axin (lanes 3-6 and 7-10 in A, and lanes 4-6 Journal of Cell Science Accepted manuscript and 7-9 in B). Loading control: -actin. Relative levels of axin (black) and conductin (grey) were determined by densitometric measurements and normalisation to levels of overexpressed proteins based on the anti-GFP blot. Error bars: s.e.m. of five independent experiments. (C) Immunofluorescence staining of -catenin (red) in SW480 cells transfected with GFPConductin or GFP-Axin (green). Dotted lines mark transfected cells. Scale bar: 20µm. (D) Quantification of -catenin fluorescence intensities of three independent experiments as in (C). Ut, untransfected. Error bars: s.e.m. (n=20); * p<0.001 (t-test). (E) Blotting of GFP versus -catenin intensities of individual cells from (C). (F) Western blotting for Flag and GFP in lysates of SW480 cells transfected with indicated plasmids. Numbers below Flag blot show densitometric measurements of Flag--catenin normalised to empty GFP. (-), empty vector. Fig. 2. Impact of depletion of axin and conductin on Wnt signalling (A) MDA-MB-231 cells transfected with indicated siRNAs were treated with Wnt3a for 6 hrs (+Wnt) or left untreated. -Catenin staining at DAPI stained nuclei was quantified in individual cells. Error bars: s.e.m. of three independent experiments (n>1200); * p<0.05 (ANOVA). (B-D) Western blotting for dephosphorylated (active) -catenin (ABC), axin and conductin in lysates from MDA-MB-231 (B), U2OS (C; upper three panels: hypotonic lysates, lower two panels: Triton X-100 lysates) and HEK293T cells (D) transfected with indicated siRNAs, and incubated with or without Wnt3a for 6 hrs. Loading control: -actin. Bar chart in (D) shows fold changes of TCF/-catenin dependent reporter activity. Error bars: s.e.m. of three independent experiments (n=6); * p<0.001 (t-test). 11 Fig. 3. Differential association of axin and conductin with Dvl mediated by DIX-DIX domain interactions (A) Western blotting for GFP and HA in membrane (M) and cytoplasmic (C) fractions of HEK293T cells transfected with indicated plasmids. Tubulin and PanCadherin blots show purity of fractions. *, unspecific band. (B) Immunofluorescence staining of HEK293T cells cotransfected with GFP-Axin or GFPAxinCdtDIX (green) together with either empty HA or HA-Dvl2-CAAX (red). Inlayed boxes are magnified on the right. Scale bar: 20µm. Cells with uniform membrane recruitment of the Journal of Cell Science Accepted manuscript GFP construct in the presence of Dvl2-CAAX were quantified. Error bars: s.e.m. of three independent experiments (n=600). (C) Immunofluorescence staining of indicated Flag constructs (red) in U2OS cells coexpressing CFP-Dvl2 (green). Scale bar: 20µm. Quantification of cells with diffuse CFPDvl2 localisation. Error bars: s.e.m. of three independent experiments (n=400). Schemes on the right show interaction models of DIX domains of axin and conductin (red) and Dvl2 (green). “X” indicates defective DIX heads due to M3 mutation. Fig. 4. Differential inhibition of axin and conductin by Dvl via DIX domains (A) Immunofluorescence staining of -catenin (red) in SW480 cells transfected with GFPAxin (a-c), GFP-AxinCdtDIX (d,e), GFP-Conductin (Cdt) (f,g) or GFP-CdtAxinDIX (h,i) (green) together with either empty HA vector (-), HA-Dvl2-CAAX or HA-Dvl2M2-CAAX. Dotted lines mark transfected cells. Scale bar: 20µm. (B) Quantification of -catenin fluorescence intensities from (A). Error bars: s.e.m. of three independent experiments (n>65); * p<0.01, ** p<0.001 (t-test). (C) Blotting of GFP intensities versus -catenin intensities of individual cells from (A). (D, E) Western blotting for Flag, GFP and HA in lysates from SW480 cells transfected with indicated plasmids. Numbers below Flag blots show densitometric measurements of Flag-catenin normalised to empty GFP. (-), empty vector . (F) Quantification of -catenin fluorescence intensities of SW480 cells expressing indicated plasmids. (-), empty vector. Error bars: s.e.m. of three independent experiments (n>65); ** p<0.001 (t-test). (G) Fold changes of TCF/-catenin dependent reporter activity in HEK293T cells transfected with indicated siRNAs together with empty vector or HA-Dvl2. Error bars: s.e.m. of three independent experiments (n=6); * p<0.01 (t-test). 12 Journal of Cell Science Accepted manuscript Journal of Cell Science Accepted manuscript Journal of Cell Science Accepted manuscript Journal of Cell Science Accepted manuscript












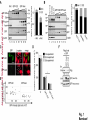
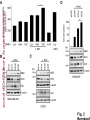
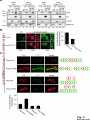
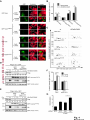











![Basic region of residues 228-231 of protein kinase CK1[alpha] is](http://s1.studyres.com/store/data/015745345_1-81da768c37e720623b4b1898472e2f2e-150x150.png)